Anticancer Activity of Anthopleura anjunae Oligopeptides in Prostate Cancer DU-145 Cells
Abstract
:1. Introduction
2. Results
2.1. Effect of AAP-H on Cell Proliferation
2.2. Effect of AAP-H on Cell Proliferation
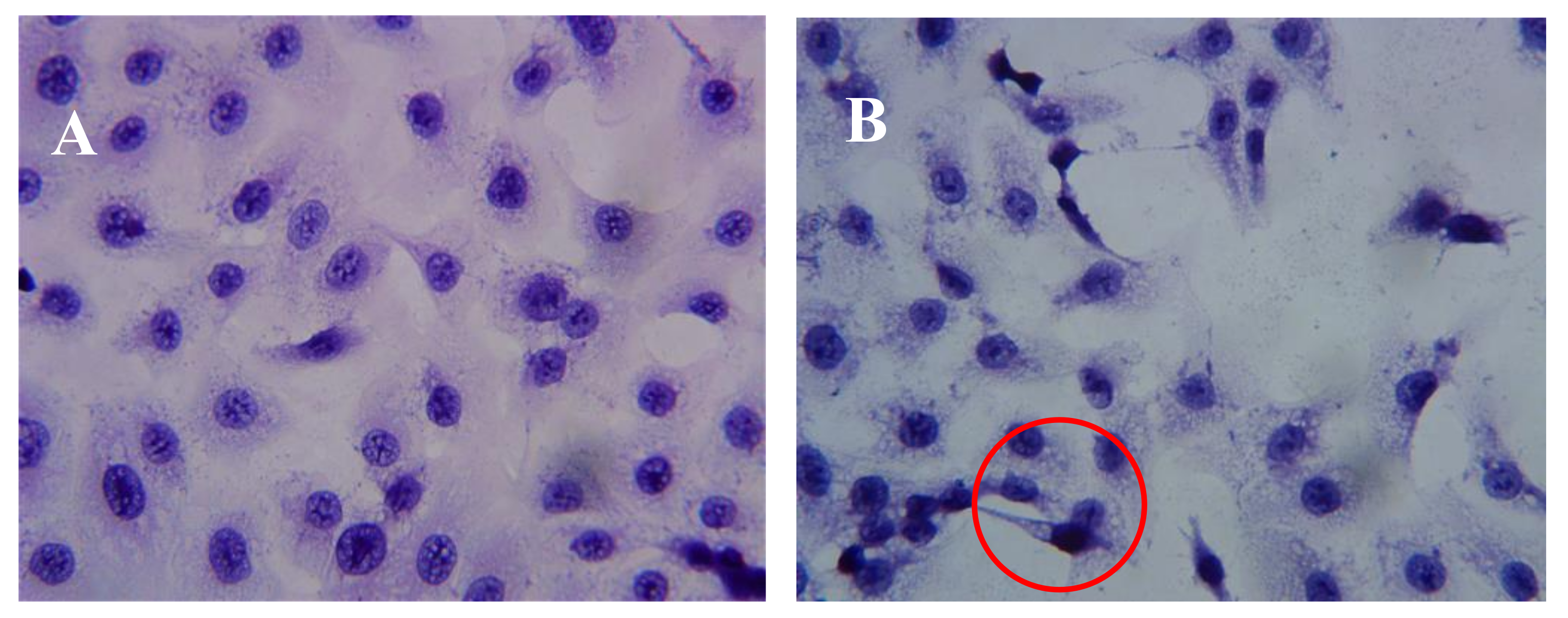
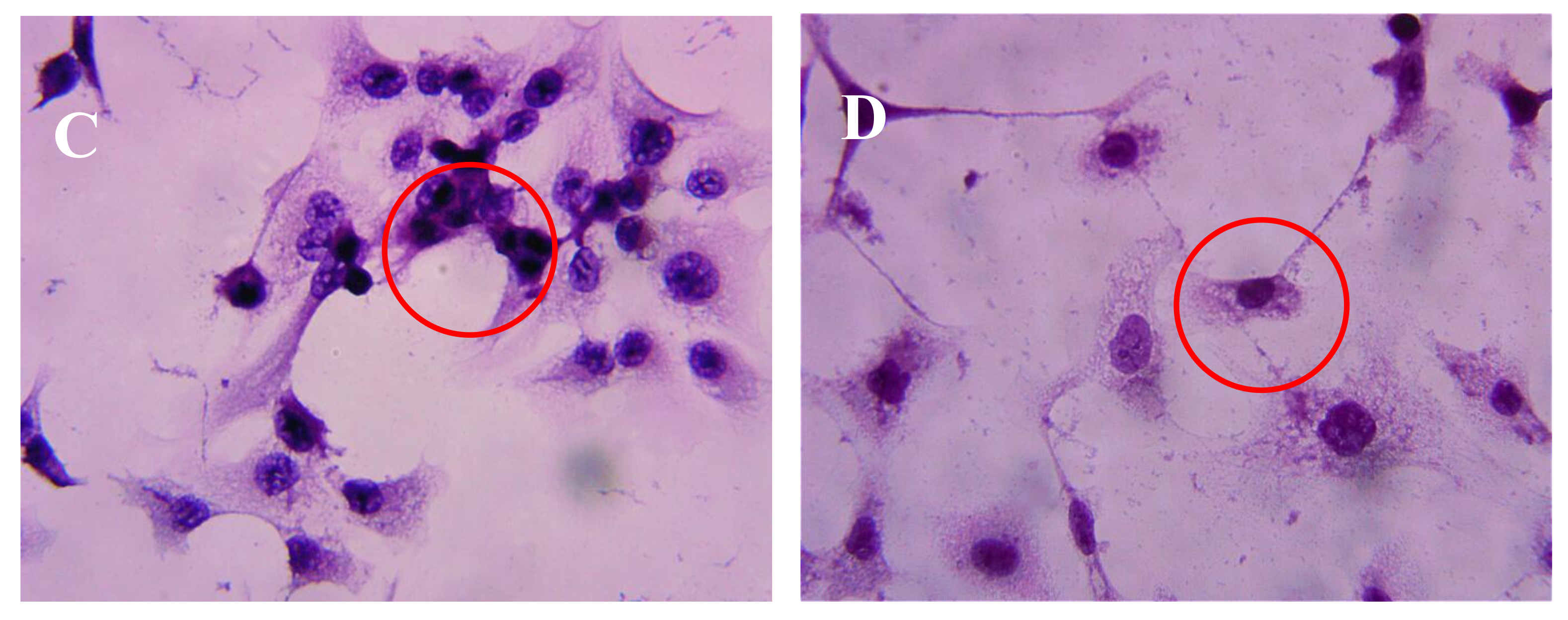
2.3. Effect of AAP-H on DU-145 Cell Morphology
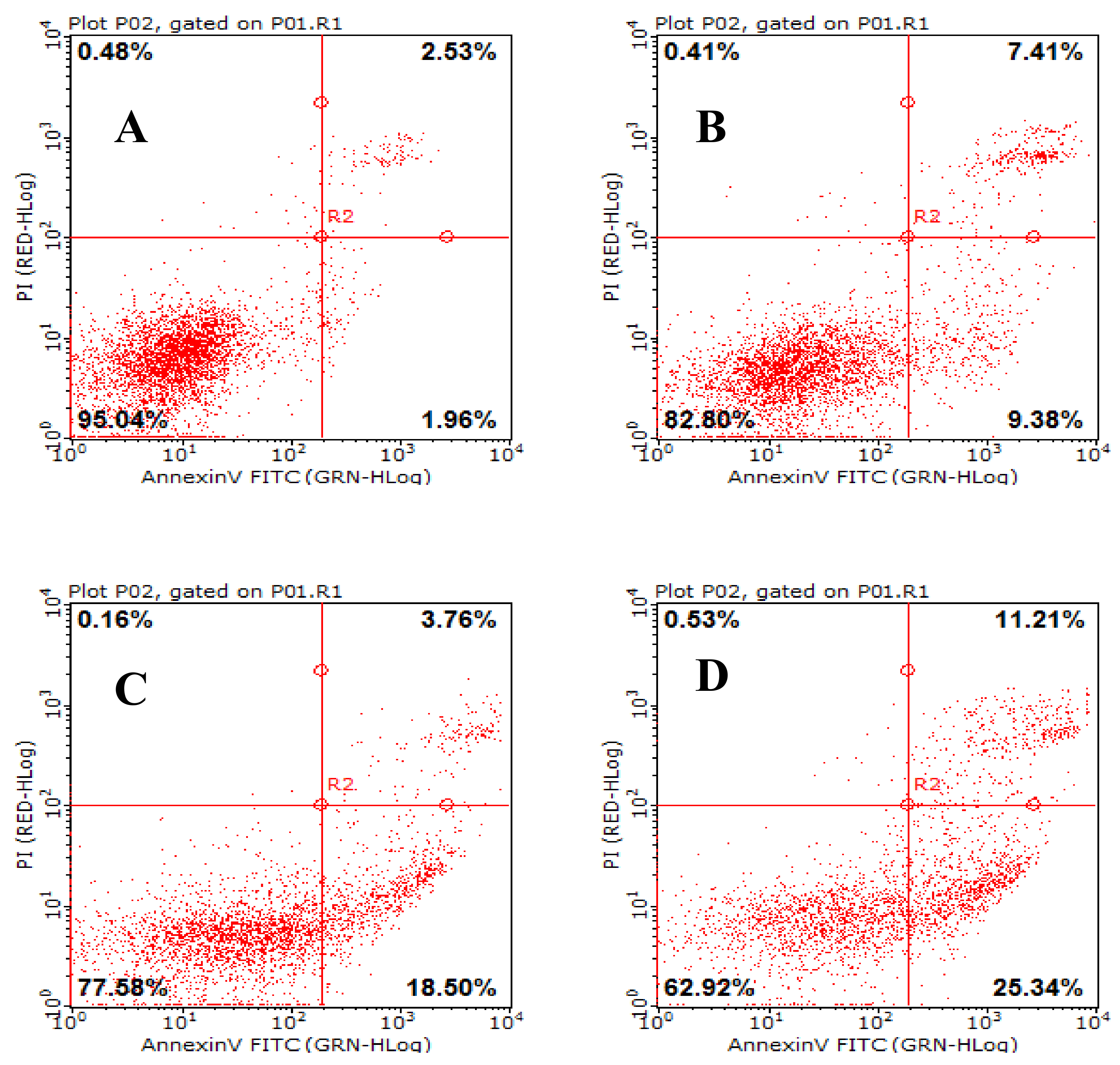
2.4. Effects of AAP-H on Early- and Late-Stage Apoptosis
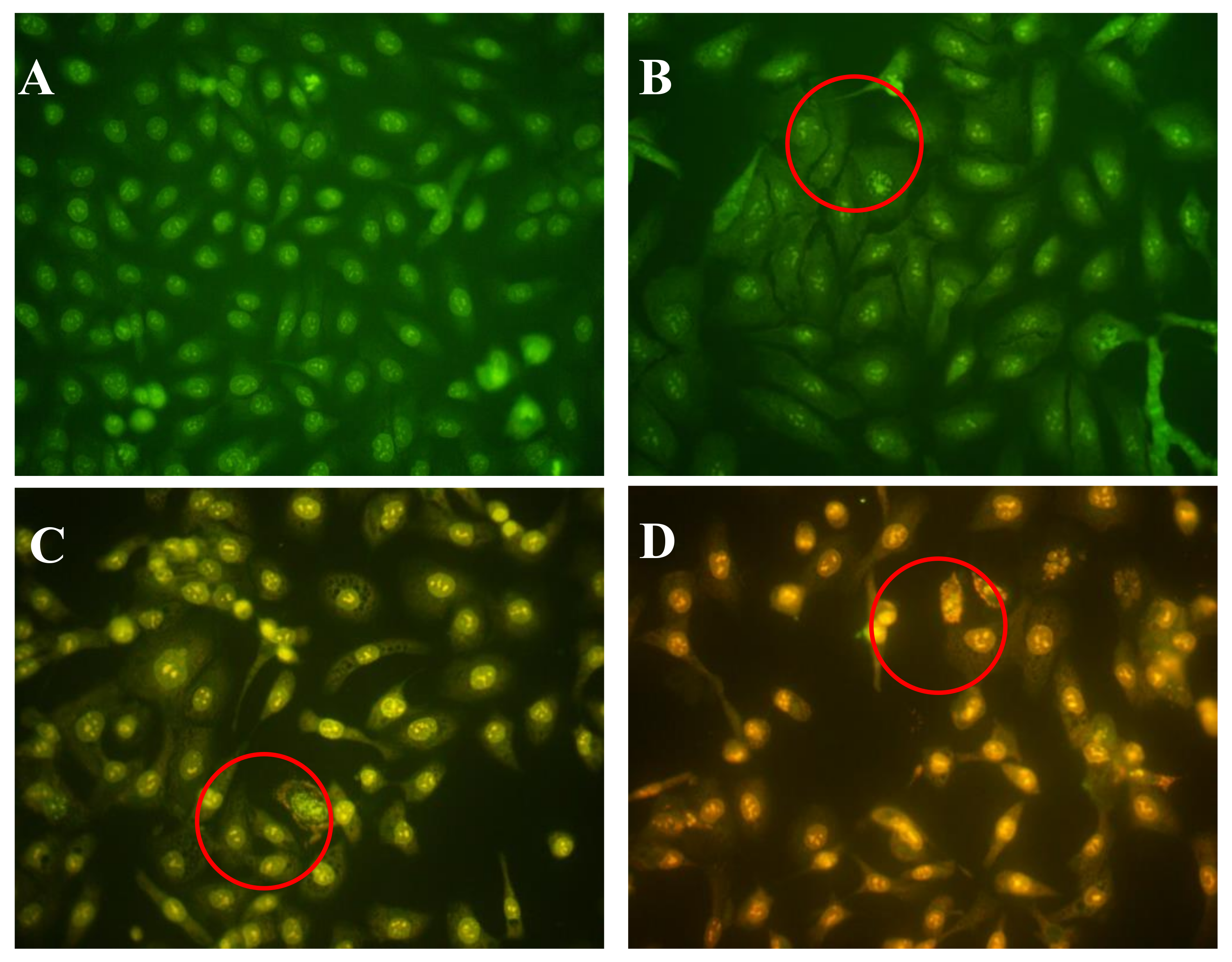
2.5. Effects of AAP-H on DU-145 Cell Nuclei
2.6. Scanning Electron Microscopy Results
2.7. Effects of AAP-H on Mitochondrial Membrane Potential (Δψm) in DU-145 Cancer Cells
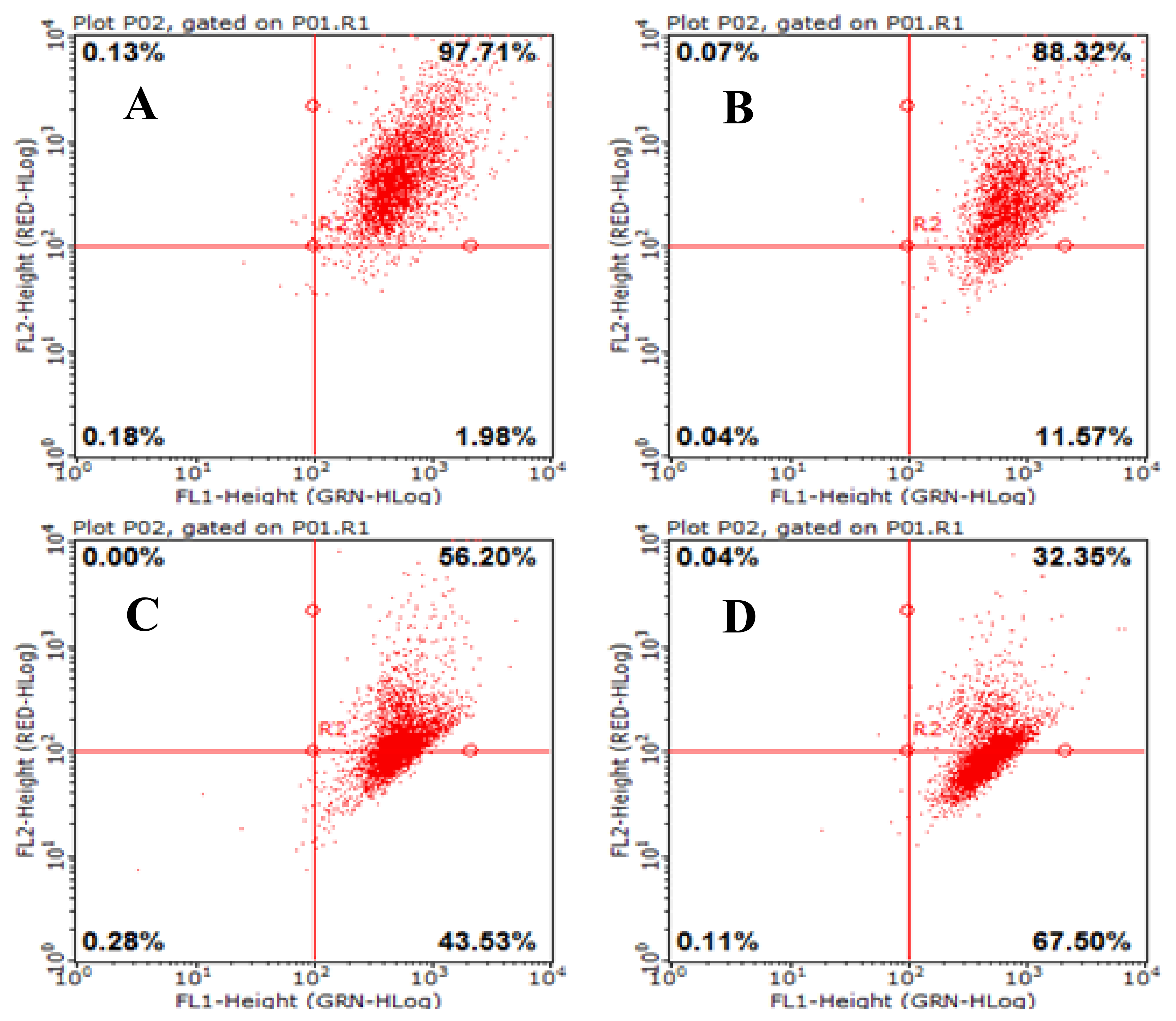
2.8. AAP-H Induces Apoptosis of DU-145 Cells
2.9. Effects of AAP-H on Apoptosis-Associated Protein Levels in DU-145 Cells
3. Discussion
4. Materials and Methods
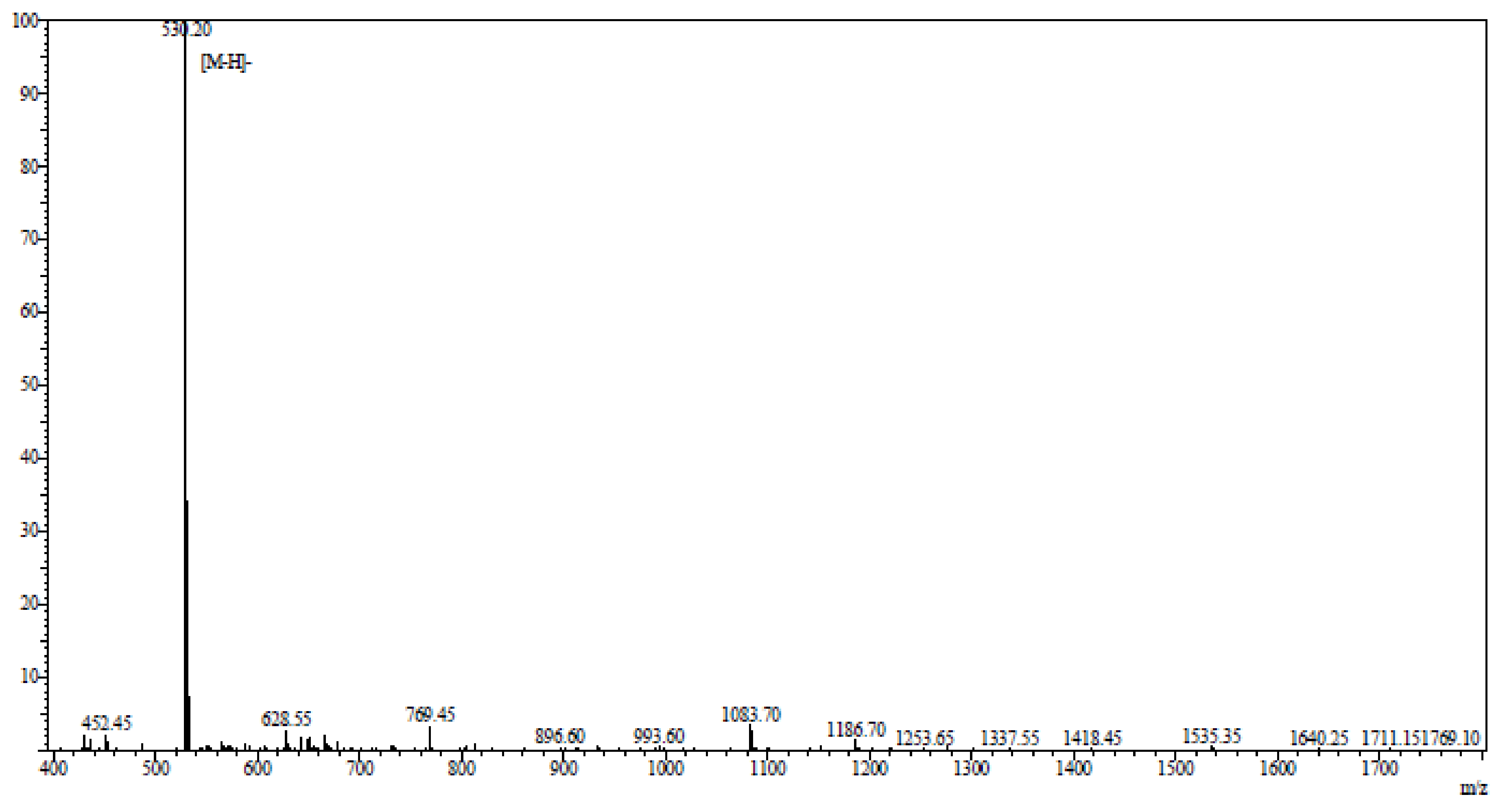
4.1. Reagents and Preparation of Anthopleura anjunae Anti-Tumor Peptide
4.2. Cell Culture
4.3. MTT Assay
4.4. Morphological Analysis
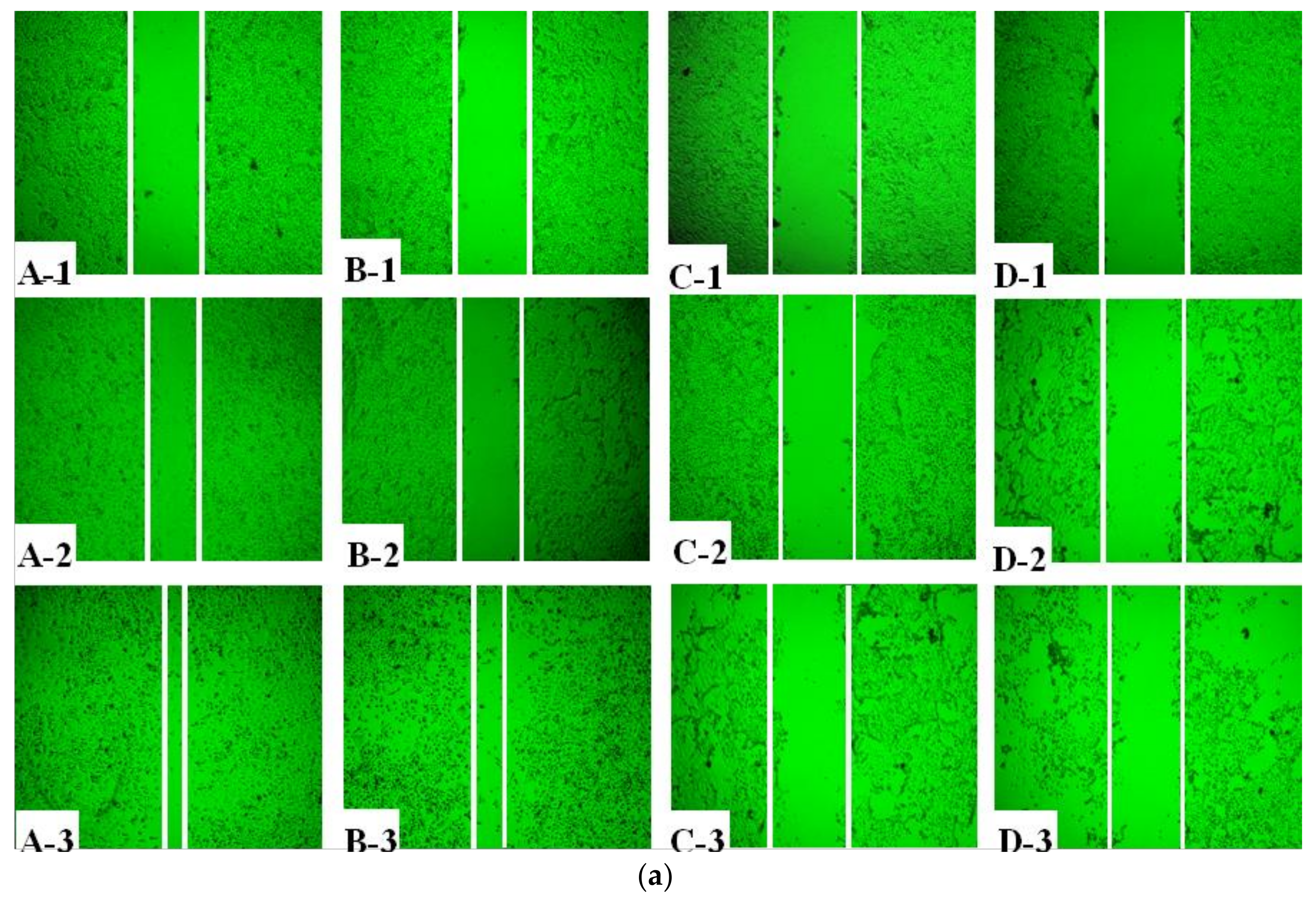
4.4.1. Wound Healing Assay
4.4.2. Hematoxylin-Eosin Staining
4.4.3. Morphological Analysis by Acridine Orange/Ethidium Bromide Fluorescent Staining
4.4.4. Hoechst 33258 Fluorescent Staining
