Spirulina platensis Improves Mitochondrial Function Impaired by Elevated Oxidative Stress in Adipose-Derived Mesenchymal Stromal Cells (ASCs) and Intestinal Epithelial Cells (IECs), and Enhances Insulin Sensitivity in Equine Metabolic Syndrome (EMS) Horses
Abstract
1. Introduction
2. Materials and Methods
2.1. Quantification of Spirulina Platensis Functional Bioactive Components
2.1.1. Determination of the Total Phenols Content (TPC)
2.1.2. Extraction and Quantification of Fatty Acids
2.1.3. Analysis of Free and Protein-Bound Amino Acids
2.1.4. Quantitative Analysis of Phycocyanin
2.1.5. Determination of Vitamin C Concentration
2.1.6. Gas Chromatographic Assay of Tocopherol Content
2.2. In Vitro Study
2.2.1. Animals Qualification
2.2.2. EqASC Isolation and Culture
2.2.3. IECs Isolation and Culture
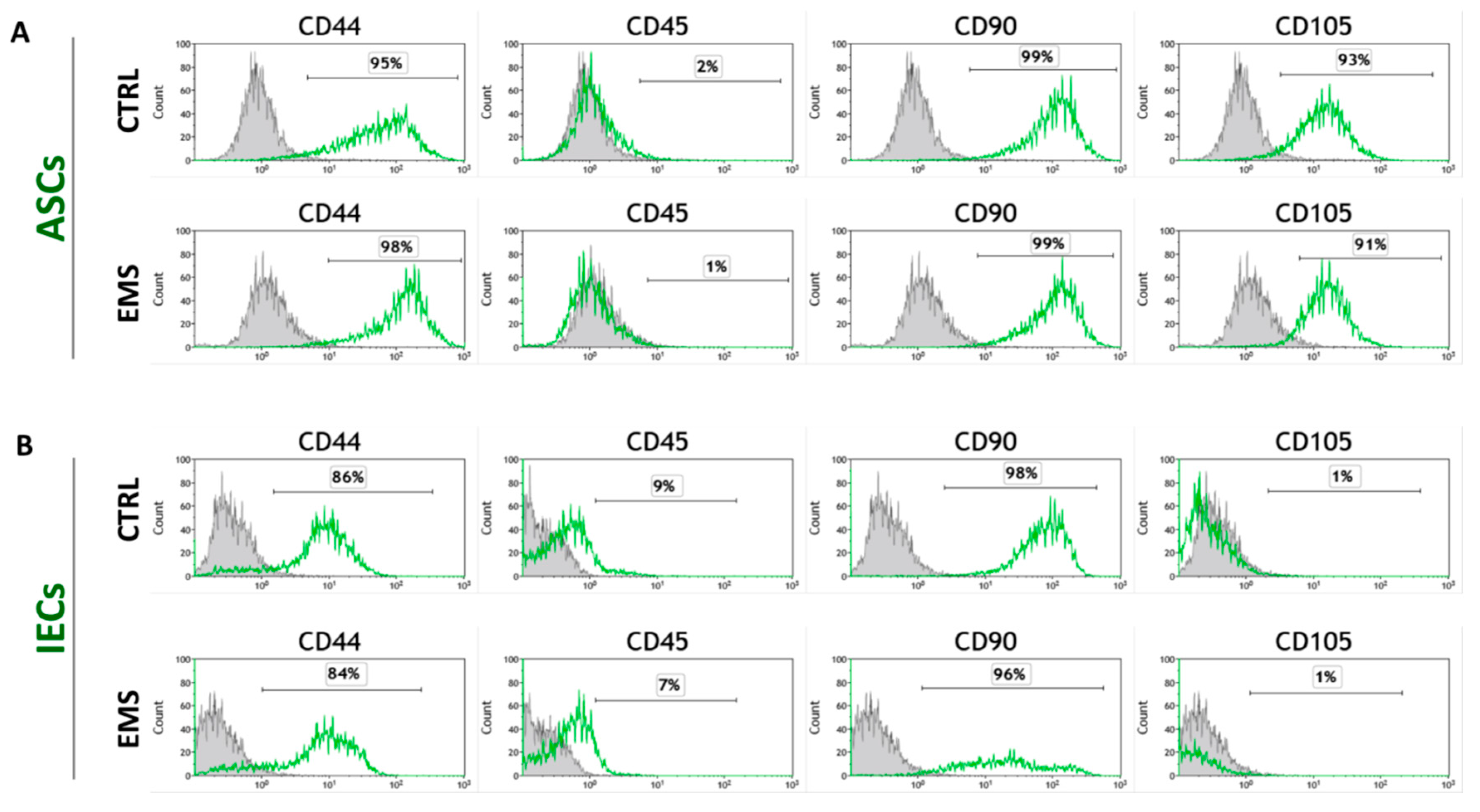
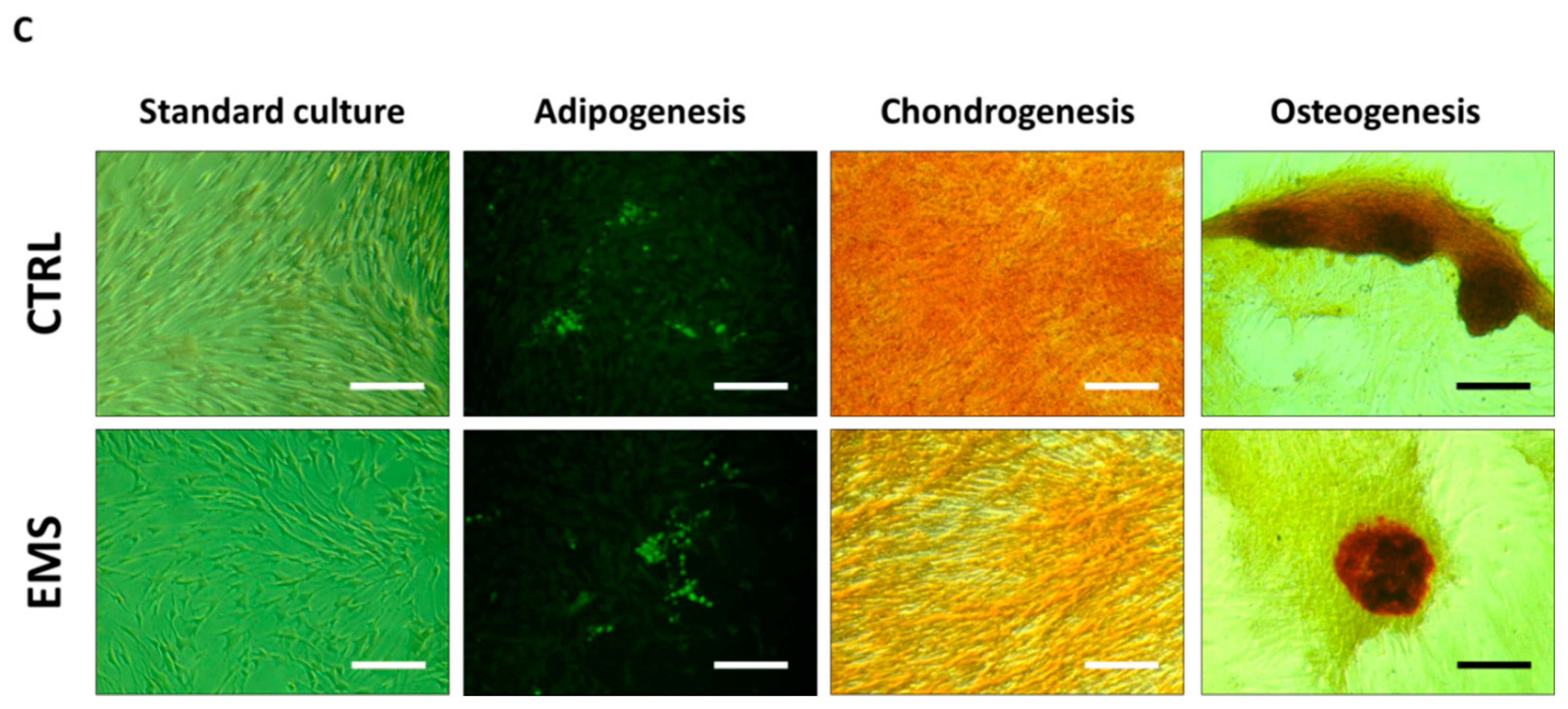
2.2.4. Immunophenotyping Characterization and Multipotency Assay
2.2.5. Preparation of Spirulina Platensis Water Extract
2.2.6. Cell Propagation with Spirulina Extract
2.2.7. Visualization of Cell Morphology
2.2.8. Cell Viability Assay—TOX8
2.2.9. BrdU Cell Proliferation Assay
2.2.10. Evaluation of Cellular Senescence and Apoptosis
2.2.11. Flow Cytometric Analysis of JC-1 and H2DCF-DA Stained Cells
2.2.12. Evaluation of Extracellular Oxidative Stress
2.2.13. Collection and Stimulation of Murine Peritoneal Macrophages
2.2.14. Determination of Nitric Oxide Production by Peritoneal Macrophages
2.2.15. Measurement of TNF-α and p53 Levels by ELISA
2.2.16. Analysis of Gene Expression
2.2.17. Statistical Analysis
2.3. In Vivo Study
2.3.1. Animals Classification
2.3.2. Dietary Protocol
2.3.3. Statistical Analysis
3. Results
3.1. Spirulina Platensis as a Source of Bioactive Compounds
3.2. Immunophenotype and Multipotent Properties of Isolated Cells
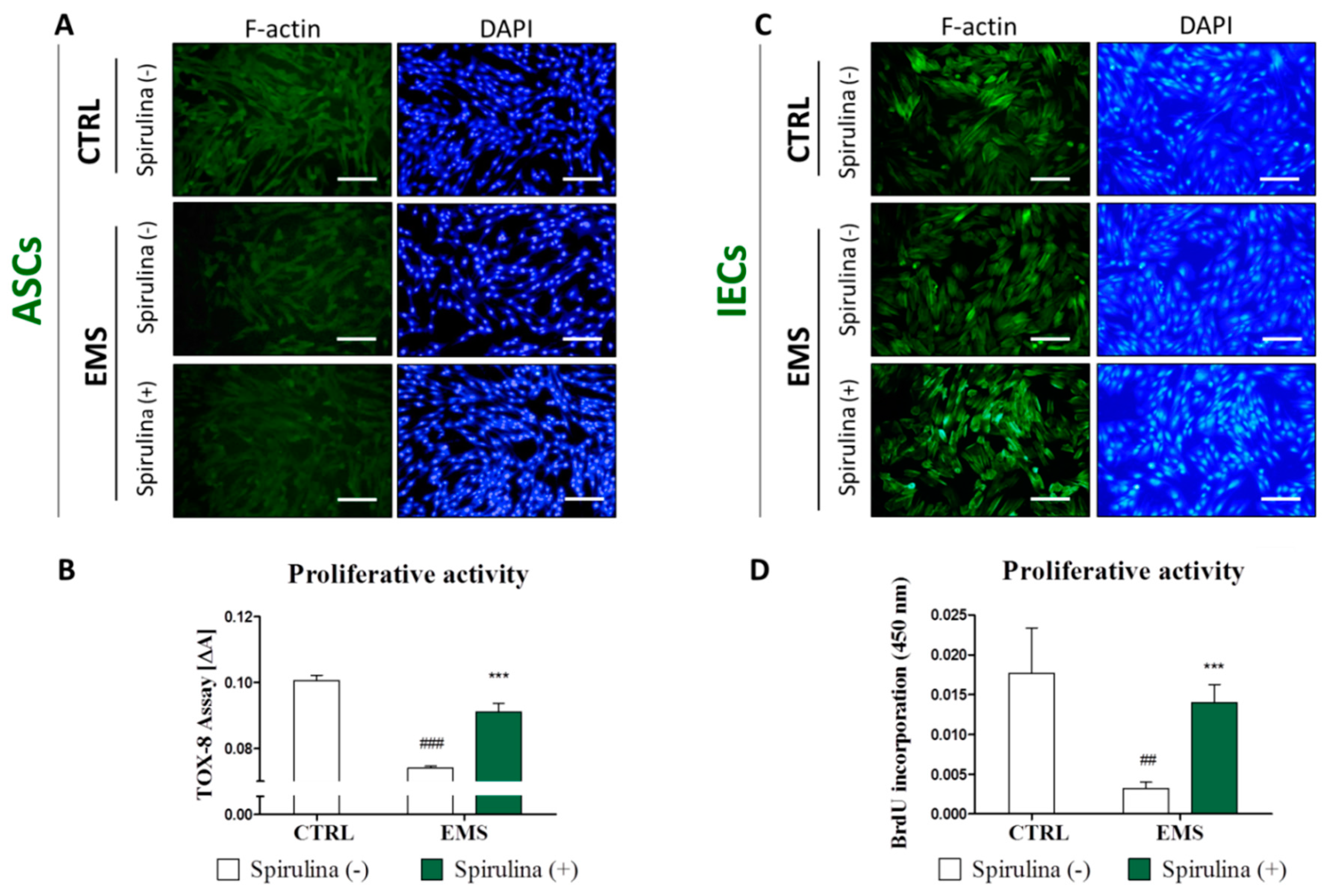
3.3. Cell Exposition to Spirulina Extract Improves ASCs’ and IECs’ Morphology and Proliferative Activity
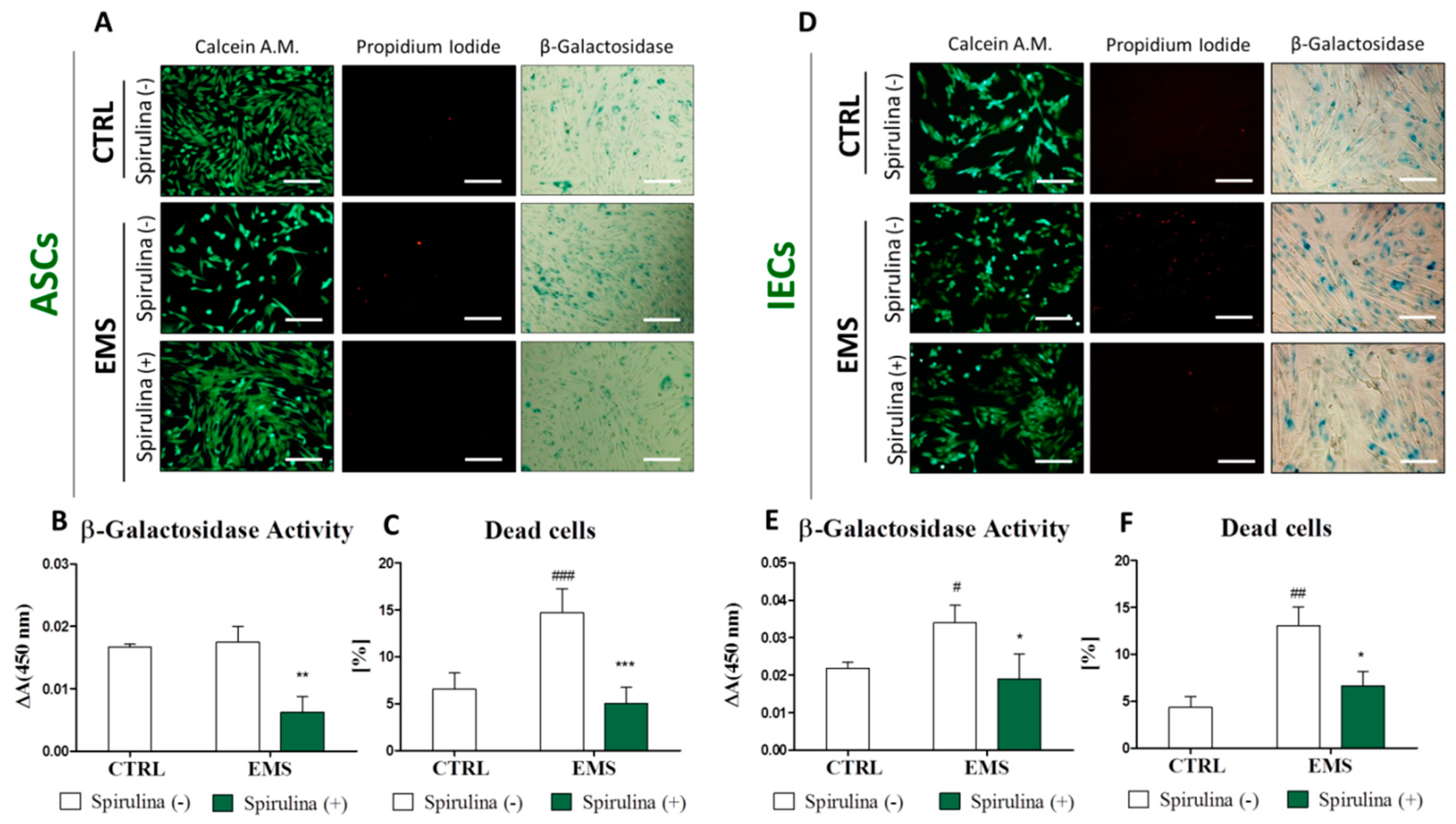
3.4. Spirulina Supresses Cellular Senescence and Apoptosis of Equine ASCs and IECs Derived from EMS-Diagnosed Horses
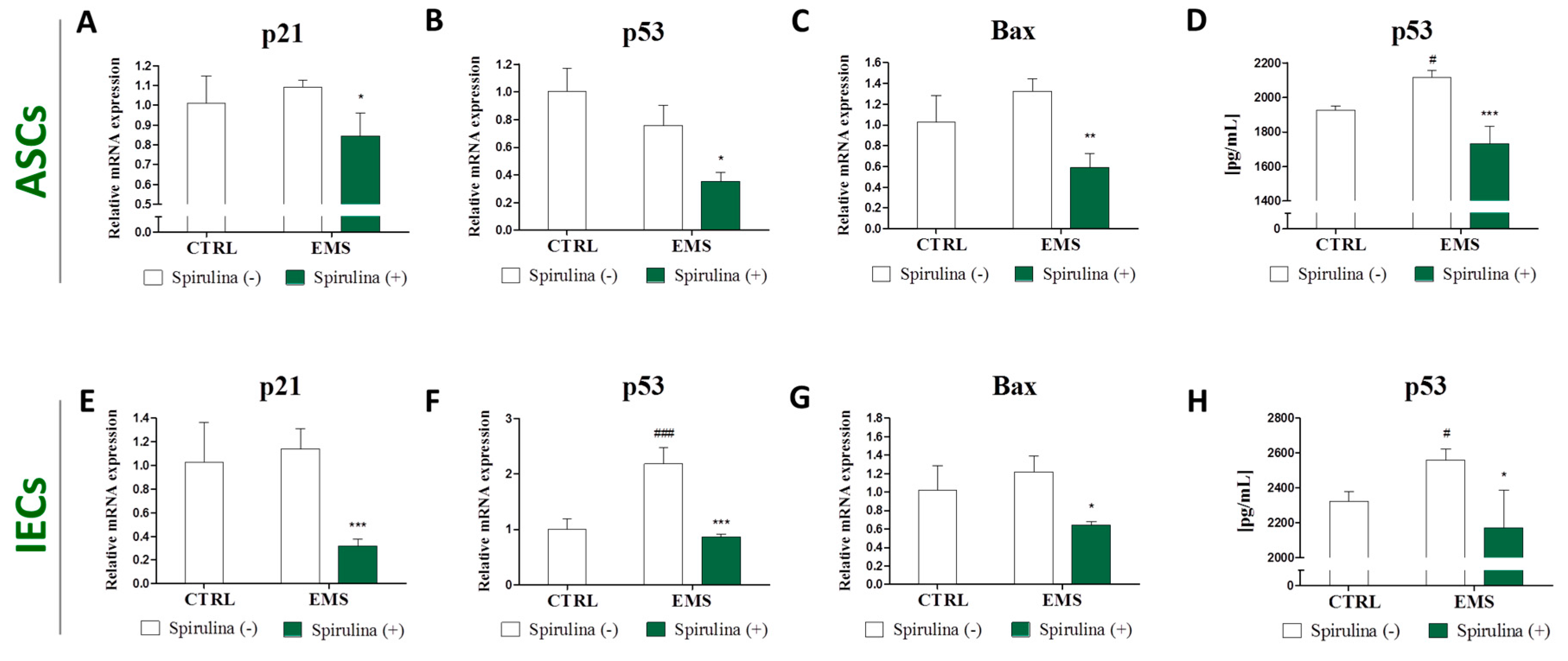
3.5. Quantitative Analysis of Apoptosis-Related Gene and p53 Protein Expression
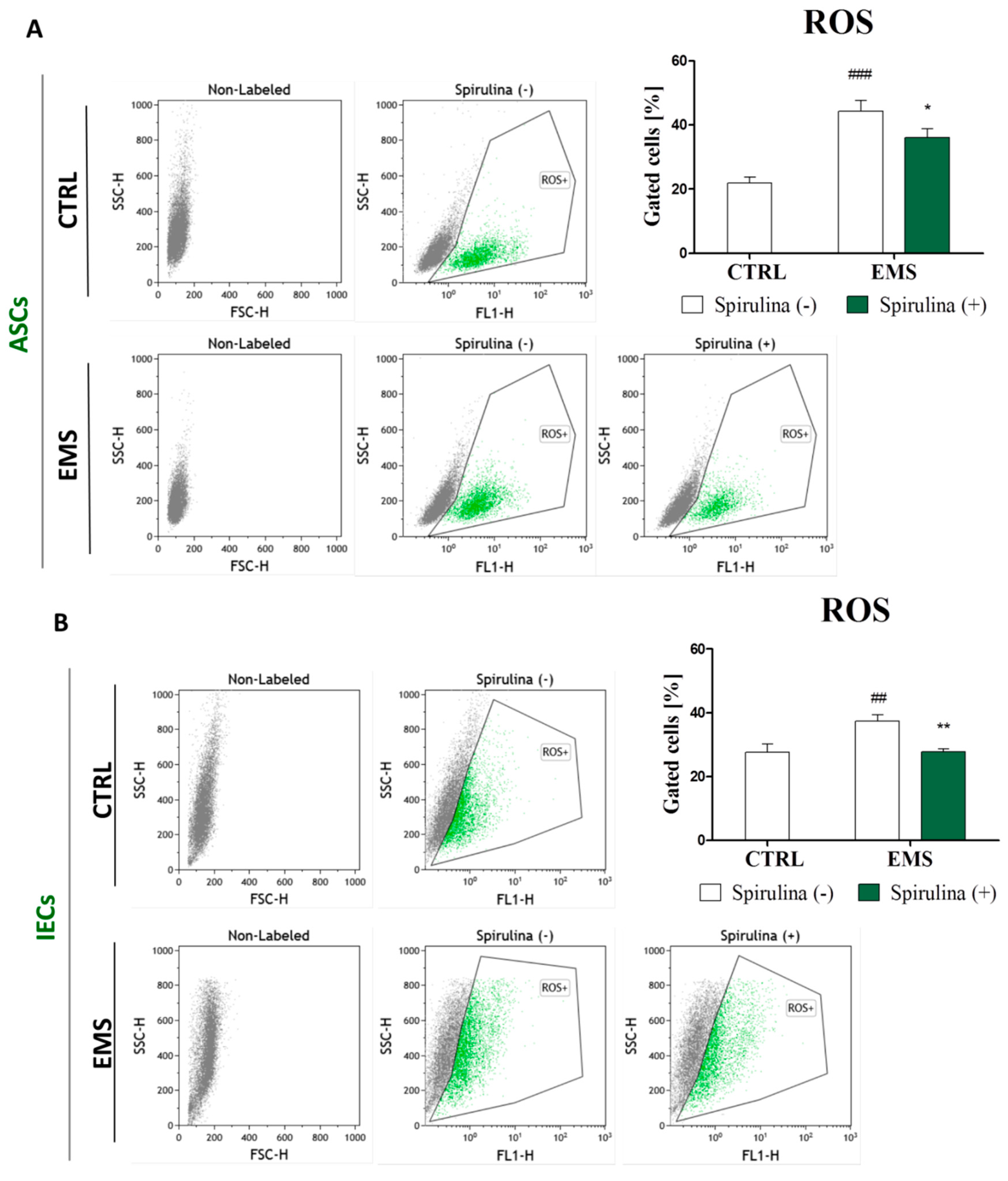
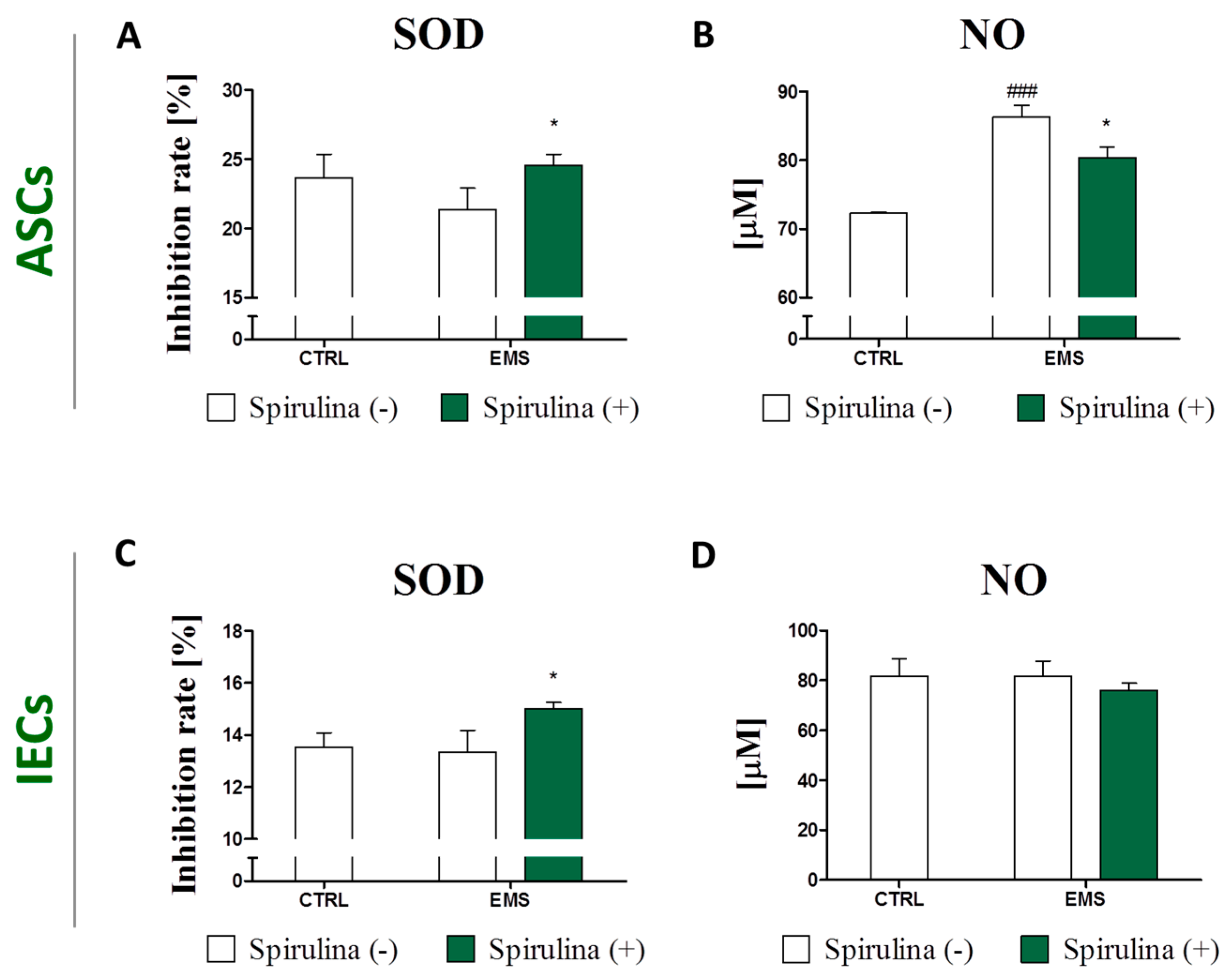
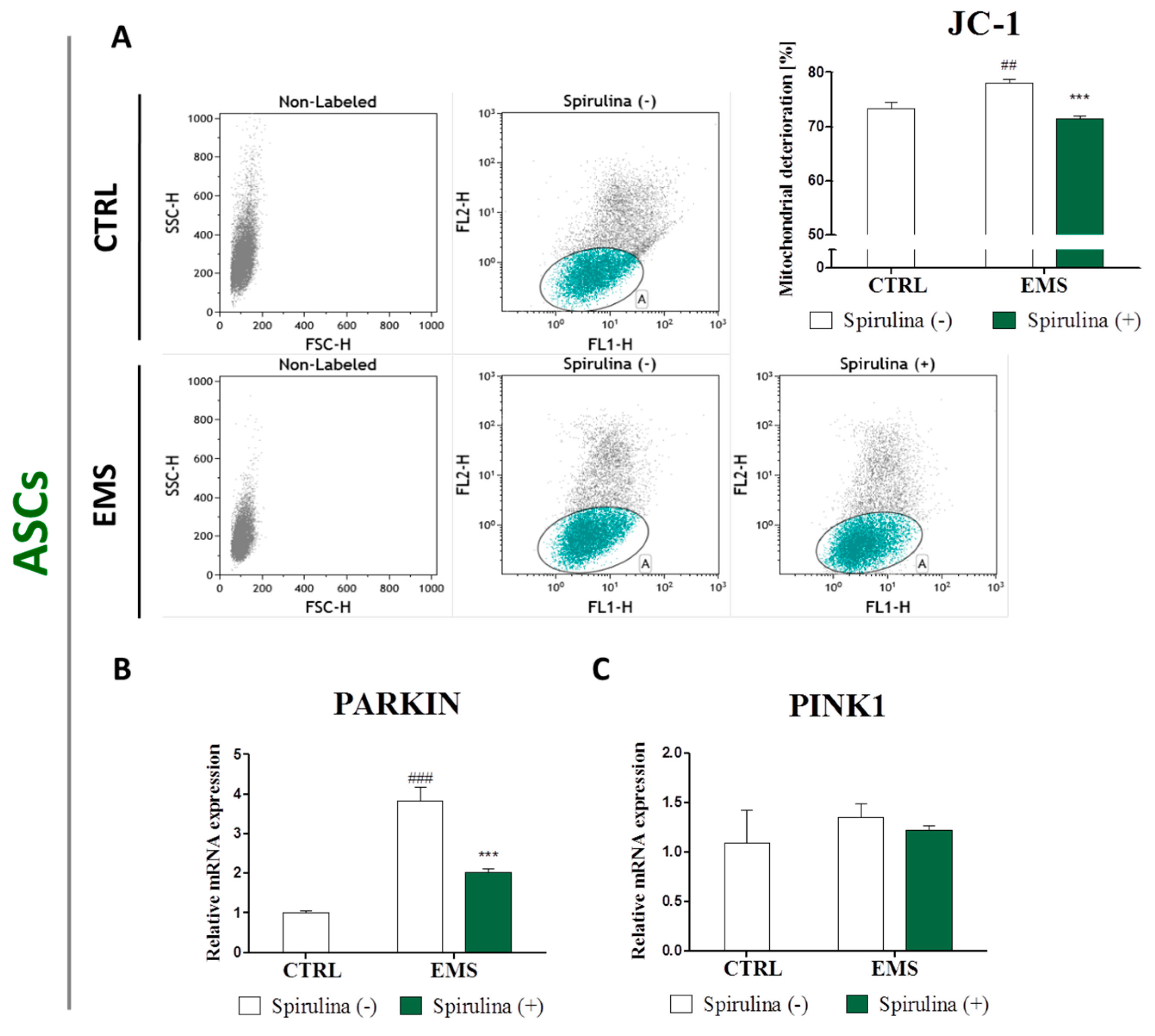
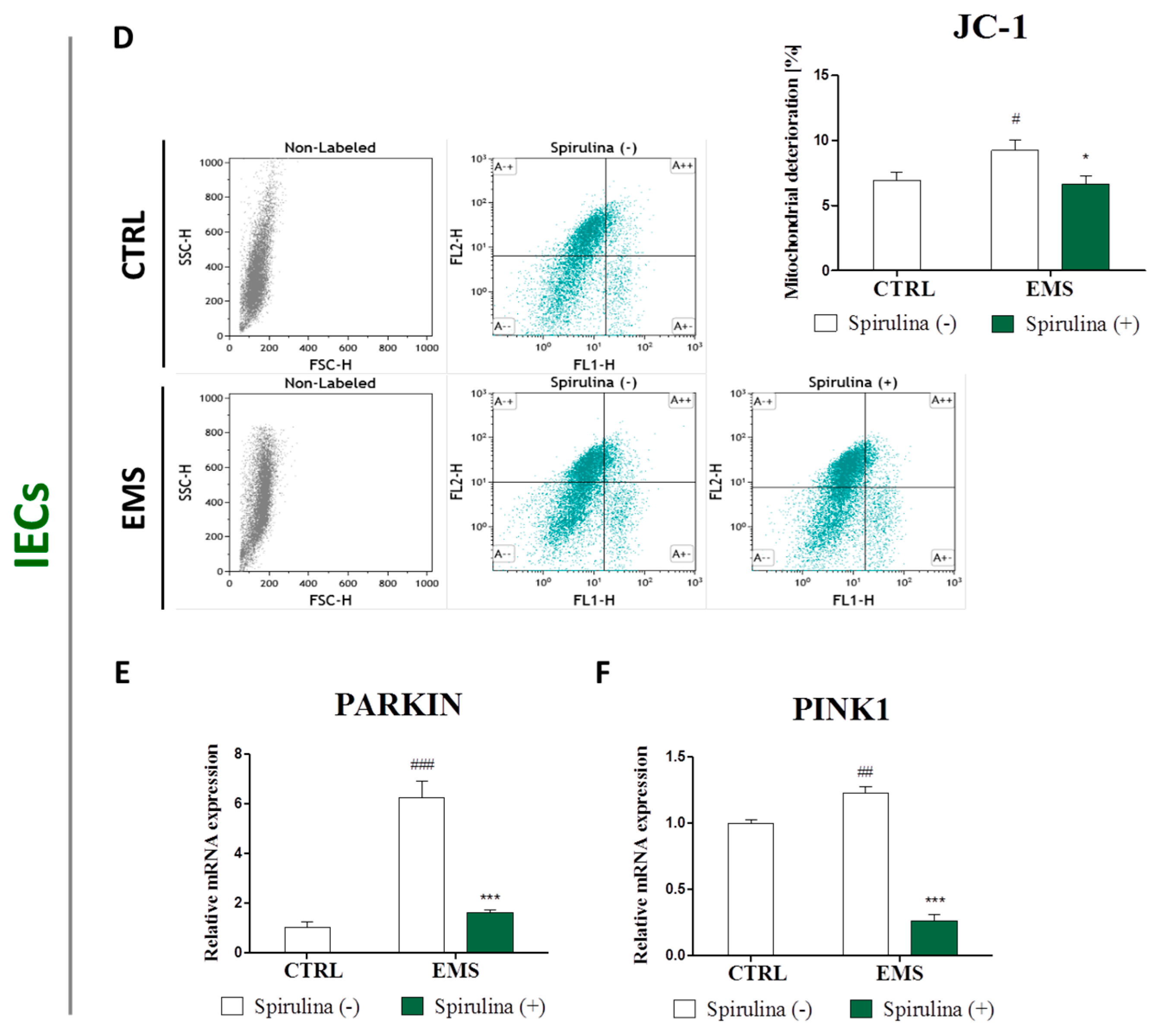
3.6. Spirulina Treatment Reduces Cellular Oxidative Stress and Protects Against Mitochondrial Dysfunction and Degeneration
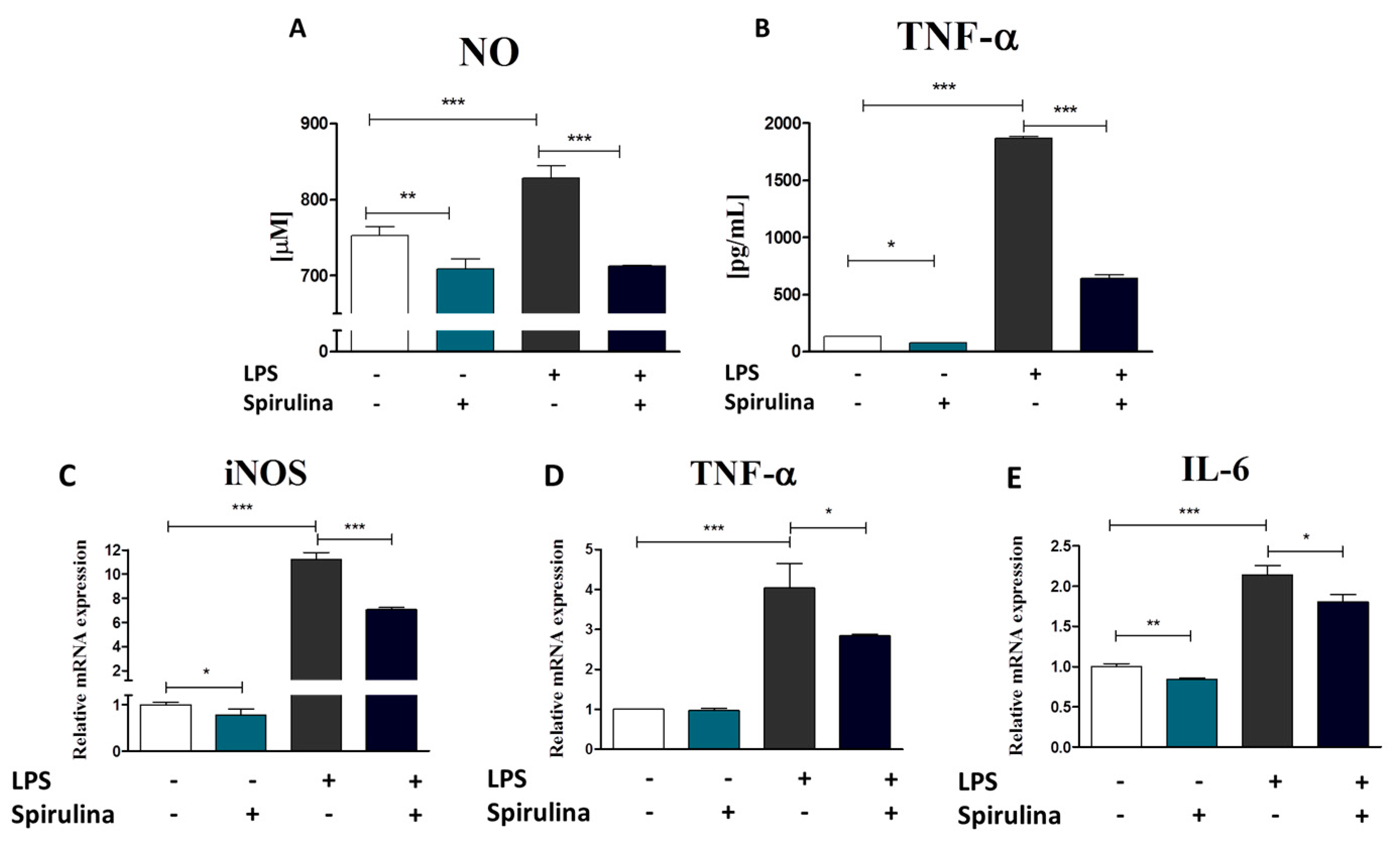
3.7. Regulation of Nitric Oxide and Inflammatory Cytokines Production in LPS-Stimulated Murine Peritoneal Cavity Macrophages by Spirulina Platensis
3.8. Spirulina Platensis Reduces Insulin Resistance and Promotes Weight Loss in Equine Metabolic Syndrome-Affected Horses
