Abstract
The recent development of analogs of brefeldin A (BFA), a fungal metabolite, for the improvement of BFA apoptosis-inducing activity is described. BFA has been isolated from various soil or, more recently, marine fungi and has shown versatile beneficial activities. More importantly, the apoptosis-inducing activity of BFA in cancer cells highlights the possibility of further developing this natural product as an anticancer agent. Besides its biological importance, its structural features have also gathered tremendous interest from both medicinal and synthetic chemists. By a medicinal chemistry and total synthesis approach, numerous analogs from BFA have been developed to improve its inferior bioavailability and its antiproliferative ability. In this review, the recent medicinal chemistry efforts in relation to the production of BFA analogs are extensively presented.
1. Introduction
Since its first isolation from Penicillium decumbens in 1958 [1], brefeldin A (BFA) has been touted as a promising lead molecule in the world of drug development because of its potent biological activity in the antitumor [2], antifungal [3], and antiviral [4] fields. More importantly, its apoptosis-inducing properties [5,6] in various cancer cell lines and its ability to disrupt the cis-Golgi apparatus [7,8] drove medicinal chemists to discover more advanced pharmaceutical compounds (APIs) derived from BFA [9]. This medicinal promise drove the recent isolation of BFA from marine fungi, such as Penicillium sp. PSU-F44 or Penicillium sp. DT-F29, along with its other various structural analogs which were expected to be promising hits for drug development [10,11]. Unfortunately, its poor physical and pharmacokinetic (PK) properties hampered further development [12,13,14]. In response to these hurdles, a tremendous synthetic and medicinal chemistry effort has been put forth to improve its physical and PK properties, along with its own biological activities [15,16].
From a structural point of view, this natural product features a unique trisubstituted cyclopentane skeleton [17] with a 13-membered macrolactone ring that possesses two trans-alkene moieties. Because BFA also contains two hydroxyl groups, esterification was carried out at first for the purpose of producing prodrugs. (Group 1 in Figure 1) Differentiation of these two hydroxyl groups with similar steric demands was tried and showed a structure–activity relationship (SAR) on BFA. The electron-deficient alkene in the 13-membered lactone also provided a variety of synthetic opportunity for prodrug production. (Group 2 in Figure 1) Other approaches showed that structural changes on the terminal methyl group or skeletal changes on the BFA framework (Group 3 in Figure 1) could improve BFA activity efficiently. For this purpose, a systematic study from the simple esterification to the development of a synthetic route for BFA itself was carried out. Herewith, recent structural modifications of BFA and its SAR study will be presented.
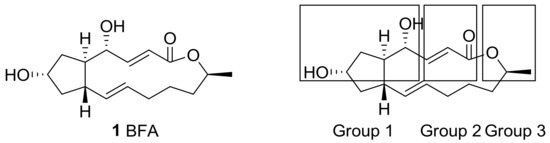
Figure 1.
Structure of brefeldin A (BFA) and groups of its analogs based on structural modifications.
2. Results
2.1. Modification of BFA
Early synthetic derivatization was executed of BFA itself to improve its physical properties. The production of a prodrug of BFA employing esterification or simple alkylation of two hydroxyl groups was usually pursued [14], although distinguishing between these two secondary hydroxyl groups was difficult in normal conditions, as shown in Scheme 1. For further SAR studies, however, this research by Hori group provided the basic information on the two hydroxyl groups [18]. Acetylation of BFA using acetic anhydride in refluxing pyridine afforded 4-acetyl BFA 2 and 4,7-diacetyl BFA 3 simultaneously. The compound 7-acetyl BFA 4 could be isolated after the treatment of BFA in refluxing acetic acid [19]. A similar modification was also observed in an O-methylated BFA synthesis. The compounds 4-O-methylated BFA 5 and 4,7-di-O-methylated BFA 6 could be obtained together and purified after treatment of BFA with silver oxide and iodomethane.
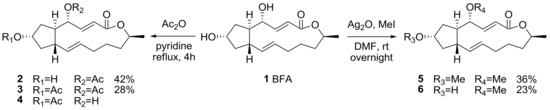
Scheme 1.
Modification of the two hydroxyl groups in BFA. DMF: dimethylformamide.
Hori group also reported an oxidation–reduction protocol to afford BFA analogs in different oxidation states [20], as shown in Scheme 2. Conventional hydrogenation of BFA using H2, Pd/C produced conformationally free 13-membered lactone 7 in good yield. Pyridinium chlorochromate (PCC) oxidation of BFA also gave 7-keto BFA 8 in short time, while this condition gave isomerized 4,7-diketo BFA 9 over a prolonged reaction time. To add conformational rigidity, oxirane functionality was introduced into an electron-rich alkene in BFA, employing meta-chloroperoxybenzoic acid (mCPBA). This reaction proceeded in good yield and in a stereoselective manner:
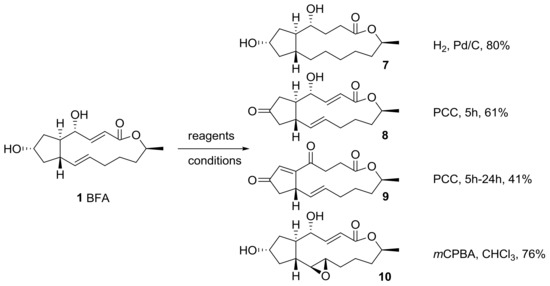
Scheme 2.
Oxidation and reduction of BFA. PCC: pyridinium chlorochromate; mCPBA: meta-chloroperoxybenzoic acid.
An oxidation–reduction protocol was also utilized to inverse the chirality of the 4-hydroxyl group in BFA. The selective oxidation of allylic alcohol followed by a chemoselective reduction of BFA was performed, as shown in Scheme 3. Although each reaction condition produced BFA itself with no reaction from MnO2 oxidation (41%) or undesired reduction from l-selectride (52%), this reaction sequence produced 4S-BFA 12, which is important for a systematic, original SAR study.