4.4.5. Scanning Electron Microscopy
4.5. Mitochondrial Membrane Potential Change Effected by AAP-H
4.6. AAP-H Induced Apoptosis
4.7. Western Blotting Analysis of Specific Proteins
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Swank, R.T.; Munkres, K.D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal. Biochem. 1971, 39, 462–477. [Google Scholar] [CrossRef]
- Aneiros, A.; Garateix, A. Bioactive peptides from marine sources: Pharmacological properties and isolation procedures. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 803, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Ravichandran, Y.D.; Khan, S.B.; Kim, Y.T. Prospective of the cosmeceuticals derived from marine organisms. Biotechnol. Bioprocess Eng. 2008, 13, 511–523. [Google Scholar] [CrossRef]
- Kim, S.-K.; Wijesekara, I. Development and biological activities of marine-derived bioactive peptides: A review. J. Funct. Foods 2010, 2, 1–9. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Laughton, M.J.; Halliwell, B. Carnosine, homocarnosine and anserine: could they act as antioxidants in vivo? Biochem. J. 1989, 264, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, H.; Pihlanto, A. Bioactive peptides: Production and functionality. Int. Dairy J. 2006, 16, 945–960. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, Y.; Sheng, J.; Fang, W.; Yuan, Z.; Lin, X.; Mi, S. Antitumor Peptides from Marine Organisms. Marine Drugs 2011, 9, 1840–1859. [Google Scholar] [CrossRef] [PubMed]
- Maček, P. Polypeptide cytolytic toxins from sea anemones (Actiniaria). FEMS Microbiol. Immunol. 1992, 5, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Monroyestrada, H.I.; Segurapuertas, L.; Galvánarzate, S.; Santamaría, A.; Sánchezrodríguez, J. The crude venom from the sea anemone Stichodactyla helianthus induces haemolysis and slight peroxidative damage in rat and human erythrocytes. Toxicol. In Vitro 2007, 21, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Pazos, F.; Valle, A.; Martínez, D.; Ramírez, A.; Calderón, L.; Pupo, A.; Tejuca, M.; Morera, V.; Campos, J.; Fando, R. Structural and functional characterization of a recombinant sticholysin I (rSt I) from the sea anemone Stichodactyla helianthus. Toxicon Offic. J. Int. Soc. Toxinol. 2006, 48, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Ramezanpour, M.; da Silva, K.D.; Sanderson, B.J. Differential susceptibilities of human lung, breast and skin cancer cell lines to killing by five sea anemone venoms. J. Venomous Anim. Toxins Including Trop. Dis. 2012, 18, 157–163. [Google Scholar] [CrossRef]
