Abstract
Tetrodotoxin (TTX), the mode of action of which has been known since the 1960s, is widely used in pharmacology as a specific inhibitor of voltage-gated sodium channels (Nav channels). This toxin has contributed to the characterization of the allosteric model of the Nav channel, and to discriminating TTX-sensitive and TTX-resistant subtypes. In addition to its role as a pharmacological tool, TTX is now considered a therapeutic molecule, and its development should lead to its use in certain pathologies involving Nav channels, particularly in the field of pain. Specifically, the blockade of Nav channels expressed in nociceptive fibres is one strategy for alleviating pain and its deleterious consequences on health. Recent work has identified, in addition to the Nav1.7, 1.8 and 1.9 channels, the Nav1.1 subtype on dorsal root ganglion (DRG) neurons as a crucial player in mechanical and non-thermal pain. The sensitivity of Nav1.1 to TTX could be exploited at the therapeutic level, especially in chronic pain conditions.
1. Introduction
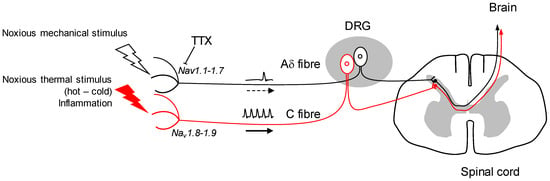
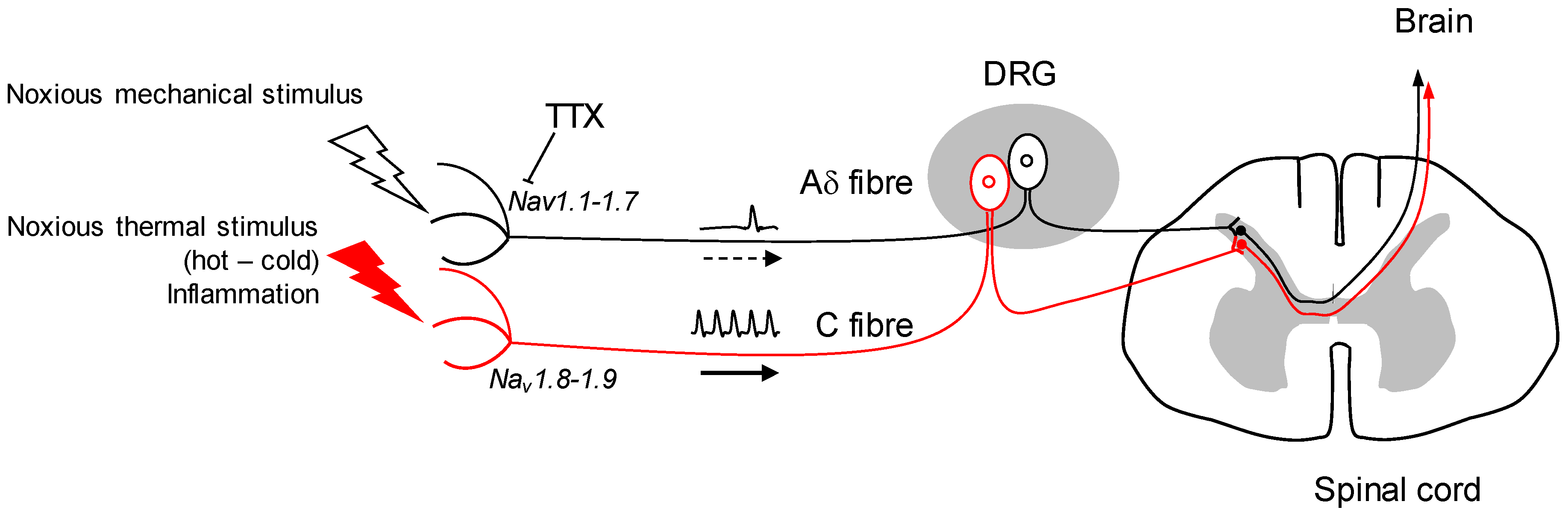
The mode of action of tetrodotoxin (TTX) was characterised in the early 1960s by the pioneering work of the Narahashi team, which made it clear that this non-peptidyl toxin inhibits voltage-gated Na+ channels (Nav) at very low concentrations [1]. Since then, the interaction between this toxin and the Nav channels has been dissected at the cellular and molecular level, and TTX is used as a pharmacological tool for Nav-dependent mechanisms [2]. In this respect, the blocking it exerts on these channels and its ability to inhibit the rise of the action potential of excitable cells are the cause of human intoxication after eating ‘fugu’ puffer fish. The second advance was the discrimination of TTX to certain subtypes of Nav channels. While blocking most Nav channels with high affinity, heart Nav1.5 and dorsal root ganglion (DRG) sensory fibres Nav1.8-1.9 subtypes show relative sensitivity or even resistance to TTX, respectively [3,4,5]. This discrimination that can be made between TTX-sensitive (Nav1.1, 1.2, 1.3, 1.4, 1.6, 1.7) and insensitive (Nav1.5, 1.8, 1.9) channels is undoubtedly a way of progress in the use of TTX as a therapeutic tool, particularly in the field of pain. It is indeed possible to specifically target the nociceptors, i.e., neurons responsible for converting high-intensity thermal, chemical or mechanical information into an electrical message. The transmission of this message is ensured by the propagation of the action potential, which depends on the opening of certain Nav channels. Blocking these channels located in nociceptors can relieve pain. In addition, TTX does not cross the blood brain barrier, or very poorly, reducing the probability of central system depression and any adverse effects [6].