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Frank, N. Equine metabolic syndrome. Vet. Clin. N. Am. Equine Pract. 2011, 27, 73–92. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.; Azevedo, I. Chronic inflammation in obesity and the metabolic syndrome. Mediat. Inflamm. 2010, 2010, 289645. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Kornicka, K.; Basinska, K.; Czyrek, A. Equine Metabolic Syndrome affects viability, senescence, and stress factors of equine adipose-derived mesenchymal stromal stem cells: New insight into EqASCs isolated from EMS horses in the context of their aging. Oxid. Med. Cell. Longev. 2015, 2016, e4710326. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, N.; Talwar, P.; Parimisetty, A.; Lefebvre D’Hellencourt, C.; Ravanan, P. A molecular web: Endoplasmic reticulum stress, inflammation, and oxidative stress. Front. Cell. Neurosci. 2014, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.S.; Kaufman, R.J. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid. Redox Signal. 2014, 21, 396–413. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Lund, P.K. Role of intestinal inflammation as an early event in obesity and insulin resistance. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-A.; Gu, W.; Lee, I.-A.; Joh, E.-H.; Kim, D.-H. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS ONE 2012, 7, e47713. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-J.; McAllister, M.J.; Slusher, A.L.; Webb, H.E.; Mock, J.T.; Acevedo, E.O. Obesity-related oxidative sstress: The impact of physical activity and diet manipulation. Sports Med. Open 2015, 1, 32. [Google Scholar] [CrossRef] [PubMed]
- Tchkonia, T.; Morbeck, D.E.; von Zglinicki, T.; van Deursen, J.; Lustgarten, J.; Scrable, H.; Khosla, S.; Jensen, M.D.; Kirkland, J.L. Fat tissue, aging, and cellular senescence. Aging Cell 2010, 9, 667–684. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhabi, N.A.; Valan Arasu, M. Quantification of phytochemicals from commercial Spirulina products and their antioxidant activities. Evid. Based Complement. Altern. Med. 2016, 2016, e7631864. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Chow, T.-J. Hypolipidemic, antioxidant and antiinflammatory activities of microalgae Spirulina. Cardiovasc. Ther. 2010, 28, e33–e45. [Google Scholar] [CrossRef] [PubMed]
- Pak, W.; Takayama, F.; Mine, M.; Nakamoto, K.; Kodo, Y.; Mankura, M.; Egashira, T.; Kawasaki, H.; Mori, A. Anti-oxidative and anti-inflammatory effects of spirulina on rat model of non-alcoholic steatohepatitis. J. Clin. Biochem. Nutr. 2012, 51, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, L.; Miron, A.; Klímová, B.; Wan, D.; Kuča, K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: An overview. Arch. Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H.; Klímová, B.; Wan, D.; Kuča, K. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Davidson, I. Hydrolysis of samples for amino acid analysis. Methods Mol. Biol. 1997, 64, 119–129. [Google Scholar] [PubMed]
- Moraes, C.C.; Sala, L.; Cerveira, G.P.; Kalil, S.J. C-phycocyanin extraction from Spirulina platensis wet biomass. Braz. J. Chem. Eng. 2011, 28, 45–49. [Google Scholar] [CrossRef]
- Horváth, H.; Kovács, A.W.; Riddick, C.; Présing, M. Extraction methods for phycocyanin determination in freshwater filamentous cyanobacteria and their application in a shallow lake. Eur. J. Phycol. 2013, 48, 278–286. [Google Scholar] [CrossRef]