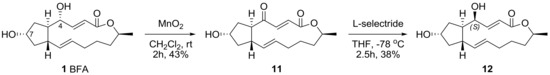
Scheme 3.
Chiral inversion of the 4-hydroxyl group in BFA. l-selectride: lithium tri-sec-butyl(hydrido)borate; THF: tetrahydrofuran.
The 13-membered lactone skeleton was hydrolyzed to find the relationship between ring conformation and activity in BFA (Scheme 4). BFA was hydrolyzed in basic conditions to produce seco acid 13 in good yield. The seco acid 13 was esterified again under O-methylation or in the Fischer esterification condition to make methyl ester 14 or benzyl ester 15, which were tested for their biological activity.
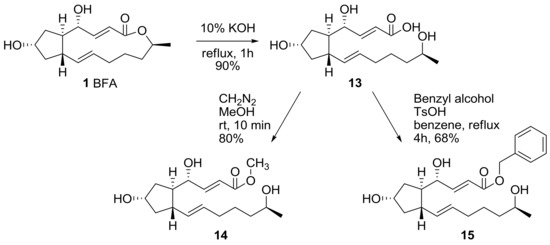
Scheme 4.
Modification of the carbonyl group in BFA.
The above synthesized analogs were tested for their apoptosis-inducing activity using HCT-116 cells, as shown in Table 1. After treatment with the analogs, characteristic DNA ladder formation was observed, as found also following treatment with BFA. The minimum effective concentrations (EC) of the analogs showed that 7-acetyl BFA 4 was almost equipotent to BFA, while 4(S)-BFA 12 lost BFA activity. The cytotoxicity of the analogs and their IC50 were also evaluated. On the basis of the similar cytotoxicity of mono-acetyl BFA derivatives such as 2 or 4 compared to BFA, it can be envisioned that a prodrug approach of the hydroxyl group in BFA would be a promising strategy to find advanced cytotoxic agents. It also seems that the active conformation needs two alkene groups and a 13-membered lactone framework, since the saturated BFA 7, epoxy BFA 10, ring-opened BFA 13, 14, and 15 showed lower apoptotic or cytotoxic activities compared to BFA.

Table 1.
Biological activity of the BFA analogs 2–15.
Another medicinal chemistry approach was employed to produce a water-soluble prodrug. For this purpose, Cushman group tried to change the C2–C3 alkene functional group of BFA with various sulfide moieties to produce a latent leaving group via in vivo oxidation and syn-elimination [21], as shown in Scheme 5. The addition of various thiols was tried in order to improve BFA activity and water solubility, to proceed with a study of facial selectivity.
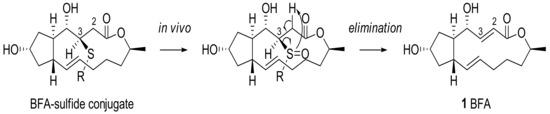
Scheme 5.
Sulfide conjugate approach for BFA.
BFA was treated with various thiols under basic conditions to provide conjugated sulfide adducts which were oxidized to sulfoxides in the presence of alkene (Scheme 6). Although excess amounts or a prolonged reaction time created a correspondingly overoxidized product, such as sulfone or epoxide, careful treatment of the reaction usually produced the desired sulfoxide in moderate yield. The newly generated stereocenters at C3 and sulfur were determined to be (R) and (S), respectively, on the basis of X-ray crystallography (Nonius Kappa CCD diffractometer) of 22 and 32 [22]. Because the addition process occurs very selectively, a diastereomeric mixture of these additional products could be easily purified by silica gel chromatography. (S)- or (R)-configured side chain preparations of the adducts 33 or 34 were obtained by the corresponding chiral thiol addition.
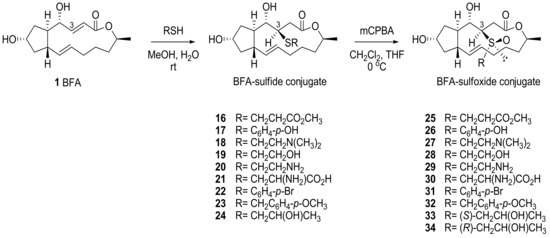
Scheme 6.
Synthesis of sulfide- and sulfoxide-conjugated BFA analogs.
For in vitro assays, these analogs were used to treat various cancer cell lines from the National Cancer Institute repository [23]. As shown in Table 2, the sulfide analogs were less potent than the corresponding sulfoxide analogs. This tendency means that the sulfoxide analogs would easily be converted to BFA in the cells, while the sulfide analogs would need additional oxidation to provide BFA. When the elimination rate was analyzed in a crude NMR study, 33 (t1/2 23 min) and 34 (t1/2 26 min) were more rapidly converted to BFA than 31 (t1/2 440 min). Although the sulfide analogs did not show potent anticancer activity in in vitro assays, the results suggested the possibility of another prodruggable strategy because the sulfide moiety can be easily oxidized with CYP450 or Flavin monooxygenase in vivo. It is important to note that the water solubility of these analogs improved in relation to BFA. For example, cysteine-conjugated analog 21 was more soluble (35 mg/mL) than BFA itself (2.8 mg/mL).

Table 2.
Biological activity of BFA and its analogs 16–33.
For more promising analogs, selenide-conjugated analogs were also prepared using a similar strategy. In Scheme 7, mCPBA oxidation of the selenides 35 produced the corresponding selenoxides (61%) along with BFA (38%), and this emphasized the higher lability of the selenide functional group for syn-elimination. This reactivity could be utilized for the production of another potential BFA prodrug.