3. Therapeutic Use of TTX
Other studies will confirm Nav1.1’s role in mechanical nociception or other forms of acute and chronic pain, but it appears to be a potential therapeutic target. The TTX targeting of mechanical pain by the inhibition of Nav1.1 in positive A fibres, while leaving the functional integrity of Nav1.8-1.9-expressing C fibres, seems a potential strategy in the future (Figure 1). Further research will determine whether Nav1.1 is up-regulated in chronic forms of mechanical pain, and if so, TTX could be a useful tool to alleviate such conditions. The use of TTX as an analgesic drug in neuropathic and inflammatory pain shows undeniable benefits in animal model studies [21]. It has also been tested by intramuscular injection to reduce cancer-related pain with significant effects [22,23]. Moreover, it has been challenged for its potential against drug addiction behaviours: TTX exhibits a reduction of cue-induced increases in heroin craving and drug-associated anxiety with no sign of cardiovascular side effects [24].
Until now, this use of TTX was based on its ability to block Nav channels, the expression of which is modified over time, implying these Nav channels―i.e., Nav 1.3, 1.7―to be therapeutic targets for chronic pain. In fact, TTX is in phase III clinical development for the management of cancer-related and neuropathic pain. Several preclinical and clinical studies have reported its ability to reduce pain conditions, although data may be a matter for debate [21]. Studies in humans show a benefit of TTX by local intramuscular or subcutaneous injection in the management of cancer pain [22,25]. A recent study has shown that a subcutaneous injection of TTX significantly reduces the reversal of localised mechanical hypersensitivity by capsaicin in a visceral mouse pain model, without implication of Nav1.7 [26], confirming data generated on Wistar rats, where TTX reduces mechanical allodynia in somatic neuropathic pain tests [27]. The management of pain with TTX might be considered in the future for its capacity as not only blocking several Nav channels, but also to target up-regulated Nav in pathological conditions.
4. Conclusions
Several elements support the therapeutic use of TTX: on the one hand, when someone experiences pain (tendonitis, osteoarthritis, neuropathic pain), especially when it is prolonged in time, as in the case of hyperalgesia/mechanical allodynia, he/she wants to be relieved because chronic pain generates a disability to normal existence, which makes pain a debilitating condition. Elimination of pain may therefore be accompanied by adverse effects that may locally affect non-nociceptive sensory fibres. Several clinical studies conducted in patients with chronic pain have shown a long-term benefit of subcutaneous or intramuscular injection of TTX, despite adverse effects that resolved rather quickly through the treatment [22,23]. The TTX-mediated reduction of visceral mechanical pain did not induce any motor deficiency/incoordination [26]. These data suggest that there is a clear benefit of TTX over pain, and few consequences on sensory functions. On the other hand, the therapeutic use of TTX is made possible because the conditions associated with certain chronic pain show precisely a differential expression of the TTX-sensitive and TTX-resistant Nav channels at the nociceptors. Notably, tissue inflammation induces overexpression of TTX-sensitive Nav1.3 and Nav1.7 channels in nociceptors [28,29]: blocking these channels by TTX would reduce pain without affecting the neighbouring sensory functions. As for Nav1.1, it has yet to be determined whether it is differentially expressed in the case of chronic mechanical pain states.
