Marine Longilenes, Oxasqualenoids with Ser-Thr Protein Phosphatase 2A Inhibition Activity
Abstract
1. Introduction
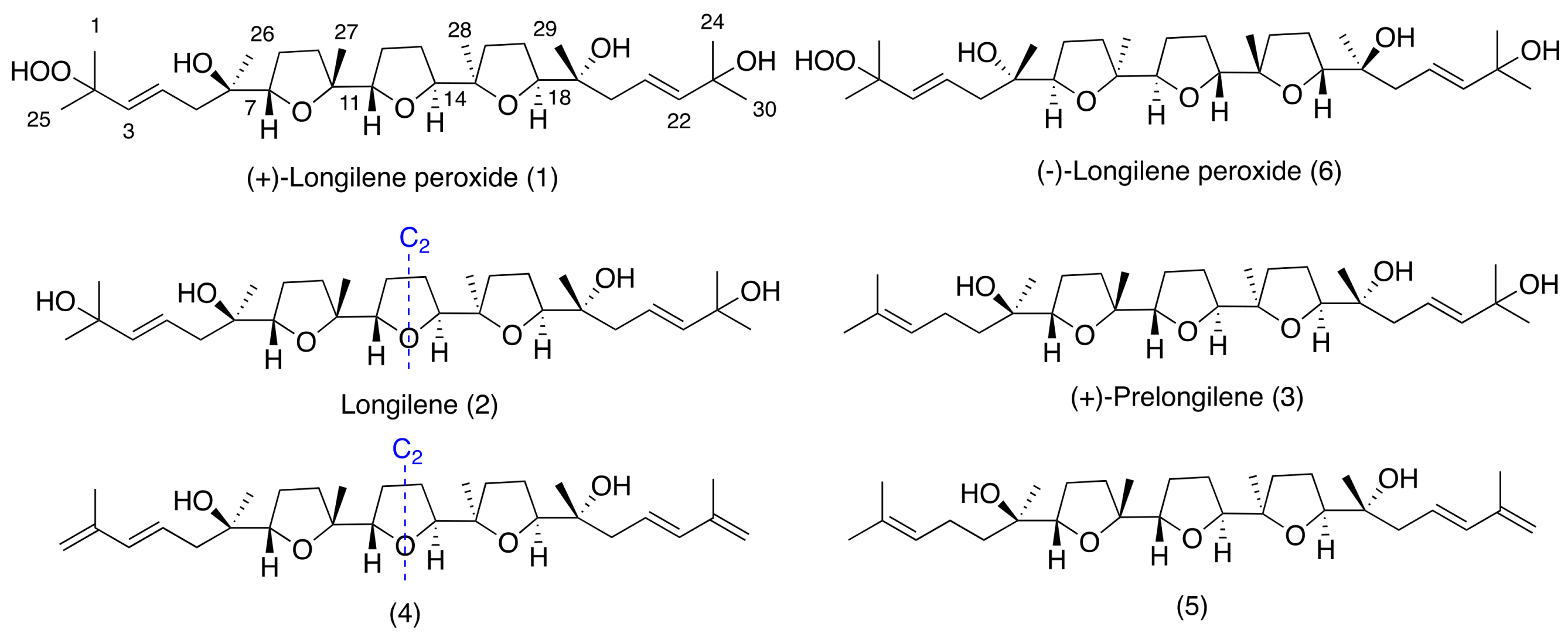
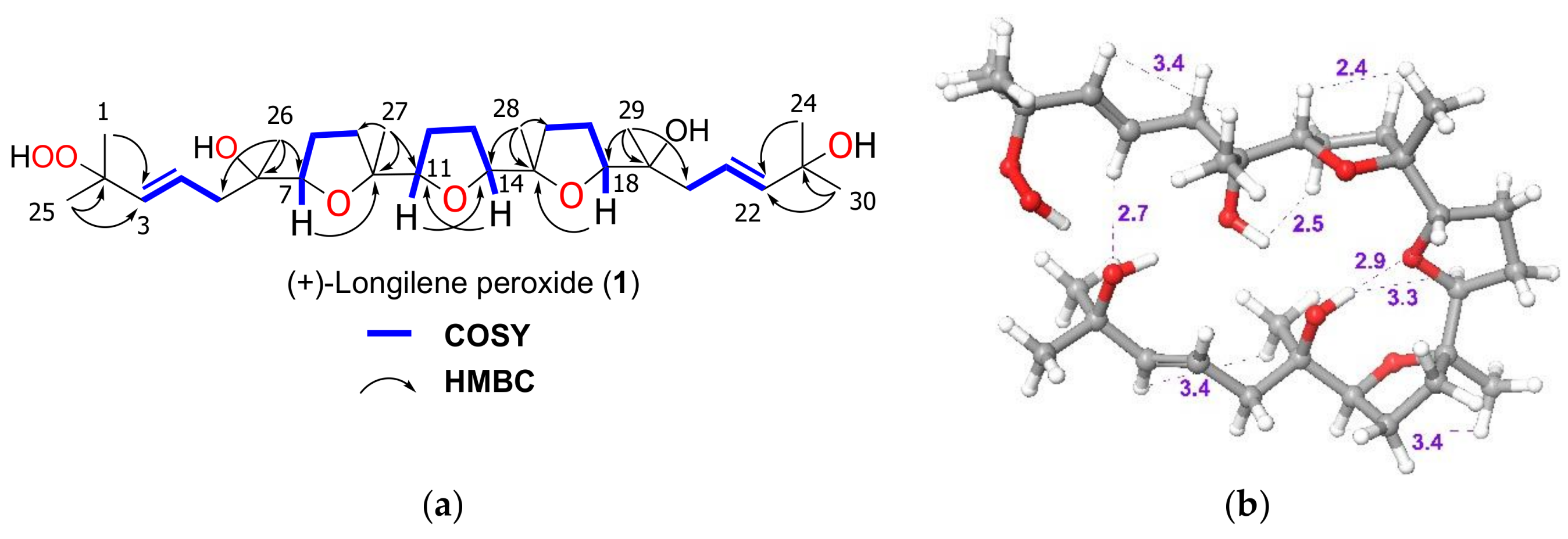
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Biological Material
3.3. Extraction and Isolation
3.4. Transformation of (+)-Prelongilene (3) into Compound 5
3.5. Protein Phosphatase 2A Inhibition Assay
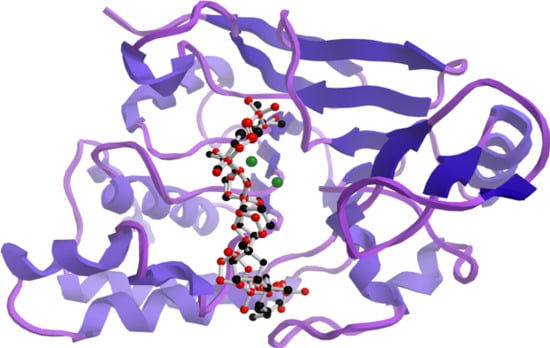
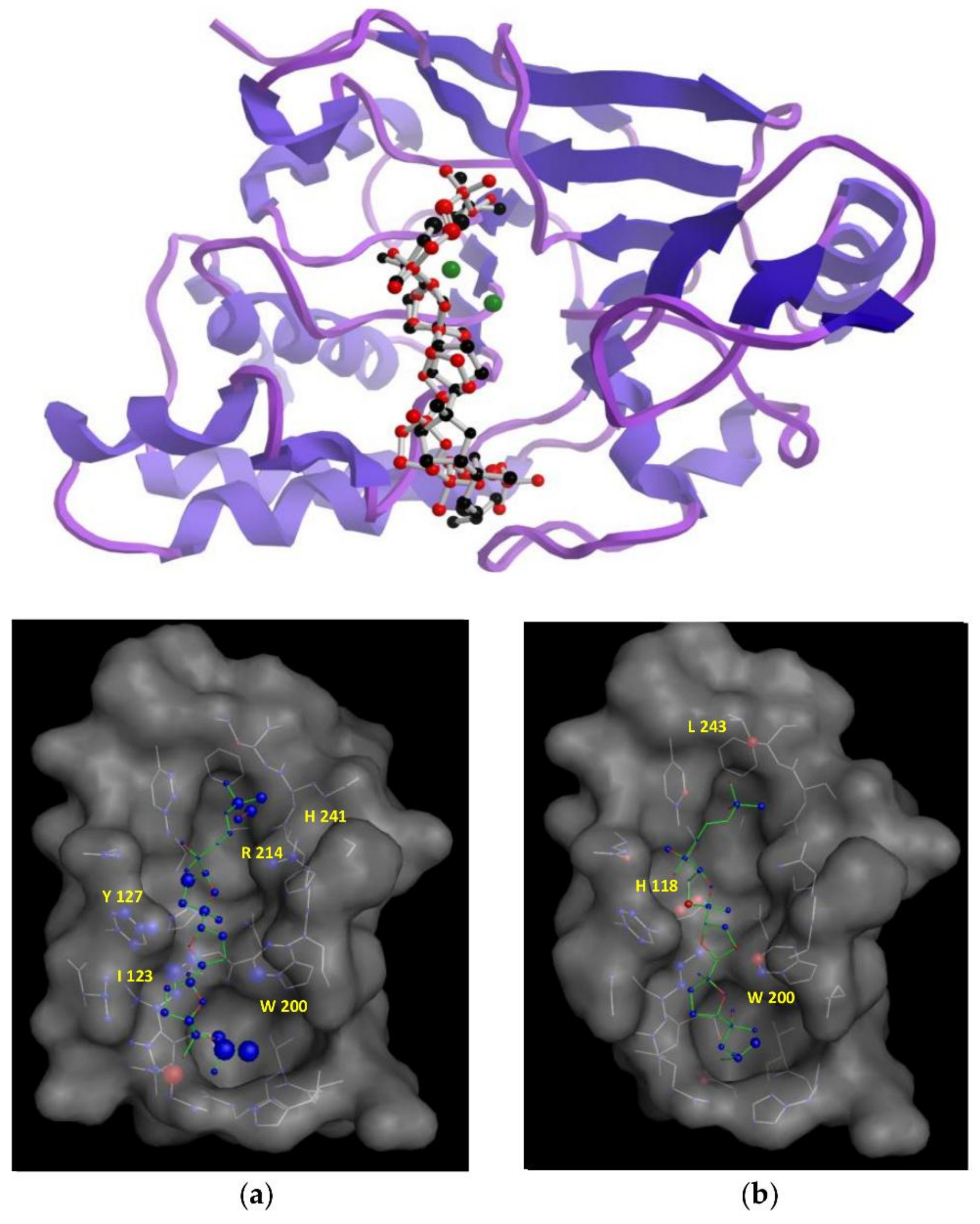
3.6. Docking Studies
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zolnierowicz, S. Type 2A protein phosphatase, the complex regulator of numerous signaling pathways. Biochem. Pharmacol. 2000, 60, 1225–1235. [Google Scholar] [CrossRef]
- Wera, S.; Hemmings, B.A. Serine/threonine protein phosphatases. Biochem. J. 1995, 311, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Hunter, T. Protein kinases and phosphatases: The Yin and Yang of protein phosphorylation and signaling. Cell 1995, 80, 225–236. [Google Scholar] [CrossRef]
- McCluskey, A.; Sim, A.T.R.; Sakoff, J.A. Serine-Threonine Protein Phosphatase Inhibitors: Development of Potential Therapeutic Strategies. J. Med. Chem. 2002, 45, 1151–1175. [Google Scholar] [CrossRef] [PubMed]
- Honkanen, R.E.; Golden, T. Regulators of serine/threonine protein phosphatases at the dawn of a clinical era? Curr. Med. Chem. 2002, 9, 2055–2075. [Google Scholar] [CrossRef] [PubMed]