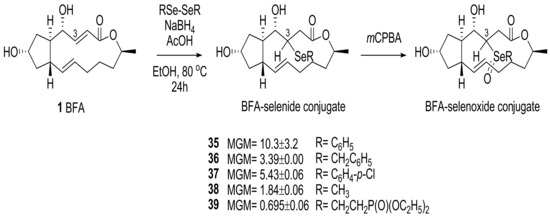
Scheme 7.
Synthesis of selenide-conjugated BFA analogs and their MGMs.
Cushman group also reported an ester analog of BFA [24], as shown in Scheme 8. It was envisioned that a long lipid chain may present the possibility to use an affinity column preparation to directly find a binding site. Because the biological response of BFA was unique and complex depending on the cancer cell line, a target fishing strategy using an affinity column was desired to find BFA’s possible biological mechanisms of action. However, the presence of two secondary hydroxyl groups made it difficult to synthesize a specific and active affinity matrix. In addition, the modification of the two hydroxyl groups could affect the SAR study. So, various alkyl chains should be linked to BFA not only to find target sites, but also to discover active analogs. A succinyl or a glutaryl group was attached to BFA via reaction condition screening.
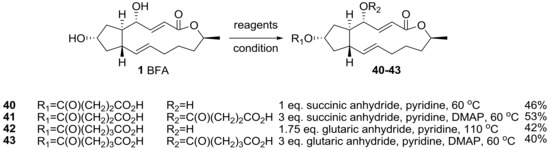
Scheme 8.
Synthesis of succinyl or glutaryl BFA analogs.
Further modifications for SAR studies and affinity matrix preparation were executed as shown in Scheme 9. A simple esterification with TMSCH2N2 was carried out to give ester analog 44 in good yield. More importantly, a longer lipid chain was introduced by the activation of the carboxylic acid in 42 and amide coupling with 6-aminohexanol, to provide hydroxyl analog 45. This analog could be utilized for affinity matrix preparation using biotin or resin with the purpose of finding a target site in cells so long as this analog does not lose BFA own biological effect on cancer cell lines. Bisfunctionalization of 4,7-dihydroxyl BFA was also carried out via a simple esterification reaction.
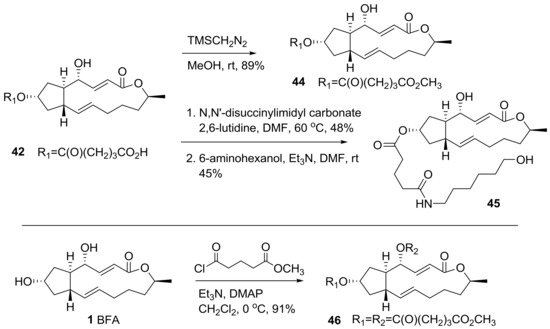
Scheme 9.
Synthesis of BFA analogs with long lipid chains. TMS: trimethylsilyl; DMAP: 4-dimethylaminopyridine.
Scheme 10 describes the monofunctionalization at the 4-hydroxyl group in BFA. A protection reaction was necessary for selective modification, because the 7-hydroxyl group was more reactive than the 4-hydroxyl group. t-butyldimethylsilyl (TBS) protection afforded monosubstituted BFA 47 in low yield. With this 7-protected BFA prepared, similar functionalizations were executed to give the desired monosubstituted analogs at the 4-hydroxyl group in BFA after conventional TBS deprotection.
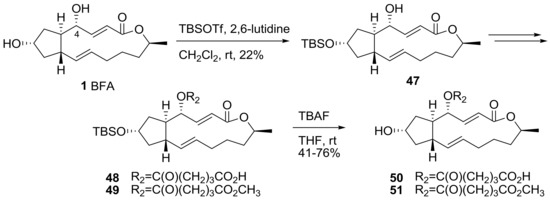
Scheme 10.
Synthesis of 4-acyl BFA analogs. TBS: t-butyldimethylsilyl, Tf: trifluoromethanesulfonyl, TBAF: tetra-n-butylammonium fluoride.
The biological evaluation of the synthesized ester analogs is depicted in Table 3. The mean graph midpoint (MGM) of the GI50 value for various cancer cell lines was calculated and reported. Although a solid SAR could be established from this result, the cell permeability of the analogs is important for their anticancer activity, as demonstrated by the fact that methyl ester analogs were usually superior than their carboxylic acid analogs. It is impressive that the anticancer activity was still maintained in the long chain-extended analog 45. This analog could be utilized in affinity matrix preparation.

Table 3.
Biological activity of BFA and its ester analogs.
More various functional groups, including aromatic esters, were also attached to BFA to evaluate their anticancer activity. Zhu group reported the synthesis of a BFA-based prodrug via simple esterification with various aromatic or alkyl groups, as shown in Scheme 11 [9]. These analogs were evaluated in TE-1 cell line, showing moderate activity for the analogs 52a, 53a, and 53h. Because the in vivo stability of BFA hampered its further development, a stability test of the analog 53h was also carried out to find if it could be utilized for BFA prodrug production.
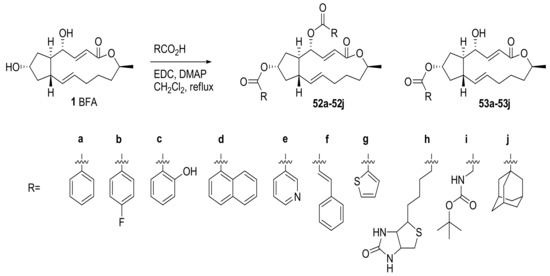
Scheme 11.
Synthesis of BFA analogs with ester functional groups. EDC: 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide.