- Keller, T.; Shewager, H. Air pollution and ascorbic acid. Eur. J. For. Pathol. 1997, 7, 338–350. [Google Scholar] [CrossRef]
- Basinska, K.; Marycz, K.; Śmieszek, A.; Nicpoń, J. The production and distribution of IL-6 and TNF-α in subcutaneous adipose tissue and their correlation with serum concentrations in Welsh ponies with equine metabolic syndrome. J. Vet. Sci. 2015, 16, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Grzesiak, J.; Marycz, K.; Wrzeszcz, K.; Czogała, J. Isolation and morphological characterization of ovine adipose-derived mesenchymal stem cells in culture. Int. J. Stem Cells 2011, 4, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Śmieszek, A.; Grzesiak, J.; Donesz-Sikorska, A.; Krzak-Roś, J. Application of bone marrow and adipose-derived mesenchymal stem cells for testing the biocompatibility of metal-based biomaterials functionalized with ascorbic acid. Biomed. Mater. 2013, 8, 065004. [Google Scholar] [CrossRef] [PubMed]
- Maredziak, M.; Marycz, K.; Tomaszewski, K.A.; Kornicka, K.; Henry, B.M. The Influence of Aging on the Regenerative Potential of Human Adipose Derived Mesenchymal Stem Cells. Stem Cells Int. 2016, 2152435. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Dittel, B.N. Isolation of mouse peritoneal cavity cells. J. Vis. Exp. 2010, 35, 1488. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-S.; Xin, Z.-C.; Dai, J.; Lue, T.F. Commonly used mesenchymal stem cell markers and tracking labels: Limitations and challenges. Histol. Histopathol. 2013, 28, 1109–1116. [Google Scholar] [PubMed]
- Kuilman, T.; Michaloglou, C.; Mooi, W.J.; Peeper, D.S. The essence of senescence. Genes Dev. 2010, 24, 2463–2479. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J.; D’Adda di Fagagna, F. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell. Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, Y.; Shimizu, S. Role of the mitochondrial membrane permeability transition in cell death. Apoptosis Int. J. Program. Cell Death 2007, 12, 835–840. [Google Scholar] [CrossRef] [PubMed]
- De Pablo-Latorre, R.; Saide, A.; Polishhuck, E.V.; Nusco, E.; Fraldi, A.; Ballabio, A. Impaired parkin-mediated mitochondrial targeting to autophagosomes differentially contributes to tissue pathology in lysosomal storage diseases. Hum. Mol. Genet. 2012, 21, 1770–1781. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Goncalves, R.; Mosser, D.M. The Isolation and characterization of murine macrophages. Curr. Protoc. Immunol. 2008. [Google Scholar] [CrossRef]
- Davies, L.C.; Jenkins, S.J.; Allen, J.E.; Taylor, P.R. Tissue-resident macrophages. Nat. Immunol. 2013, 14, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Choi, M.-S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Sun, S.; Xu, A.; Zheng, S.; Xue, M.; Wu, P.; Zeng, J.H.; Bai, L. Advanced oxidation protein products induce intestine epithelial cell death through a redox-dependent, c-jun N-terminal kinase and poly (ADP-ribose) polymerase-1-mediated pathway. Cell Death Dis. 2014, 5, e1006. [Google Scholar] [CrossRef] [PubMed]
