Briarane Diterpenoids Isolated from Octocorals between 2014 and 2016
Abstract
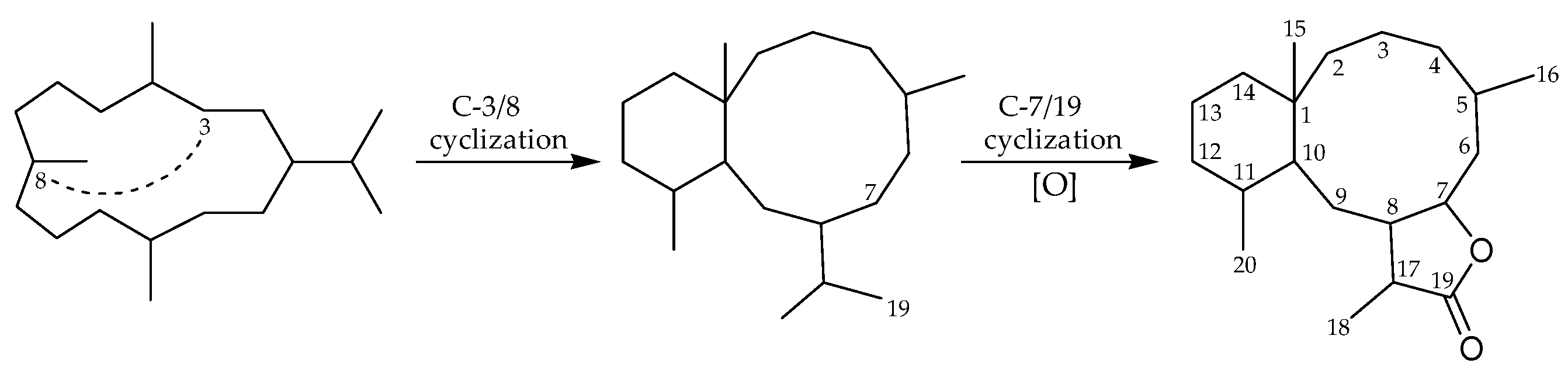
:1. Introduction
2. Alcyonacea