A recent impressive BFA analog was developed using a nitric oxide (NO)-producing moiety [25], as shown in Scheme 12. Since an NO donor hybrid was used for chemotherapy, it has been employed to strengthen the cytotoxicity level of anticancer agents such as evodiamine or spirolactone-type diterpenoid. Furoxan, a NO-producing subunit, was attached to BFA on the basis of SAR. After extensive screening and optimization of conditions, the compound 54 was found to be active against prostate, colon, and liver cancer cell lines with IC50 of nanomolar concentration. This cytotoxicity is consistent with the high NO-producing ability of 54 in HepG2 (liver cancer) cells. It is also important that 54 induced apoptosis of HepG2 cells in a dose-dependent manner, and apoptosis was reduced when hemoglobin, an NO scavenger, was present. This means that an NO-producing ability is important for the induction of apoptosis in cancer cell lines. The employment of a protein array indicated that survivin and heme oxygenase-1 and 2 are related to this apoptotic response of BFA analog 54.
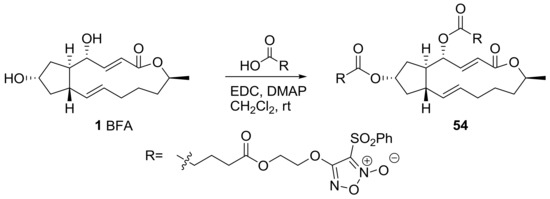
Scheme 12.
Synthesis of NO-producing ester analogs of BFA.
Amino-BFA analog 57 was prepared using reductive amination of 7-keto BFA 8 and chiral sulfinyl amine 55, followed by acidic deprotection [26], as shown in Scheme 13. However, when 57 was used to treat HeLa cells to observe its disrupting effect on the Golgi complex, it showed less activity than BFA itself.
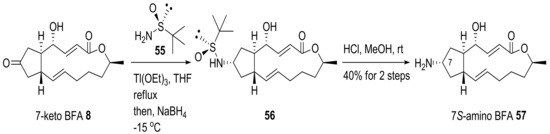
Scheme 13.
Synthesis of 7-amino-BFA analogs.
2.2. Modification of BFA Based on Total Synthesis
BFA gathered tremendous attention from synthetic chemists because of its unique structure. A fused bicyclic skeleton with trisubstituted cyclopentane and 13-membered lactone has been always a synthetic challenge for full synthetic approaches. In addition, the presence of two alkenes and four stereocenters was also a formidable hurdle in the synthesis of BFA. To solve these problems, many synthetic routes have been developed by employing unique methodologies or multiple-step synthesis, which also provided analogs of BFA. It is worth noting that synthetic analogs from total synthesis of BFA may possess a skeleton different from the BFA’s original one. Since conventional medicinal chemistry approaches used BFA as a starting material, simple modifications, such as esterification–hydrolysis, oxidation–reduction, and addition reactions of BFA were possible. The construction of the BFA skeleton via total synthesis from simple starting materials, however, could change the BFA’s skeleton itself. This dramatic change may lead to precious information for an SAR study.
Wu group reported a conjugate addition of vinyl cuprate 59 to substituted cyclopentenone 58 to build another side chain in the BFA framework [27], as shown in Scheme 14. While an achiral reduction afforded the undesired epimer 62 as a major product, a chiral reduction using (S)-CBS reagent 63 was carried out to produce the desired secondary alcohol 61 as a major product. The desired alcohol 61 and its epimer 62 were transformed into BFA 1 and 7-epi-BFA 64, following the conventional reaction route.
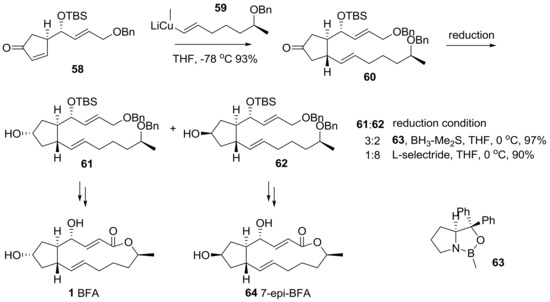
Scheme 14.
Total synthesis of BFA and 7-epi-BFA using the conjugated addition route.
After the development of a synthetic route to BFA, its variation using different synthon was tried to make BFA analogs, as shown in Scheme 15 [28]. Desmethyl BFA 67 and its 7-epimer 68 were prepared from a similar reaction route, employing desmethyl side chain 65 instead of 59. A similar strategy was also tried for 13-oxo-BFA analog 71 synthesis [29]. Using vinylborane 69 from lactic acid, an Rh-mediated conjugate addition was carried out to attach a side chain in the BFA skeleton. Following a similar reaction pathway, 13-oxo-BFA analog 71 could be obtained uneventfully. It is interesting that the same strategy was also applied to pursue 13-thio-BFA, which could not be synthesized via an Rh-mediated coupling reaction.
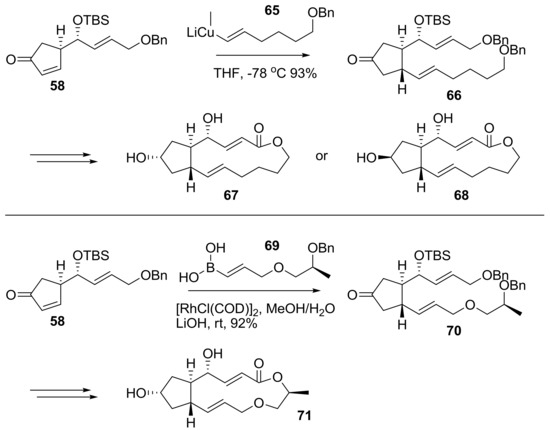
Scheme 15.
Synthesis of NO-producing ester analogs of BFA. COD: cyclooctadiene.