- Belay, A.; Ota, Y.; Miyakawa, K.; Shimamatsu, H. Current knowledge on potential health benefits of Spirulina. J. Appl. Phycol. 1993, 5, 235–241. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Asker, M.M.S.; Ibrahim, Z.K. Functional bioactive compounds and biological activities of Spirulina platensis lipids. Czech J. Food Sci. 2008, 26, 211–222. [Google Scholar]
- Sanberg, C.D.; Bickford, P.C.; Sanberg, P.R.; Tan, J.; Shytle, R.D. Compounds for Stimulating Stem Cell Proliferation Including Spirulina. U.S. Patent 20,080,085,330 A1, 10 April 2008. Available online: http://www.google.com/patents/US20080085330 (accessed on 4 January 2017).
- Deo, S.K.; Pandey, R.; Jha, S.K.; Singh, J.; Sodhi, K.S. Spirulina: The single cell protein. IAJP 2014, 4, 2211–2217. [Google Scholar]
- Chu, W.-L.; Lim, Y.-W.; Radhakrishnan, A.K.; Lim, P.-E. Protective effect of aqueous extract from Spirulina platensis against cell death induced by free radicals. BMC Complement. Altern. Med. 2010, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-L.; Xu, G.; Chen, T.; Wong, Y.-S.; Zhao, H.-L.; Fan, R.-R.; Gu, X.-M.; Tong, P.C.Y.; Chan, J.C.N. Phycocyanin protects INS-1E pancreatic beta cells against human islet amyloid polypeptide-induced apoptosis through attenuating oxidative stress and modulating JNK and p38 mitogen-activated protein kinase pathways. Int. J. Biochem. Cell Biol. 2009, 41, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.F.; Ali, D.A.; Fernando, A.; Abdraboh, M.E.; Gaur, R.L.; Ibrahim, W.M.; Raj, M.H.G.; Ouhtit, A. Chemoprevention of rat liver toxicity and carcinogenesis by Spirulina. Int. J. Biol. Sci. 2009, 5, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Jiang, J.; Tan, W.; Xia, Y.; Cao, H.; Meng, Y.; Da, Z.; Liu, H.; Cheng, C. p53/p21 pathway involved in mediating cellular senescence of bone marrow-derived mesenchymal stem cells from systemic lupus erythematosus patients. Clin. Dev. Immunol. 2013, 2013, 134243. [Google Scholar] [CrossRef] [PubMed]
- Armesilla-Diaz, A.; Elvira, G.; Silva, A. p53 regulates the proliferation, differentiation and spontaneous transformation of mesenchymal stem cells. Exp. Cell Res. 2009, 315, 3598–3610. [Google Scholar] [CrossRef] [PubMed]
- Kamble, S.P.; Gaikar, R.B.; Padalia, R.B.; Shinde, K.D. Extraction and purification of C-phycocyanin from dry Spirulina powder and evaluating its antioxidant, anticoagulation and prevention of DNA damage activity. J. Appl. Pharm. Sci. 2013, 3, 149–153. [Google Scholar]
- Linjawi, S.A. Protective effect of Spirulina against mitomycin C-Induced genotoxic damage in male rats. J. Am. Sci. 2011, 7, 922–931. [Google Scholar]
- Jürgensmeier, J.M.; Xie, Z.; Deveraux, Q.; Ellerby, L.; Bredesen, D.; Reed, J.C. Bax directly induces release of cytochrome c from isolated mitochondria. Proc. Natl. Acad. Sci. USA 1998, 95, 4997–5002. [Google Scholar] [CrossRef] [PubMed]
- Finucane, D.M.; Bossy-Wetzel, E.; Waterhouse, N.J.; Cotter, T.G.; Green, D.R. Bax-induced caspase activation and apoptosis via cytochrome c release from mitochondria is inhibitable by Bcl-xL. J. Biol. Chem. 1999, 274, 2225–2233. [Google Scholar] [CrossRef] [PubMed]