- Bialojan, C.; Takai, A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem. J. 1988, 256, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Dilip de Silva, E.; Williams, D.E.; Andersen, R.J.; Klix, H.; Holmes, C.F.B.; Allen, T.M. Motuporin, a Potent Protein Phosphatase Inhibitor Isolated from the Papua New Guinea Sponge Theonella swinhoei Gray. Tetrahedron Lett. 1992, 33, 1561–1564. [Google Scholar] [CrossRef]
- Williams, D.E.; Lapawa, M.; Feng, X.D.; Tarling, T.; Roberge, M.; Andersen, R.J. Spirastrellolide A: Revised structure, progress toward the relative configuration, and inhibition of protein phosphatase 2A. Org. Lett. 2004, 6, 2607–2610. [Google Scholar] [CrossRef] [PubMed]
- Cen-Pacheco, F.; Nordström, L.; Souto, M.L.; Martín, M.N.; Fernández, J.J.; Daranas, A.H. Studies on Polyethers Produced by Red Algae. Mar. Drugs 2010, 8, 1178–1188. [Google Scholar] [CrossRef] [PubMed]
- Cen-Pacheco, F.; Villa-Pulgarin, J.A.; Mollinedo, F.; Norte, M.; Daranas, A.H.; Fernández, J.J. Cytotoxic oxasqualenoids from the red alga Laurencia viridis. Eur. J. Med. Chem. 2011, 46, 3302–3308. [Google Scholar] [CrossRef] [PubMed]
- Cen-Pacheco, F.; Villa-Pulgarin, J.A.; Mollinedo, F.; Norte, M.; Fernández, J.J.; Daranas, A.H. New Polyether Triterpenoids from Laurencia viridis and Their Biological Evaluation. Mar. Drugs 2011, 9, 2220–2235. [Google Scholar] [CrossRef] [PubMed]