There is no consensus around the use of TTX as a pain killer, most likely because (i) there are many paradigms of pain, both experimental and human, (ii) the administration of TTX should be done to what might be called the “right dose”, that is, an effective concentration to eliminate the pain without eliminating other sensory modalities, (iii) the route of administration of TTX must allow it to be delivered to the site of propagation of the nociceptive message to stop it, (iv) the possible combination of TTX with other analgesic molecules, (v) and the choice of TTX analogs or metabolites to increase the efficiency of inhibition of targeted Nav channels.
However, given the recent evidence in the field of pain disorders, and in view of the current development of TTX as a therapeutic, it can be assumed that the management of chronic mechanical pain, including hyperalgesia and allodynia mechanical troubles, as well as channelopathies related to overexpression or a gain-of-function of TTX-sensitive Nav channels, could have a favourable outcome.
Conflicts of Interest
The author declares no conflict of interest.
References
- Narahashi, T.; Deguchi, T.; Urakawa, N.; Ohkubo, Y. Stabilization and rectification of muscle fiber membrane by tetrodotoxin. Am. J. Physiol. 1960, 198, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Moczydlowski, E.G. The molecular mystique of tetrodotoxin. Toxicon 2013, 63, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Baer, M.; Best, P.M.; Reuter, H. Voltage-dependent action of tetrodotoxin in mammalian cardiac muscle. Nature 1976, 263, 344–345. [Google Scholar] [CrossRef] [PubMed]
- Akopian, A.N.; Sivilotti, L.; Wood, J.N. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature 1996, 379, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Dib-Hajj, S.D.; Tyrrell, L.; Black, J.A.; Waxman, S.G. NaN, a novel voltage-gated Na channel, is expressed preferentially in peripheral sensory neurons and down-regulated after axotomy. Proc. Natl. Acad. Sci. USA 1998, 95, 8963–8968. [Google Scholar] [CrossRef] [PubMed]
- Berde, C.B.; Athiraman, U.; Yahalom, B.; Zurakowski, D.; Corfas, G.; Bognet, C. Tetrodotoxin-bupivacaine-epinephrine combinations for prolonged local anesthesia. Mar. Drugs 2011, 9, 2717–2728. [Google Scholar] [CrossRef] [PubMed]
- Eijkelkamp, N.; Linley, J.E.; Baker, M.D.; Minett, M.S.; Cregg, R.; Werdehausen, R.; Rugiero, F.; Wood, J.N. Neurological perspectives on voltage-gated sodium channels. Brain 2012, 135, 2585–2612. [Google Scholar] [CrossRef] [PubMed]
- Goldin, A.L. Evolution of voltage-gated Na+ channels. J. Exp. Biol. 2002, 205, 575–584. [Google Scholar] [PubMed]
- Nassar, M.A.; Stirling, L.C.; Forlani, G.; Baker, M.D.; Matthews, E.A.; Dickenson, A.H.; Wood, J.N. Nociceptor-specific gene deletion reveals a major role for Nav1.7 (PN1) in acute and inflammatory pain. Proc. Natl. Acad. Sci. USA 2004, 101, 12706–12711. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, K.; Leffler, A.; Babes, A.; Cendan, C.M.; Carr, R.W.; Kobayashi, J.; Nau, C.; Wood, J.N.; Reeh, P.W. Sensory neuron sodium channel Nav1.8 is essential for pain at low temperatures. Nature 2007, 447, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Amaya, F.; Wang, H.; Costigan, M.; Allchorne, A.J.; Hatcher, J.P.; Egerton, J.; Stean, T.; Morisset, V.; Grose, D.; Gunthorpe, M.J.; et al. The voltage-gated sodium channel Na(v)1.9 is an effector of peripheral inflammatory pain hypersensitivity. J. Neurosci. 2006, 26, 12852–12860. [Google Scholar] [CrossRef] [PubMed]
- Dib-Hajj, S.D.; Cummins, T.R.; Black, J.A.; Waxman, S.G. Sodium channels in normal and pathological pain. Annu. Rev. Neurosci. 2010, 33, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, R. Dravet syndrome: The main issues. Eur. J. Paediatr. Neurol. 2012, 16. [Google Scholar] [CrossRef] [PubMed]
- Dichgans, M.; Freilinger, T.; Eckstein, G.; Babini, E.; Lorenz-Depiereux, B.; Biskup, S.; Ferrari, M.D.; Herzog, J.; van den Maagdenberg, A.M.; Pusch, M.; et al. Mutation in the neuronal voltage-gated sodium channel SCN1A in familial hemiplegic migraine. Lancet 2005, 366, 371–377. [Google Scholar] [CrossRef]