Amino-BFA was also prepared in Scheme 16. To introduce nitrogen in the BFA structure, alanine 72 was chosen as the starting material for side chain 73. After efficient cross metathesis (CM) [30] with side chain 73 and bicyclic lactone 74, the desired CM adduct 75 was successfully transformed to unsaturated enone 76. For the completion of the synthesis, a Luche reduction was tried to establish a 4R chiral alcohol moiety. However, this reaction condition, surprisingly, afforded the undesired epimer 77 as a single diastereomer. After an extensive survey of the reaction conditions and structural confirmation, the synthetic program ended with the synthesis of the 4-epi-BFA lactam 78 analog [31].
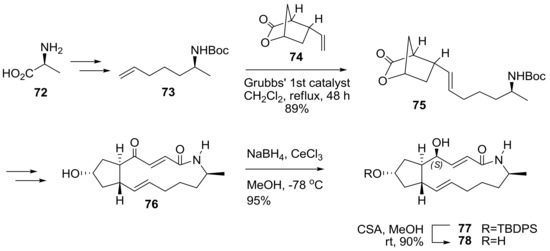
Scheme 16.
Synthesis of 4-epi-BFA lactam analogs. Boc: t-butoxycarbonyl, CSA: camphorsulfonic acid, TBDPS: t-butyldiphenylsilyl.
Another approach to prepare a lactam analog of brefeldin was tried using a different synthetic route as shown in Scheme 17 [32]. Helmchen group developed an efficient synthesis of substituted cyclopentene 79 using catalytic allylic alkylation of cyanomalonate with chiral iridium as a catalyst. After hydrogenation, semi-reduction, and epimerization, chiral aldehyde 80 was obtained quickly and submitted to Julia-Kocienski olefination with sulfonyltetrazole 81 to produce trans-alkene 82 in good yield. Treatment of 82 with acidic deprotection and Swern oxidation afforded the resulting aldehyde 83, which was then submitted to Nozaki-Hiyama-Kishi (NHK) alkenylation with iodoacrylate 84, with nickel/chrome as a catalyst. Although this reaction gave the undesired Si-face product as a major isomer, with a small amount of the desired Re-face adduct, it could be easily and successfully converted to 7-deoxy brefeldin A (brefeldin C) lactam analog 86.
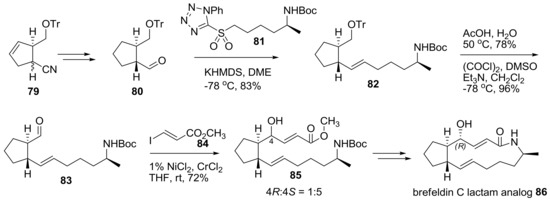
Scheme 17.
Synthesis of brefeldin C lactam analogs. Tr: triphenylmethyl, KHMDS; Potassium bis(trimethylsilyl)amide, DME: dimethoxyethane, DMSO: dimethyl sulfoxide.
Instead of hydrogenation, dihydroxylation of cyclopentene 79 was carried out to introduce a hydroxyl group at the C6 position [26], as shown in Scheme 18. Two substituents of cis-79 possessed a β-face of the cyclopentene plane, and directed catalysis of potassium osmate occurred on the α-face dominantly. With dihydroxyl cyclopentane 87 prepared, a similar conversion, as in Scheme 17, including semi-reduction, epimerization, Julia-Kocienski olefination, and Nozaki-Hiyama-Kishi alkenylation was executed to produce the (6R)-hydroxy BFA analog 89. It is impressive that Golgi complex redistribution was observed in mammalian or plant cells when 89 was used to treat the cells, as observed for BFA [33].
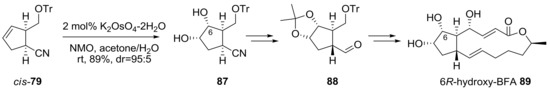
Scheme 18.
Synthesis of (6R)-hydroxy-BFA analogs. NMO: N-methylmorpholine N-oxide.
Helmchen group also reported the semi-synthesis of BFA analogs using hybridization of a BFA skeleton and artificial side chains [26], as shown in Scheme 19. Starting from BFA, protection and chemoselective olefin cleavage of the electron-rich olefin were tried to obtain properly substituted cyclopentanyl aldehyde 90 in a large scale. Employing olefination with sulfonyl tetrazole 81, as previously done, the unsaturated methyl ester 91 could be obtained in one step with a formidable 4R-configured hydroxyl group. Conventional transformations, including deprotection and lactamization, finally afforded BFA lactam 92.
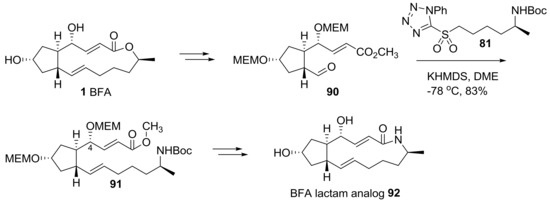
Scheme 19.
Synthesis of BFA lactam analogs. MEM: 2-methoxyethoxylmethyl.
A similar semi-synthetic approach was also applied for the preparation of C15-substituted BFA analogs, as summarized in Scheme 20 [34]. The C15 position had been so far hardly changed because it did not have any active functional group. Employing this semi-synthesis however, it was possible to efficiently introduce unprecedented modifications at the C15 methyl group of BFA. The preparation of variously substituted sulfonyl tetrazoles 94, followed by Julia–Kocienski olefination with bis-protected aldehyde 90 or 93 from BFA, produced a versatile synthesis of substituted ester 95 in good yield. Finally, deprotection–esterification afforded the C15-substituted BFA 96a–c analogs uneventfully. The vinyl group at C15 in analog 96c was also utilized for further transformation. Wacker oxidation, Suzuki coupling, or hydroboration was applied to add a carbonyl, an aromatic, or a hydroxyl group, respectively. After various transformation stages, 15 analogs were prepared for SAR study. Some of them are represented below.