- Itokawa, H.; Kishi, E.; Morita, H.; Takeya, K.; Iitaka, Y. A New Squalene-type Triterpene from the Woods of Eurycoma longifolia. Chem. Lett. 1991, 2221–2222. [Google Scholar] [CrossRef]
- Morimoto, Y.; Iwai, T.; Kinoshita, T. Total synthesis and determination of the absolute configuration of (−)-longilene peroxide. Tetrahedron Lett. 2001, 42, 6307–6309. [Google Scholar] [CrossRef]
- Fernández, J.J.; Souto, M.L.; Gil, L.V.; Norte, M. Isolation of naturally occurring dactylomelane metabolites as Laurencia constituents. Tetrahedron 2005, 61, 8910–8915. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olsen, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. Software news and update AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Velec, H.F.; Gohlke, H.; Klebe, G. DrugScore(CSD)-knowledge-based scoring function derived from small molecule crystal data with superior recognition rate of near-native ligand poses and better affinity prediction. J. Med. Chem. 2005, 48, 6296–6303. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Xu, Y.; Chen, Y.; Jeffrey, P.D.; Chao, Y.; Lin, Z.; Strack, Z.L.J.; Stock, J.B.; Shi, Y. Structure of Protein Phosphatase 2A Core Enzyme Bound to Tumor-Inducing Toxins. Cell 2006, 127, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Kita, A.; Matsunaga, S.; Takai, A.; Kataiwa, H.; Wakimoto, T.; Fusetani, N.; Isobe, M.; Miki, K. Crystal structure of the complex between calyculin A and the catalytic subunit of protein phosphatase 1. Structure 2002, 10, 715–724. [Google Scholar] [CrossRef]