- Narendra, D.; Walker, J.E.; Youle, R. Mitochondrial quality control mediated by PINK1 and Parkin: Links to Parkinsonism. Cold Spring Harb. Perspect. Biol. 2012, 4, a011338. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.M.; Youle, R.J. PINK1- and Parkin-mediated mitophagy at a glance. J. Cell Sci. 2012, 125, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Xu, H.; Wang, Z.; Mao, Y.; Yuan, L.; Luo, W.; Cui, Z.; Cui, T.; Wang, L.; Shen, Y. PINK1-Parkin-mediated mitophagy protects mitochondrial integrity and prevents metabolic stress-induced endothelial injury. PLoS ONE 2015, 10, e0132499. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Du, J.; Stitham, J.; Atteya, G.; Lee, S.; Xiang, Y.; Wang, D.; Jin, Y.; Leslie, K.L.; Spollett, G.; et al. Inducing mitophagy in diabetic platelets protects against severe oxidative stress. EMBO Mol. Med. 2016, 8, 779–795. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Kornicka, K.; Grzesiak, J.; Smieszek, A.; Szlapka, J. Macroautophagy and selective mitophagy ameliorate chondrogenic differentiation potential in adipose stem cells of equine metabolic syndrome: New findings in the field of progenitor cells differentiation. Oxid. Med. Cell. Longev. 2016, 2016, e3718468. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Kornicka, K.; Marędziak, M.; Golonka, P.; Nicpoń, J. Equine metabolic syndrome impairs adipose stem cells osteogenic differentiation by predominance of autophagy over selective mitophagy. J. Cell. Mol. Med. 2016, 20, 2384–2404. [Google Scholar] [CrossRef] [PubMed]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C.S. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.K.; Sindhu, K.K. Oxidative stress and metabolic syndrome. Life Sci. 2009, 84, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Selivanov, V.A.; Votyakova, T.V.; Pivtoraiko, V.N.; Zeak, J.; Sukhomlin, T.; Trucco, M.; Roca, J.; Cascante, M. Reactive oxygen species production by forward and reverse electron fluxes in the mitochondrial respiratory chain. PLoS Comput. Biol. 2011, 7, e1001115. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chen, Y.; St. Clair, D.K. ROS and p53: Versatile partnership. Free Radic. Biol. Med. 2008, 44, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Guo, R.; Hui, L.; Ye, Y.; Xiang, J.; Wan, C.; Zou, M.; Ma, R.; Sun, X.; Yang, S.; et al. Stanniocalcin-1 protects bovine intestinal epithelial cells from oxidative stress-induced damage. J. Vet. Sci. 2014, 15, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, H.; Halliwell, B. Damage to DNA by reactive oxygen and nitrogen species: Role in inflammatory disease and progression to cancer. Biochem. J. 1996, 313, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Datta, K.; Suman, S.; Kallakury, B.V.S.; Fornace, A.J. Exposure to heavy ion radiation induces persistent oxidative stress in mouse intestine. PLoS ONE 2012, 7, e42224. [Google Scholar] [CrossRef] [PubMed]
- Denu, R.A.; Hematti, P. Effects of oxidative stress on mesenchymal stem cell biology. Oxid. Med. Cell. Longev. 2016, 2016, e2989076. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, S.; Herrero, M.; Senorans, F.J.; Cifuentes, A.; Ibáñez, E.; Jaime, L. Functional characterization of pressurized liquid extracts of Spirulina platensis. Eur. Food Res. Technol. 2006, 224, 75–81. [Google Scholar] [CrossRef]