- Soletti, R.C.; de Faria, G.P.; Vernal, J.; Terenzi, H.; Anderluh, G.; Borges, H.L.; Moura-Neto, V.; Gabilan, N.H. Potentiation of anticancer-drug cytotoxicity by sea anemone pore-forming proteins in human glioblastoma cells. Anti-Cancer Drugs 2008, 19, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, S.; Dyshlovoy, S.; Monastyrnaya, M.; Shubina, L.; Leychenko, E.; Kozlovskaya, E.; Jin, J.O.; JongYoung, K.; Bode, A.M.; Dong, Z.G. The anticancer effects of actinoporin RTX-A from the sea anemone Heteractis crispa (=Radianthus macrodactylus). Toxicon: Offic. J. Int. Soc. Toxinol. 2010, 55, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Monroyestrada, H.I.; Chirino, Y.I.; Soriamercado, I.E.; Sánchezrodríguez, J. Toxins from the Caribbean sea anemone Bunodeopsis globulifera increase cisplatin-induced cytotoxicity of lung adenocarcinoma cells. J. Venomous Anim. Toxins Including Trop. Dis. 2013, 19, 12. [Google Scholar]
- Ramezanpour, M.; Da, S.K.; Sanderson, B.J. The effect of sea anemone (H. magnifica) venom on two human breast cancer lines: Death by apoptosis. Cytotechnology 2013, 66, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive peptides from marine processing waste and shellfish: A review. J. Funct. Foods 2012, 4, 6–24. [Google Scholar] [CrossRef]