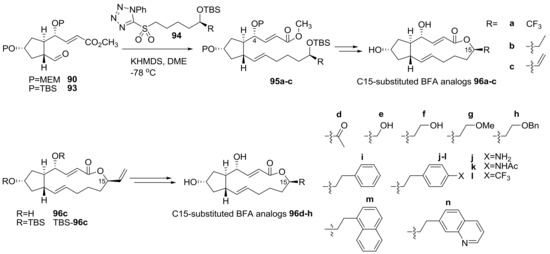
Scheme 20.
Synthesis of C15-substituted BFA analogs.
The biological evaluation of the synthesized analogs was performed using 60 cancer cell lines, such as lung, colon, CNS, prostate, renal, ovarian, and breast cells, as well as leukemia and melanoma cells from the National Cancer Institute (Table 4). While some analogs did not show impressive anticancer activity, 96b, c, i, and j showed potent cell growth inhibition. Importantly, these analogs showed more potent anticancer activity in specific cancer cells such as breast cancer cells. It means that advances in the modification of BFA based on medicinal chemistry and SAR study are continuing successfully.

Table 4.
Biological activity of the BFA and its ester analogs.
An asymmetric desymmetrization route of the BFA skeleton was also developed, as summarized in Scheme 21 [35]. Cyclopentylsuccinic anhydride 97 was submitted to asymmetric methanolysis using quinidine, a chiral natural amine, for the stereogenic differentiation of two carbonyl groups. A resulting chiral carboxylic acid was treated for epimerization, reduction, and Swern oxidation to produce trans-substituted cyclopentane 99 in good yield. After Takai olefination of aldehyde 99, followed by Suzuki coupling with chiral borane 101, trans-alkene 102 was obtained. By employing three carbon homologations, using acetylide addition or NHK reaction and usual transformations, brefeldin C 103 could be obtained successfully.
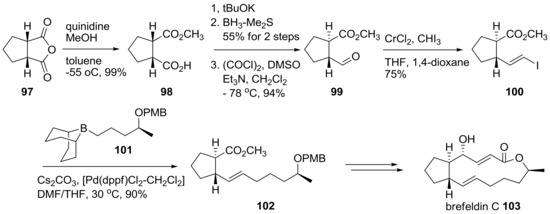
Scheme 21.
Synthesis of brefeldin C and its analogs. PMB: para-methoxybenzyl; dppf: 1,1′-bis(diphenylphosphino)ferrocene.
This strategy was also utilized to synthesize desmethyl BFA analogs 108 and 109 [36], as shown in Scheme 22. By employing the same strategy for chiral desymmetrization of anhydride 97, oxocyclopentyl anhydride 104 could be efficiently converted to chiral ketone 105. Instead of 101, desmethyl side chain 107 was used to synthesize C15-desmethyl BFA analogs 108 and 109, following the procedure developed in Scheme 21.
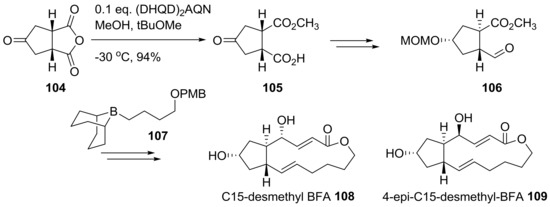
Scheme 22.
Synthesis of C15-desmethyl BFA analogs. (DHQD)2AQN: Hydroquinidine (anthraquinone-1,4-diyl) diether.
A recent synthetic study employing a [3+2]-cycloaddition route was reported to enable a versatile synthesis of BFA 1 and 4-epi-BFA 12 from BFA, in a unified strategy [37]. In Scheme 23, cycloaddition of unsaturated enone 110 with allenylsulfone 111 afforded cyclopentane 112 in a stereoselective manner. After conversion of sulfonylalkene to the corresponding ketone, and following the reaction route, bis-protected BFA skeleton 113 could be obtained efficiently. Finally, BFA 1 and its C4-epimer 12 were prepared in the reaction conditions shown below. Direct global deprotection of 113 afforded 4-epi-BFA 12 in moderate yield, while selective TBS deprotection, followed by Mitsunobu inversion and sequential deprotection, yielded BFA 1 in a straightforward manner. With this synthetic route developed, a more promising synthetic analog of BFA can be anticipated in the future.
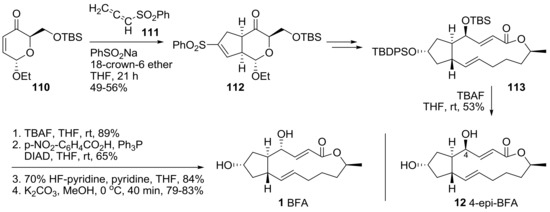
Scheme 23.
Synthesis of BFA and its 4-epimer through cycloaddition of allenylsulfone strategy.
3. Conclusions
The apoptotic activity of BFA has been deeply researched and studied from a medicinal chemistry point of view. Based on an early report that a simple esterification of BFA still maintains its biological activity, a lot of prodrugs or esterified analogs, such as Nitric oxide-releasing BFA and long linker-containing BFA have been synthesized. Although some analogs showed better activity than BFA, the problem related to its physical properties is not solved yet, despite numerous advances in this sense. Hopefully, more soluble sulfide-conjugated BFA analogs have been developed for the purpose of producing BFA prodrugs. In addition, advanced synthetic technologies have been developed to more efficiently obtain the BFA skeleton. Studies on the mechanism of action of BFA were also carried out using these BFA analogs. By employing molecular studies it was shown that esterified BFA analogs were tolerated when inserting between ADP ribosylation factor (ARF) and ARF nucleotide binding site opener (ARNO) proteins, although this molecular interaction did not correspond to the disruption of the Golgi complex [24]. Co-crystalization of synthetic analogs with their target proteins would give meaningful information on their mode of action [38,39]. This synthetic and mechanistic studies would make it possible to change the BFA skeleton efficiently and provide more promising analogs of BFA that possess enhanced apoptotic activity in cancer cells.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the ministry of Education, Science and Technology (NRF-2016R1C1B2006699)
Conflicts of Interest
The authors declare no conflict of interest.