- Cestèle, S.; Schiavon, E.; Rusconi, R.; Franceschetti, S.; Mantegazza, M. Nonfunctional Nav1.1 familial hemiplegic migraine mutant transformed into gain of function by partial rescue of folding defects. Proc. Natl. Acad. Sci. USA 2013, 110, 17546–17551. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, T.; Kobayashi, K.; Yamanaka, H.; Obata, K.; Dai, Y.; Noguchi, K. Comparative study of the distribution of the alpha-subunits of voltage-gated sodium channels in normal and axotomized rat dorsal root ganglion neurons. J. Comp. Neurol. 2008, 510, 188–206. [Google Scholar] [CrossRef] [PubMed]
- Osteen, J.D.; Herzig, V.; Gilchrist, J.; Emrick, J.J.; Zhang, C.; Wang, X.; Castro, J.; Garcia-Caraballo, S.; Grundy, L.; Rychkov, G.Y.; et al. Selective spider toxins reveal a role for the Nav1.1 channel in mechanical pain. Nature 2016, 534, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Basbaum, A.; Bautista, D.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Tsunozaki, M.; Lennertz, R.C.; Vilceanu, D.; Katta, S.; Stucky, C.L.; Bautista, D.M. A ‘toothache tree’ alkylamide inhibits Aδ mechanonociceptors to alleviate mechanical pain. J. Physiol. 2013, 591, 3325–3340. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Atianjoh, F.; Gauda, E.B.; Yaster, M.; Li, Y.; Tao, Y.X. Increased expression of sodium channel subunit Nav1.1 in the injured dorsal root ganglion after peripheral nerve injury. Anat. Rec. (Hoboken) 2011, 294, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Nieto, F.R.; Cobos, E.J.; Tejada, M.Á.; Sánchez-Fernández, C.; González-Cano, R.; Cendán, C.M. Tetrodotoxin (TTX) as a therapeutic agent for pain. Mar. Drugs 2012, 10, 281–305. [Google Scholar] [CrossRef] [PubMed]
- Hagen, N.A.; Fisher, K.M.; Lapointe, B.; du Souich, P.; Chary, S.; Moulin, D.; Sellers, E.; Ngoc, A.H. Canadian Tetrodotoxin Study Group. An open-label, multi-dose efficacy and safety study of intramuscular tetrodotoxin in patients with severe cancer-related pain. J. Pain Symptom Manag. 2007, 34, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Hagen, N.A.; Lapointe, B.; Ong-Lam, M.; Dubuc, B.; Walde, D.; Gagnon, B.; Love, R.; Goel, R.; Hawley, P.; Ngoc, A.H.; du Souich, P. A multicentre open-label safety and efficacy study of tetrodotoxin for cancer pain. Curr. Oncol. 2011, 18, e109–e116. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Liu, T.T.; Wang, X.; Epstein, D.H.; Zhao, L.Y.; Zhang, X.L.; Lu, L. Tetrodotoxin reduces cue-induced drug craving and anxiety in abstinent heroin addicts. Pharmacol. Biochem. Behav. 2009, 92, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Hagen, N.A.; Cantin, L.; Constant, J.; Haller, T.; Blaise, G.; Ong-Lam, M.; du Souich, P.; Korz, W.; Lapointe, B. Tetrodotoxin for moderate to severe cancer-related pain: A multicentre, randomized, double-blind, placebo-controlled, parallel-design trial. Pain Res. Manag. 2017, 2017, 7212713. [Google Scholar] [CrossRef] [PubMed]
- González-Cano, R.; Tejada, M.Á.; Artacho-Cordón, A.; Nieto, F.R.; Entrena, J.M.; Wood, J.N.; Cendán, C.M. Effects of tetrodotoxin in mouse models of visceral pain. Mar. Drugs 2017, 15. [Google Scholar] [CrossRef] [PubMed]
- Marcil, J.; Walczak, J.S.; Guindon, J.; Ngoc, A.H.; Lu, S.; Beaulieu, P. Antinociceptive effects of tetrodotoxin (TTX) in rodents. Br. J. Anaesth. 2006, 96, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Djouhri, L.; Newton, R.; Levinson, S.R.; Berry, C.M.; Carruthers, B.; Lawson, S.N. Sensory and electrophysiological properties of guinea-pig sensory neurones expressing Nav 1.7 (PN1) Na+ channel alpha subunit protein. J. Physiol. 2003, 546, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Black, J.A.; Liu, S.; Tanaka, M.; Cummins, T.R.; Waxman, S.G. Changes in the expression of tetrodotoxin-sensitive sodium channels within dorsal root ganglia neurons in inflammatory pain. Pain 2004, 108, 237–247. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).