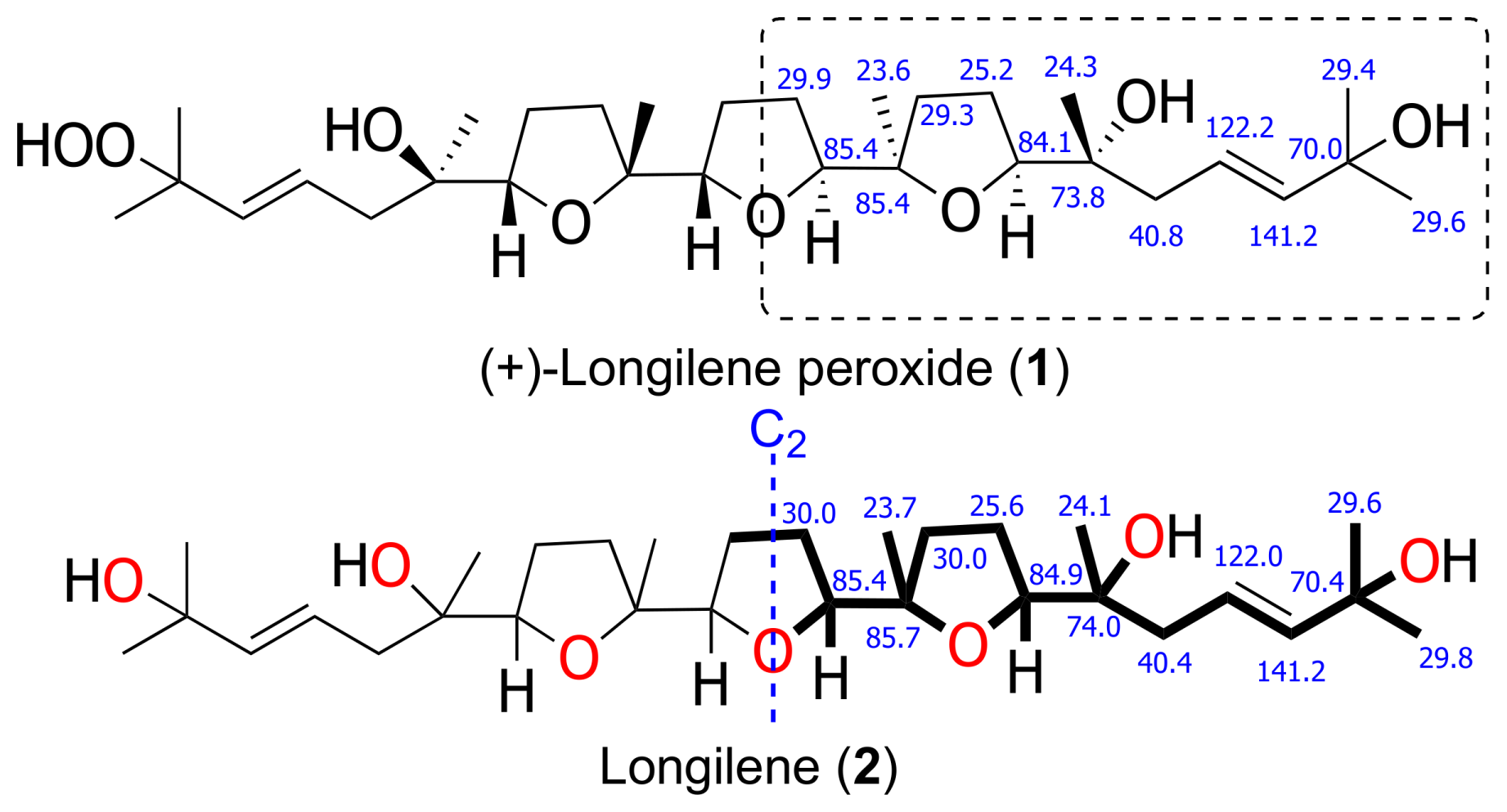
| (+)-Longilene Peroxide (1) | Longilene (2) | |||
|---|---|---|---|---|
| Position | δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) |
| 1 | 26.9, CH3 | 1.19, s | 29.6, CH3 | 1.29, s |
| 2 | 80.1, C | 70.4, C | ||
| 3 | 137.0, CH | 5.43, d (15.6) | 141.2, CH | 5.62, d (15.6) |
| 4 | 125.8, CH | 5.81, ddd (6.6, 8.5, 15.6) | 122.0, CH | 5.77, ddd (7.0, 7.4, 15.6) |
| 5 | 41.3, CH2 | 1.78, dd (8.5, 13.3) 2.20, dd (6.6, 13.3) | 40.4, CH2 | 1.78, dd (7.0, 13.4) 2.15, dd (7.4, 13.4) |
| 6 | 73.9, C | 74.0, C | ||
| 7 | 85.1, CH | 3.72, m | 84.9, CH | 3.70, dd (6.4, 6.6) |
| 8 | 25.8, CH2 | 1.89, m 2.06, m | 25.6, CH2 | 1.91, m 2.03, m |
| 9 | 29.7, CH2 | 1.49, m 2.06, m | 30.0, CH2 | 1.47, m 2.06, m |
| 10 | 85.8, C | 85.7, C | ||
| 11 | 85.8, CH | 4.09, m | 85.4, CH | 4.11, dd (5.5, 5.6) |
| 12 | 30.1, CH2 | 1.50, m 2.01, m | 30.0, CH2 | 1.49, m 2.01, m |
| 13 | 29.9, CH2 | 1.50, m 2.01, m | 30.0, CH2 | 1.49, m 2.01, m |
| 14 | 85.4, CH | 4.09, m | 85.4, CH | 4.11, dd (5.5, 5.6) |
| 15 | 85.4, C | 85.7, C | ||
| 16 | 29.3, CH2 | 1.46, m 2.03, m | 30.0, CH2 | 1.47, m 2.06, m |
| 17 | 25.2, CH2 | 1.89, m 2.03, m | 25.6, CH2 | 1.91, m 2.03, m |
| 18 | 84.1, CH | 3.72, m | 84.9, CH | 3.70, dd (6.4, 6.6) |
| 19 | 73.8, C | 74.0, C | ||
| 20 | 40.8, CH2 | 1.88, dd (6.8, 13.4) 2.20, dd (7.0, 13.4) | 40.4, CH2 | 1.78, dd (7.0, 13.4) 2.15, dd (7.4, 13.4) |