- Dartsch, P.C. Antioxidant potential of selected Spirulina platensis preparations. Phytother. Res. 2008, 22, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Romay, C.; Armesto, J.; Remirez, D.; González, R.; Ledon, N.; García, I. Antioxidant and anti-inflammatory properties of C-phycocyanin from blue-green algae. Inflamm. Res. 1998, 47, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Romay, C.; Ledón, N.; González, R. Further studies on anti-inflammatory activity of phycocyanin in some animal models of inflammation. Inflamm. Res. 1998, 47, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Giugliano, D. The metabolic syndrome and inflammation: Association or causation? Nutr. Metab. Cardiovasc. Dis. 2004, 14, 228–232. [Google Scholar] [CrossRef]
- Joventino, I.P.; Alves, H.G.R.; Neves, L.C.; Pinheiro-Joventino, F.; Leal, L.K.A.M.; Neves, S.A.; et al. The microalga Spirulina platensis presents anti-inflammatory action as well as hypoglycemic and hypolipidemic properties in diabetic rats. J. Complement. Integr. Med. 2012, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.X.; Park, Y.-K.; Lee, J.-Y. Anti-inflammatory effects of Spirulina platensis extract via the modulation of histone deacetylases. Nutrients 2016, 8, 381. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.X.; Lee, J.-Y. The anti-inflammatory effects of Spirulina platensis extract are mediated, in part, through the induction of an endotoxin tolerance-like mechanism. FASEB J. 2016, 30 (Suppl. 916.15). [Google Scholar]
- Quader, S.H.; Islam, S.U.; Saifullah, A.; Majumder, F.U.; Hannan, J. In-vivo studies of the anti-inflammatory effects of Spirulina platensis. Pharma Innov. 2013, 2, 70–80. [Google Scholar]
- Abdel-Daim, M.M.; Farouk, S.M.; Madkour, F.F.; Azab, S.S. Anti-inflammatory and immunomodulatory effects of Spirulina platensis in comparison to Dunaliella salina in acetic acid-induced rat experimental colitis. Immunopharmacol. Immunotoxicol. 2015, 37, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Singh, S.; Patro, N.; Patro, I. Evaluation of protective efficacy of Spirulina platensis against collagen-induced arthritis in rats. Inflammopharmacology 2009, 17, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Parikh, P.; Mani, U.; Iyer, U. Role of Spirulina in the control of glycemia and lipidemia in type 2 diabetes mellitus. J. Med. Food 2001, 4, 193–199. [Google Scholar] [CrossRef] [PubMed]
| Clinical Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|
| No. | Group | Sex | Bw (kg) | BCS [1–9] | CNS [1–5] | Fasting insulin (mU/mL) | LEP (ng/mL) | CGIT:GLU in 45 min (mg/dL) |
| 1 | Healthy | F | 610 | 6 | 1 | 7 | 3.21 | 74/n |
| 2 | F | 644 | 7 | 2 | 12 | 4.12 | 69/n | |
| 3 | F | 627 | 7 | 2 | 9 | 2.87 | 71/n | |
| 4 | M | 609 | 6 | 1 | 8 | 1.86 | 89/n | |
| 5 | M | 649 | 7 | 2 | 14 | 3.56 | 80/n | |
| 6 | M | 639 | 6 | 2 | 13 | 2.91 | 74/n | |
| Mean ± SD | 629.7 ± 15.7 | 6.5 ± 0.5 | 1.7 ± 0.5 | 10.5 ± 2.6 | 3.1 ± 0.7 | 76.2 ± 6.7 | ||
| 1 | EMS | F | 710 | 8 | 3 | 83 | 4.89 | 138/p |
| 2 | F | 726 | 9 | 3 | 67 | 5.19 | 141/p | |