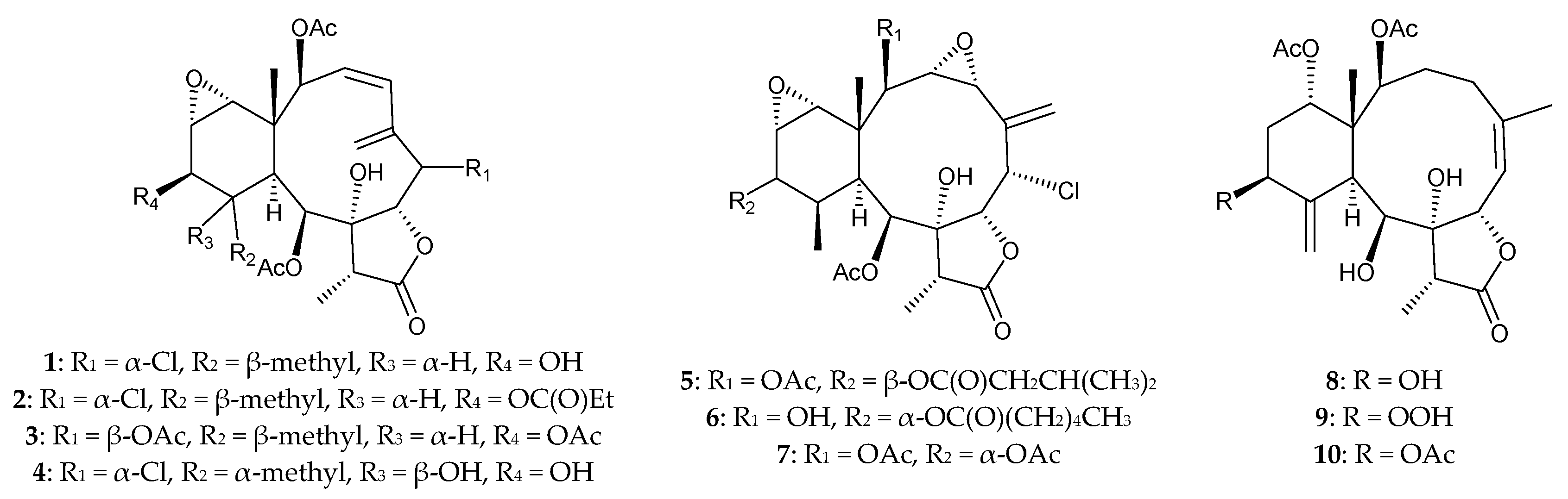
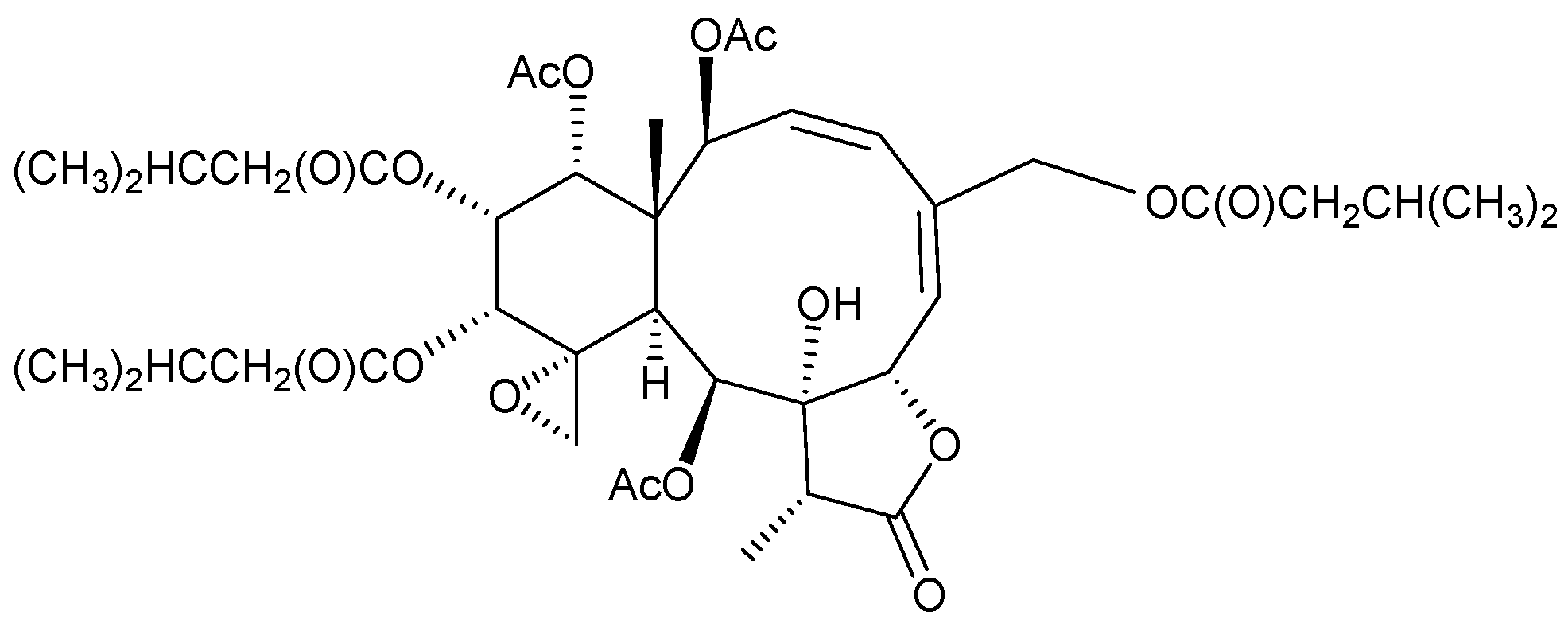