- Samaranayaka, A.G.P.; Li-Chan, E.C.Y. Food-derived peptidic antioxidants: A review of their production, assessment, and potential applications. J. Funct. Foods 2011, 3, 229–254. [Google Scholar] [CrossRef]
- Gherghi, I.C.; Girousi, S.T.; Voulgaropoulos, A.N.; Tzimou-Tsitouridou, R. Study of interactions between DNA-ethidium bromide (EB) and DNA-acridine orange (AO), in solution, using hanging mercury drop electrode (HMDE). Talanta 2003, 61, 103–112. [Google Scholar] [CrossRef]
- Chen, H.L.; Jian-Hua, L.I.; Wang, S.Q. Correlative Study between Mitochondrial Transmembrane Potential and Apoptosis. Med. Recapitul. 2007, 13, 1041–1043. [Google Scholar]
- Ding, G.F.; Huang, F.F.; Yang, Z.S.; Di, Y.U.; Yang, Y.F. Anticancer Activity of an Oligopeptide Isolated from Hydrolysates of Sepia Ink. Chin. J. Nat. Med. 2011, 9, 151–155. [Google Scholar]
- Cairns, R.B.; Harris, I.S.; Mak, T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Egeblad, M.; Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2002, 2, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Nemec, K.N.; Khaled, A.R. Therapeutic Modulation of Apoptosis: Targeting the BCL-2 Family at the Interface of the Mitochondrial Membrane. Yonsei Med. J. 