| 21 | 122.2, CH | 5.75, ddd (6.8, 7.0, 15.6) | 122.2, CH | 5.77, ddd (7.0, 7.4, 15.6) |
| 22 | 141.2, CH | 5.61, d (15.6) | 141.2, CH | 5.62, d (15.6) |
| 23 | 70.0, C | 70.4, C | ||
| 24 | 29.4, CH3 | 1.27, s | 29.6, CH3 | 1.29, s |
| 25 | 24.2, CH3 | 1.37, s | 29.8, CH3 | 1.31, s |
| 26 | 24.3, CH3 | 1.20, s | 24.1, CH3 | 1.24, s |
| 27 | 24.2, CH3 | 1.09, s | 23.7, CH3 | 1.10, s |
| 28 | 23.6, CH3 | 1.07, s | 23.7, CH3 | 1.10, s |
| 29 | 24.3, CH3 | 1.27, s | 24.1, CH3 | 1.24, s |
| 30 | 29.6, CH3 | 1.31, s | 29.8, CH3 | 1.31, s |
| -OOH | 10.57, s | |||
| -OH-6 | 5.24, s | |||
| -OH-19 | 5.03, s | |||
| -OH-23 | 3.29, s | |||
| (+)-Prelongilene (3) | |||||
|---|---|---|---|---|---|
| Position | δC Type | δH (J in Hz) | C | δC Type | δH (J in Hz) |
| 1 | 17.7, CH3 | 1.60, s | 16 | 30.0, CH2 | 1.46, m 2.06, m |
| 2 | 131.1, C | 17 | 25.5, CH2 | 1.93, m 2.14, m | |
| 3 | 124.8, CH | 5.08, t (6.9) | 18 | 85.0, CH | 3.81, dd (4.4, 8.1) |
| 4 | 22.5, CH2 | 1.97, m 2.04, m | 19 | 74.1, C | |
| 5 | 39.0, CH2 | 1.29, m 1.43, m | 20 | 40.8, CH2 | 1.83, m 2.17, m |
| 6 | 72.5, C | 21 | 122.7, CH | 5.74, ddd (6.2, 8.6, 15.2) | |
| 7 | 83.4, CH | 3.73, dd (6.9, 7.6) | 22 | 141.0, CH | 5.62, d (15.2) |
| 8 | 25.4, CH2 | 1.80, m 1.91, m | 23 | 70.3, C | |
| 9 | 30.0, CH2 | 1.46, m 2.04, m | 24 | 29.8, CH3 | 1.31, s |
| 10 | 84.7, C | 25 | 25.7, CH3 | 1.66, s | |
| 11 | 85.0, CH | 4.07, dd (5.4, 10.3) | 26 | 24.9, CH3 | 1.27, s |