References
- Singleton, V.L.; Bohonos, N.; Ullstrup, A.J. Decumbin, a New Compound from a Species of Penicillium. Nature 1958, 181, 1072. [Google Scholar] [CrossRef] [PubMed]
- Betina, V. Effects of the Macrolide Antibiotic Cyanein on HeLa Cells Growth and Metabolism. Neoplasma 1969, 16, 23–32. [Google Scholar] [PubMed]
- Haerri, E.; Loeffler, W.; Sigg, H.P.; Stähelin, H.; Tamm, C.H. Isolation of New Metabolic Products from Penicillium Brefeldianum. Helv. Chim. Acta 1963, 46, 1235–1243. [Google Scholar]
- Tamura, G.; Ando, K.; Suzuki, S.; Takatsuki, A.; Arima, K. Antiviral Activity of Brefeldin A and Verrugarin A. J. Antibiot. 1968, 21, 160–161. [Google Scholar] [CrossRef] [PubMed]
- Larsson, D.E.; Wickstrom, M.; Hassan, S.; Oberg, K.; Granberg, D. The Cytotoxic Agents NSC-95397, Brefeldin A, Bortezomib and Sanguinarine Induce Apoptosis in Neuroendocrine Tumors in Vitro. Anticancer Res. 2010, 30, 149–156. [Google Scholar] [PubMed]
- Moon, J.L.; Kim, S.Y.; Shin, S.W.; Park, J. Regulation of Brefeldin A-Induced ER Stress and Apoptosis by Mitochondrial NADP+-Dependent Isocitrate Dehydrogenase. Biochem. Biophys. Res. Commun. 2012, 417, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Lippincott-Schwartz, J.; Yuan, L.C.; Bonifacino, J.S.; Klausner, R.D. Rapid Redistribution of Golgi Proteins into the ER in Cells Treated with Brefeldin A: Evidence for Membrane Cycling from Golgi to ER. Cell 1989, 56, 801–813. [Google Scholar] [CrossRef]
- Dinter, A.; Berger, E.G. Golgi-Disturbing Agents. Histochem. Cell Biol. 1998, 109, 571–590. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Wang, Y.; Zheng, Y.; Chen, W.; Zhu, Q. Synthesis and Cytotoxic Evaluation of Acylated Brefeldin a Derivatives as Potential Anticancer Agents. Chem. Biol. Drug Des. 2013, 82, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Trisuwan, K.; Rukachaisirikul, V.; Sukpondma, Y.; Phongpaichit, S.; Preedanon, S.; Sakayaroj, J. Lactone Derivatives from the Marine-Derived Fungus Penicillium sp. PSU-F44. Chem. Pharm. Bull. 2009, 57, 1100–1102. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Qin, L.; Ding, W.; Liu, Y.; Ma, Z. New Analogues of Brefeldin A from Sediment-Derived Fungus Penicillium sp. DT-F29. Nat. Prod. Res. 2016, 30, 2311–2315. [Google Scholar] [CrossRef] [PubMed]
- Bruning, A.; Ishikawa, T.; Kneusel, R.E.; Matern, U.; Lottspeich, F.; Wieland, F.T. Brefeldin A Binds to Glutathione S-Transferase and is Secreted as Glutathione and Cysteine Conjugates by Chinese Hamster Ovary Cells. J. Biol. Chem. 1992, 267, 7726–7732. [Google Scholar] [PubMed]
- Phillips, L.R.; Supko, J.G.; Malspeis, L. Analysis of Brefeldin A in Plasma by Gas Chromatography with Electron Capture Detection. Anal. Biochem. 1993, 211, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Phillips, L.R.; Wolfe, T.L.; Malspeis, L.; Supko, J.G. Analysis of Brefeldin A and the Prodrug Breflate in Plasma by Gas Chromatography with Mass Selective Detection. J. Pharm. Biomed. Anal. 1998, 16, 1301–1309. [Google Scholar] [CrossRef]
- Fuchs, M.; Fürstner, A. trans-Hydrogenation: Application to a Concise and Scalable Synthesis of Brefeldin A. Angew. Chem. Int. Ed. Engl. 2015, 54, 3978–3982. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, S.; Yelleni, M.K.R. Application of Ru(II)-Catalyzed Enyne Cyclization in the Synthesis of Brefeldin A. J. Org. Chem. 2016, 81, 10912–10921. [Google Scholar] [CrossRef] [PubMed]
- Heasley, B. Recent Developments in the Stereocontrolled Synthesis of Highly Substituted Cyclopentane Core Structures: From Drug Discovery Research to Natural Product Synthesis. Curr. Org. Chem. 2014, 18, 641–686. [Google Scholar] [CrossRef]
- Zhu, J.; Hori, H.; Nojiri, H.; Tsukuda, T.; Taira, Z. Synthesis and Activity of Brefeldin A Analogs as Inducers of Cancer Cell Differentiation and Apoptosis. Bioorg. Med. Chem. Lett. 1997, 7, 139–144. [Google Scholar] [CrossRef]
- Proksa, B.; Uhrin, D.; Adamcova, J.; Fuska, J. Oxidation of Brefeldin A. Pharmazie 1992, 47, 582–584. [Google Scholar] [PubMed]
- Zhu, J.; Nagasawa, H.; Nagura, F.; Mohamad, S.B.; Uto, Y.; Ohkura, K.; Hori, H. Elucidation of Strict Structural Requirements of Brefeldin A as an Inducer of Differentiation and Apoptosis. Bioorg. Med. Chem. 2000, 8, 455–463. [Google Scholar] [CrossRef]