2008, 49, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Del Poeta, G.; Venditti, A.; del Principe, M.I.; Maurillo, L.; Buccisano, F.; Tamburini, A.; Cox, M.C.; Franchi, A.; Bruno, A.; Mazzone, C.; et al. Amount of spontaneous apoptosis detected by Bax/Bcl-2 ratio predicts outcome in acute myeloid leukemia (AML). Blood 2003, 101, 2125–2131. [Google Scholar] [CrossRef] [PubMed]
- Stoetzer, O.J.; Nüssler, V.; Darsow, M.; Gullis, E.; Pelka-Fleischer, R.; Scheel, U.; Wilmanns, W. Association of bcl-2, bax, bcl-xL and interleukin-1 beta-converting enzyme expression with initial response to chemotherapy in acute myeloid leukemia. Leukemia 1996, 10, 18–22. [Google Scholar]
- Brentnall, M.; Rodriguezmenocal, L.; Guevara, R.L.D.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Huang, F.; Lin, H.; Xian, W. Isolation and Purification of a Peptide from Bullacta exarata and Its Impaction of Apoptosis on Prostate Cancer Cell. Mar. Drugs 2013, 11, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.F.; Yan, H.Q.; Ding, G.F. Isolation and purification of an anticancer activity peptide from protein hydrolysate of Mytilus coruscus. J. China Pharm. Univ. 2011, 42, 272–275. [Google Scholar]