| 3 | F | 760 | 9 | 4 | 98 | 9.12 | 140/p | |
| 4 | M | 709 | 8 | 3 | 73 | 8.49 | 136/p | |
| 5 | M | 716 | 8 | 4 | 69 | 7.27 | 134/p | |
| 6 | M | 746 | 9 | 4 | 82 | 8.36 | 146/p | |
| Mean ± SD | 727.8 ± 19.1 | 8.5 ± 0.5 | 3.5 ± 0.5 | 78.7 ± 10.5 | 7.2 ± 1.6 | 139.2 ± 3.8 | ||
| Gene | Sequence 5′—3′ | Amplicon Length (bp) | Accession Number |
|---|---|---|---|
| p21 | F: GAAGAGAAACCCCCAGCTCC R: TGACTGCATCAAACCCCACA | 241 | XM_003365840.2 |
| p53 | F: TACTCCCCTGCCCTCAACAA R: AGGAATCAGGGCCTTGAGGA | 252 | U37120.1 |
| Bax | F: TTCCGACGGCAACTTCAACT R: GGTGACCCAAAGTCGGAGAG | 218 | XM_005596728.1 |
| PINK1 | F: GCACAATGAGCCAGGAGCTA R: GGGGTATTCACGCGAAGGTA | 298 | XM_014737247.1 |
| Parkin | F: TCCCAGTGGAGGTCGATTCT R: CCCTCCAGGTGTGTTCGTTT | 218 | XM_005608126.2 |
| GAPDH | F: GATGCCCCAATGTTTGTGA R: AAGCAGGGATGATGTTCTGG | 250 | NM_001163856.1 |
| iNOS | F: GACAAGCTGCATGTGACATC R: GCTGGTAGGTTCCTGTTGTT | 325 | NM_001313922.1 |
| TNF-α | F: ACAGAAAGCATGATCCGCGA R: CTTGGTGGTTTGCTACGACG | 295 | NM_013693.3 |
| IL-6 | F: GAGGATACCACTCCCAACAGACC R: AAGTGCATCATCGTTGTTCATACA | 141 | NM_001314054.1 |
| β-Actin | F: CGACGATGCTCCCCGGGCTGTA R: CTCTTTGATGTCACGCACGATTTCCCTCT | 574 | NM_007393.5 |
| Fatty Acid | Content [mg/100 g of Dry Mass] |
|---|---|
| 8:0 | 5.1 ± 0.93 |
| 10:0 | 3.3 ± 0.57 |
| 12:0 | 12.9 ± 1.44 |
| 14:0 | 18.1 ± 1.01 |
| 14:1 (n-5) | n.d. |
| 15:0 | n.d. |
| 16:0 | 2423.3 ± 241.11 |
| 16:1 (n-7) | 282.7 ± 16.86 |
| 18:0 | 42.0 ± 6.13 |
| 18:1 (n-12) | 173.0 ± 14.00 |
| 18:2 (n-6) | 1053.3 ± 61.10 |
| 18:3 (n-3) | n.d. |
| 18:3 (n-6) | 744.0 ± 24.25 |
| 18:4 (n-3) | 232.0 ± 27.87 |
| 20:0 | 12.6 ± 3.03 |
| 20:2 (n-6) | 7.7 ± 0.87 |
| 22:0 | n.d. |
| Amino Acid | Free | Bound |
|---|---|---|
| Ala | 207 ± 25.2 | 5167 ± 2757 |
| Glc | 97 ± 5.8 | 3657 ± 265.0 |
| Val | 50 ± 13.2 | 3320 ± 248.8 |
| Leu | 103 ± 5.8 | 4660 ± 390.0 |
| Ile | 42 ± 2.9 | 2760 ± 144.2 |
| Asn | 0 | 6367 ± 260.3 |
| Asp | 65 ± 5.0 | |
| Gln | 82 ± 7.6 | 7937 ± 361.2 |
| Glu | 437 ± 40.4 | |
| Lys | 35 ± 5.0 | 2530 ± 130.0 |
| Arg | 0 | 3477 ± 130.5 |
| His | 217 ± 20.8 | 763 ± 51.3 |
| Phe | 33 ± 2.9 | 2270 ± 175.2 |
| Tyr | 42 ± 7.6 | 2613 ± 263.5 |
| Trp | 0 | 757 ± 55.1 |
| Ser | 0 | 3297 ± 130.5 |
| Thr | 88 ± 7.6 | 1827 ± 115.9 |
| Met | 20 ± 0 | 1000 ± 87.2 |
| Cys | 0 | 540 ± 10.0 |
| Pro | 22 ± 2.9 | 1967 ± 151.8 |
| No. | Group | Sex | Clinical Parameters | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bw (kg) | BCS [1–9] | CNS [1–5] | Fasting Insulin (mU/mL) | LEP (ng/mL) | CGIT:GLU in 45 min (mg/dL) | |||||||||
| Before Treatment | After Treatment | Before Treatment | After Treatment | Before Treatment | After Treatment | Before Treatment | After Treatmment | Before Treatment | After Treatment | Before Treatment | After Treatment | |||
| 1 | Healthy | F | 580 | 592 | 6 | 6 | 2 | 3 | 2 | 6 | 3.21 | 3.13 | 78/n | 75/n |
| 2 | F | 550 | 565 | 6 | 6 | 2 | 2 | 4 | 3 | 2.89 | 3.78 | 69/n | 73/n | |
| 3 | F | 590 | 575 | 6 | 6 | 2 | 2 | 2 | 2 | 3.14 | 2.98 | 72/n | 75/n | |
| 4 | M | 559 | 562 | 6 | 6 | 2 | 3 | 7 | 5 | 2.91 | 2.47 | 81/n | 77/n | |
| 5 | M | 528 | 543 | 6 | 6 | 2 | 2 | 5 | 4 | 3.38 | 2.94 | 79/n | 71/n | |