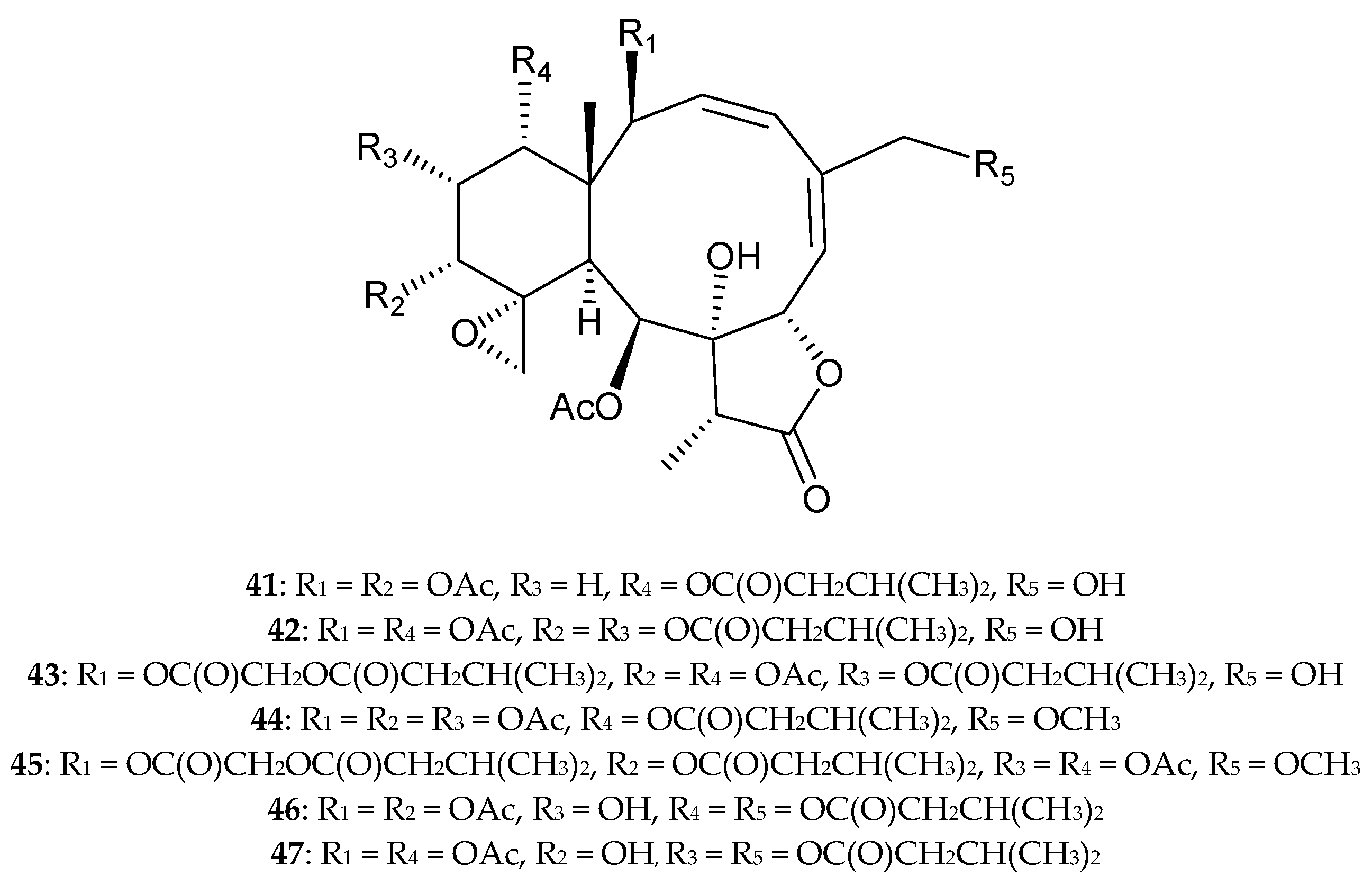
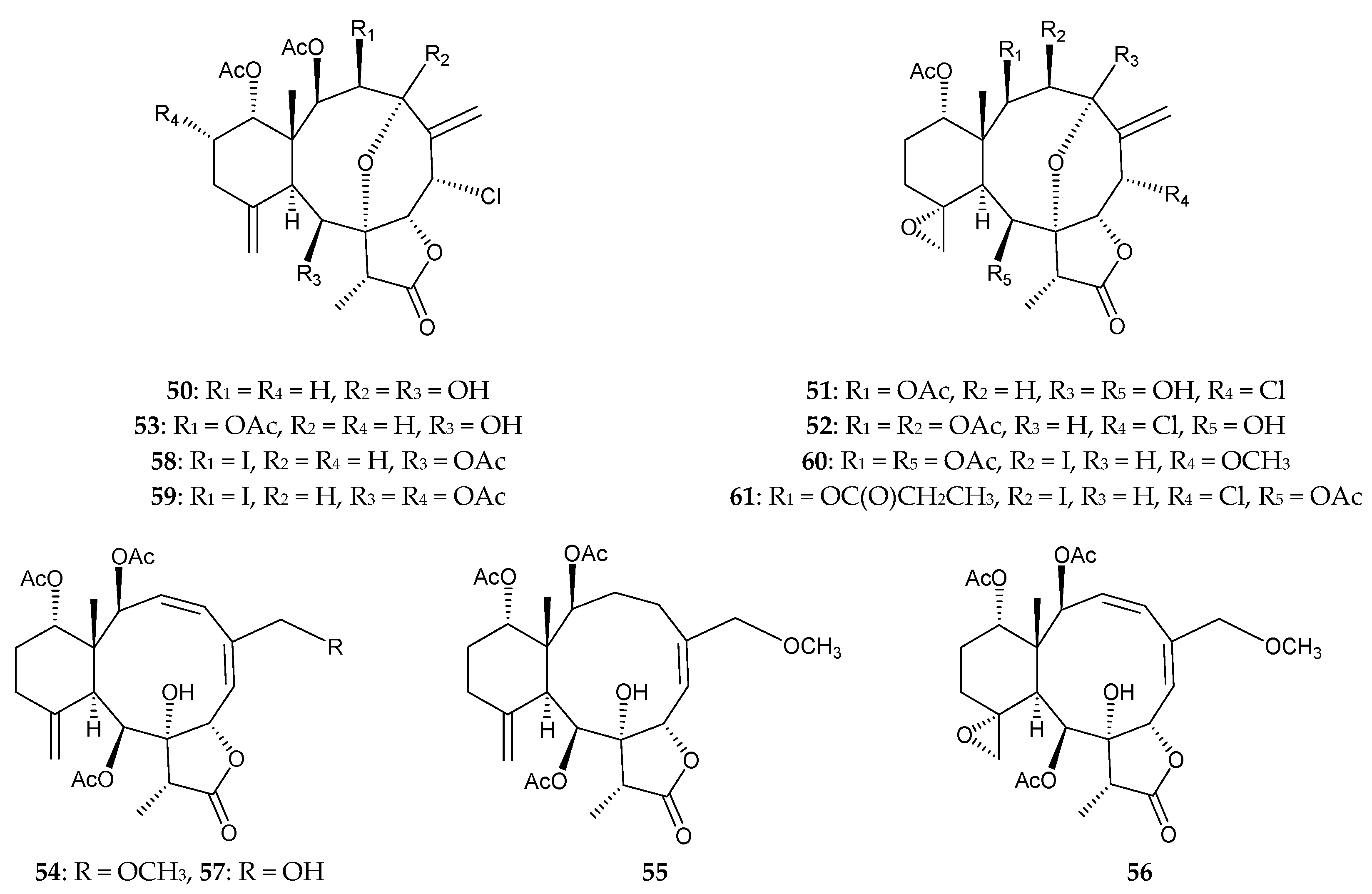