- Huang, F.; Yang, Z.; Yu, D.; Wang, J.; Li, R.; Ding, G. Sepia ink oligopeptide induces apoptosis in prostate cancer cell lines via caspase-3 activation and elevation of Bax/Bcl-2 ratio. Mar. Drugs 2012, 10, 2153–2165. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Ding, G.; Yang, Z.; Yu, F. Two novel peptides derived from Sinonovacula constricta inhibit the proliferation and induce apoptosis of human prostate cancer cells. Mol. Med. Rep. 2017, 16, 6697–6707. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Debatin, K.M. Extrinsic vs intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 2006, 25, 4798–4811. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.; Zha, J.; Jockel, J.; Boise, L.H.; Thompson, C.B.; Korsmeyer, S.J. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell 1995, 80, 285–291. [Google Scholar] [CrossRef]
- Overbeeke, R.; Yildirim, M.; Reutelingsperger, C.P.M.; Haanen, C.; Vermes, I. Sequential occurrence of mitochondrial and plasma membrane alterations, fluctuations in cellular Ca2+ and pH during initial and later phases of cell death. Apoptosis Int. J. Programm. Cell Death 1999, 4, 455–460. [Google Scholar] [CrossRef]
- Zong-Ze, W.; Guo-Fang, D.; Zui-Su, Y.; Fang-Miao, Y.; Yun-Ping, T.; Ying-Lu, J.; Yuan-Yuan, Z.; Rui, C. Enzymatic preparation of oligopeptide from anthopleura anjunae and its anti-cancer aactivity of prosctate cancer cells. Oceanol. Limnol. Sin. 2017, 48, 1114–1123. [Google Scholar]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.-Z.; Ding, G.-F.; Huang, F.-F.; Yang, Z.-S.; Yu, F.-M.; Tang, Y.-P.; Jia, Y.-L.; Zheng, Y.-Y.; Chen, R. Anticancer Activity of Anthopleura anjunae Oligopeptides in Prostate Cancer DU-145 Cells. Mar. Drugs 2018, 16, 125. https://doi.org/10.3390/md16040125
Wu Z-Z, Ding G-F, Huang F-F, Yang Z-S, Yu F-M, Tang Y-P, Jia Y-L, Zheng Y-Y, Chen R. Anticancer Activity of Anthopleura anjunae Oligopeptides in Prostate Cancer DU-145 Cells. Marine Drugs. 2018; 16(4):125. https://doi.org/10.3390/md16040125
Chicago/Turabian StyleWu, Zong-Ze, Guo-Fang Ding, Fang-Fang Huang, Zui-Su Yang, Fang-Miao Yu, Yun-Ping Tang, Ying-Lu Jia, Yuan-Yuan Zheng, and Rui Chen. 2018. "Anticancer Activity of Anthopleura anjunae Oligopeptides in Prostate Cancer DU-145 Cells" Marine Drugs 16, no. 4: 125. https://doi.org/10.3390/md16040125
APA StyleWu, Z.-Z., Ding, G.-F., Huang, F.-F., Yang, Z.-S., Yu, F.-M., Tang, Y.-P., Jia, Y.-L., Zheng, Y.-Y., & Chen, R. (2018). Anticancer Activity of Anthopleura anjunae Oligopeptides in Prostate Cancer DU-145 Cells. Marine Drugs, 16(4), 125. https://doi.org/10.3390/md16040125