| 6 | M | 560 | 551 | 6 | 6 | 2 | 2 | 3 | 3 | 2.71 | 2.01 | 67/n | 73/n | |
| Mean ± SD | 561.2 ± 22.0 | 564.7 ± 17.4 | 6.0 | 6.0 | 2.0 | 2.3 ± 0.5 | 3.8 ± 1.9 | 3.8 ± 1.5 | 3.0 ± 0.2 | 2.9 ± 0.6 | 74.3 ± 5.8 | 74.0 ± 2.1 | ||
| 1 | EMS | F | 760 | 748 | 9 | 9 | 4 | 4 | 78 | 92 | 10.41 | 9.48 | 132/p | 118/p |
| 2 | F | 732 | 728 | 9 | 8 | 4 | 4 | 91 | 83 | 9.29 | 9.22 | 126/p | 129/p | |
| 3 | F | 743 | 751 | 9 | 9 | 4 | 4 | 87 | 80 | 9.89 | 10.31 | 149/p | 138/p | |
| 4 | M | 708 | 712 | 9 | 9 | 4 | 4 | 74 | 73 | 8.78 | 9.13 | 121/p | 119/p | |
| 5 | M | 719 | 720 | 9 | 9 | 4 | 4 | 86 | 82 | 7.98 | 8.43 | 138/p | 131/p | |
| 6 | M | 698 | 692 | 9 | 9 | 4 | 4 | 92 | 89 | 8.13 | 7.88 | 136/p | 129/p | |
| Mean ± SD | 726.7 ± 23.0 | 725.2 ± 22.3 | 9.0 | 8.8 ± 0.4 | 4.0 | 4.0 | 84.7 ± 7.2 | 83.2 ± 6.7 | 9.1 ± 1.0 | 9.1 ± 0.8 | 133.7 ± 9.8 | 127.3 ± 7.6 | ||
| 1 | EMS spirulina-treated | F | 740 | 685 | 9 | 8 | 4 | 3 | 97 | 45 | 9.12 | 9.02 | 142/p | 130/p |
| 2 | F | 711 | 672 | 8 | 7 | 4 | 4 | 74 | 51 | 7.45 | 6.58 | 139/p | 78/n | |
| 3 | F | 753 | 648 | 9 | 8 | 4 | 3 | 98 | 92 | 11.02 | 10.98 | 128/p | 81/n | |
| 4 | M | 701 | 642 | 8 | 8 | 4 | 3 | 83 | 61 | 7.58 | 7.21 | 111/p | 63/n | |
| 5 | M | 749 | 679 | 8 | 7 | 4 | 3 | 79 | 82 | 8.91 | 8.70 | 129/p | 72/n | |
| 6 | M | 707 | 692 | 8 | 7 | 4 | 3 | 73 | 43 | 7.62 | 7.11 | 146/p | 79/n | |
| Mean ± SD | 726.8 ± 23.1 | 669.7 ± 20.3 ** | 8.3 ± 0.5 | 7.5 ± 0.5 * | 4.0 | 3.2 ± 0.4 *** | 84.0 ± 11.1 | 62.3 ± 20.4 * | 8.6 ± 1.4 | 8.3 ± 1.6 | 132.5 ± 12.7 | 83.8 ± 23.5 ** | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawrocka, D.; Kornicka, K.; Śmieszek, A.; Marycz, K. Spirulina platensis Improves Mitochondrial Function Impaired by Elevated Oxidative Stress in Adipose-Derived Mesenchymal Stromal Cells (ASCs) and Intestinal Epithelial Cells (IECs), and Enhances Insulin Sensitivity in Equine Metabolic Syndrome (EMS) Horses. Mar. Drugs 2017, 15, 237. https://doi.org/10.3390/md15080237
Nawrocka D, Kornicka K, Śmieszek A, Marycz K. Spirulina platensis Improves Mitochondrial Function Impaired by Elevated Oxidative Stress in Adipose-Derived Mesenchymal Stromal Cells (ASCs) and Intestinal Epithelial Cells (IECs), and Enhances Insulin Sensitivity in Equine Metabolic Syndrome (EMS) Horses. Marine Drugs. 2017; 15(8):237. https://doi.org/10.3390/md15080237
Chicago/Turabian StyleNawrocka, Daria, Katarzyna Kornicka, Agnieszka Śmieszek, and Krzysztof Marycz. 2017. "Spirulina platensis Improves Mitochondrial Function Impaired by Elevated Oxidative Stress in Adipose-Derived Mesenchymal Stromal Cells (ASCs) and Intestinal Epithelial Cells (IECs), and Enhances Insulin Sensitivity in Equine Metabolic Syndrome (EMS) Horses" Marine Drugs 15, no. 8: 237. https://doi.org/10.3390/md15080237
APA StyleNawrocka, D., Kornicka, K., Śmieszek, A., & Marycz, K. (2017). Spirulina platensis Improves Mitochondrial Function Impaired by Elevated Oxidative Stress in Adipose-Derived Mesenchymal Stromal Cells (ASCs) and Intestinal Epithelial Cells (IECs), and Enhances Insulin Sensitivity in Equine Metabolic Syndrome (EMS) Horses. Marine Drugs, 15(8), 237. https://doi.org/10.3390/md15080237