- Argade, A.B.; Devraj, R.; Vroman, J.A.; Haugwitz, R.D.; Hollingshead, M.; Cushman, M. Design and Synthesis of Brefeldin A Sulfide Derivatives as Prodrug Candidates with Enhanced Aqueous Solubilities. J. Med. Chem. 1998, 41, 3337–3346. [Google Scholar] [CrossRef] [PubMed]
- Argade, A.B.; Haugwitz, R.D.; Devraj, R.; Kozlowski, J.; Fanwick, P.E.; Cushman, M. Highly Efficient Diastereoselective Michael Addition of various Thiols to (+)-Brefeldin A. J. Org. Chem. 1998, 63, 273–278. [Google Scholar] [CrossRef]
- Fox, B.M.; Vroman, J.A.; Fanwick, P.E.; Cushman, M. Preparation and Evaluation of Sulfide Derivatives of the Antibiotic Brefeldin a as Potential Prodrug Candidates with Enhanced Aqueous Solubilities. J. Med. Chem. 2001, 44, 3915–3924. [Google Scholar] [CrossRef] [PubMed]
- Anadu, N.O.; Davisson, V.J.; Cushman, M. Synthesis and Anticancer Activity of Brefeldin A Ester Derivatives. J. Med. Chem. 2006, 49, 3897–3905. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Xu, F.; Gao, X.; Han, T.; Li, J.; Pan, H.; Zang, L.; Li, D.; Li, Z.; Uchita, T. Nitric Oxide-Releasing Derivatives of Brefeldin A as Potent and Highly Selective Anticancer Agents. Eur. J. Med. Chem. 2017, 136, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Förster, S.; Persch, E.; Tverskoy, O.; Rominger, F.; Helmchen, G.; Klein, C.; Gönen, B.; Brügger, B. Syntheses and Biological Properties of Brefeldin Analogues. Eur. J. Org. Chem. 2011, 2011, 878–891. [Google Scholar] [CrossRef]
- Wu, Y.; Shen, X.; Yang, Y.; Hu, Q.; Huang, J. Enantioselective Total Synthesis of (+)-Brefeldin A and 7-epi-Brefeldin A. J. Org. Chem. 2004, 69, 3857–3865. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Yang, Y.; Hu, Q.; Huang, J.; Gao, J.; Wu, Y. Synthesis of 15-Demethyl Brefeldin A and its 7-epi Isomer. Chin. J. Chem. 2007, 25, 802–807. [Google Scholar] [CrossRef]
- Gao, J.; Huang, Y.; Wu, Y. Enantioselective Total Synthesis of 13-O-Brefeldin A. Tetrahedron 2008, 64, 11105–11109. [Google Scholar] [CrossRef]
- Paek, S. Synthesis of Tetrasubstituted Alkenes via Metathesis. Molecules 2012, 17, 3348–3358. [Google Scholar] [CrossRef] [PubMed]
- Paek, S.; Seo, S.; Min, K.; Shin, D.M.; Chung, Y.K.; Suh, Y. Synthesis of Lactam Analog of 4-epi-Brefeldin A. Heterocycles 2007, 71, 1059–1066. [Google Scholar]
- Förster, S.; Helmchen, G. Stereoselective Synthesis of a Lactam Analogue of Brefeldin C. Synlett 2008, 2008, 831–836. [Google Scholar]
- Langhans, M.; Förster, S.; Helmchen, G.; Robinson, D.G. Differential Effects of the Brefeldin A Analogue (6R)-Hydroxy-BFA in Tobacco and Arabidopsis. J. Exp. Bot. 2011, 62, 2949–2957. [Google Scholar] [CrossRef] [PubMed]
- Seehafer, K.; Rominger, F.; Helmchen, G.; Langhans, M.; Robinson, D.G.; Özata, B.; Brügger, B.; Strating, J.R.; van Kuppeveld, F.J.; Klein, C.D. Synthesis and Biological Properties of Novel Brefeldin A Analogues. J. Med. Chem. 2013, 56, 5872–5884. [Google Scholar] [CrossRef] [PubMed]
- Archambaud, S.; Aphecetche-Julienne, K.; Guingant, A. A New Total Synthesis of (+)-Brefeldin C. Synlett 2005, 2005, 139–143. [Google Scholar] [CrossRef]
- Archambaud, S.; Legrand, F.; Aphecetche-Julienne, K.; Collet, S.; Guingant, A.; Evain, M. Total Synthesis of (+)-Brefeldin C, (+)-nor-Me Brefeldin A and (+)-4-epi-nor-Me Brefeldin A. Eur. J. Org. Chem. 2010, 2010, 1364–1380. [Google Scholar] [CrossRef]
- Xiong, Z.; Hale, K.J. Total Synthesis of the Antitumor Macrolides, (+)-Brefeldin A and 4-Epi-Brefeldin A from d-Glucose: Use of the Padwa Anionic Allenylsulfone [3+2]-Cycloadditive Elimination to Construct Trans-Configured Chiral Cyclopentane Systems. Org. Lett. 2016, 18, 4254–4257. [Google Scholar] [CrossRef] [PubMed]
- Mossessova, E.; Corpina, R.A.; Goldberg, J. Crystal Structure of ARF1·Sec7 Complexed with Brefeldin A and Its Implications for the Guanine Nucleotide Exchange Mechanism. Mol. Cell 2003, 12, 1403–1411. [Google Scholar] [CrossRef]
- Cherfils, J.; Menetrey, J.; Mathieu, M.; Le Bras, G.; Robineau, S.; Beraud-Dufour, S.; Antonny, B.; Chardin, P. Structure of the Sec7 domain of the Arf exchange factor ARNO. Nature 1998, 392, 101–105. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).