| 12 | 29.5, CH2 | 1.50, m 1.99, m | 27 | 23.5, CH3 | 1.08, s |
| 13 | 29.5, CH2 | 1.50, m 1.99, m | 28 | 24.0, CH3 | 1.11, s |
| 14 | 85.7, CH | 4.13, dd (2.1, 5.7) | 29 | 24.1, CH3 | 1.20, s |
| 15 | 85.8, C | 30 | 30.1, CH3 | 1.31, s | |
| OH-6 | 4.94, s | ||||
| OH-19 | 4.39, s | ||||
| OH-23 | 2.57, s | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cen-Pacheco, F.; Pérez Manríquez, C.; Luisa Souto, M.; Norte, M.; Fernández, J.J.; Hernández Daranas, A. Marine Longilenes, Oxasqualenoids with Ser-Thr Protein Phosphatase 2A Inhibition Activity. Mar. Drugs 2018, 16, 131. https://doi.org/10.3390/md16040131
Cen-Pacheco F, Pérez Manríquez C, Luisa Souto M, Norte M, Fernández JJ, Hernández Daranas A. Marine Longilenes, Oxasqualenoids with Ser-Thr Protein Phosphatase 2A Inhibition Activity. Marine Drugs. 2018; 16(4):131. https://doi.org/10.3390/md16040131
Chicago/Turabian StyleCen-Pacheco, Francisco, Claudia Pérez Manríquez, María Luisa Souto, Manuel Norte, José Javier Fernández, and Antonio Hernández Daranas. 2018. "Marine Longilenes, Oxasqualenoids with Ser-Thr Protein Phosphatase 2A Inhibition Activity" Marine Drugs 16, no. 4: 131. https://doi.org/10.3390/md16040131
APA StyleCen-Pacheco, F., Pérez Manríquez, C., Luisa Souto, M., Norte, M., Fernández, J. J., & Hernández Daranas, A. (2018). Marine Longilenes, Oxasqualenoids with Ser-Thr Protein Phosphatase 2A Inhibition Activity. Marine Drugs, 16(4), 131. https://doi.org/10.3390/md16040131