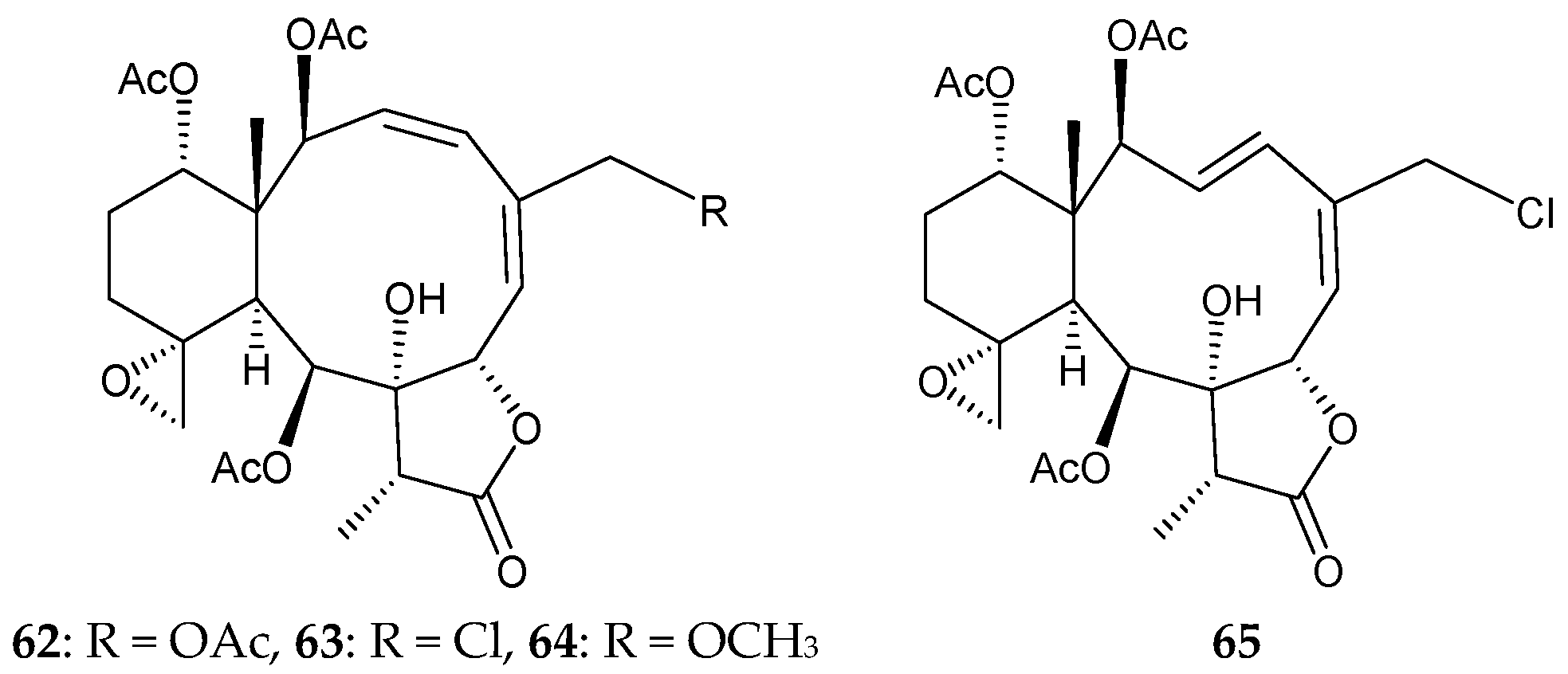
2.1. Briareum violacea (Family Briareidae)
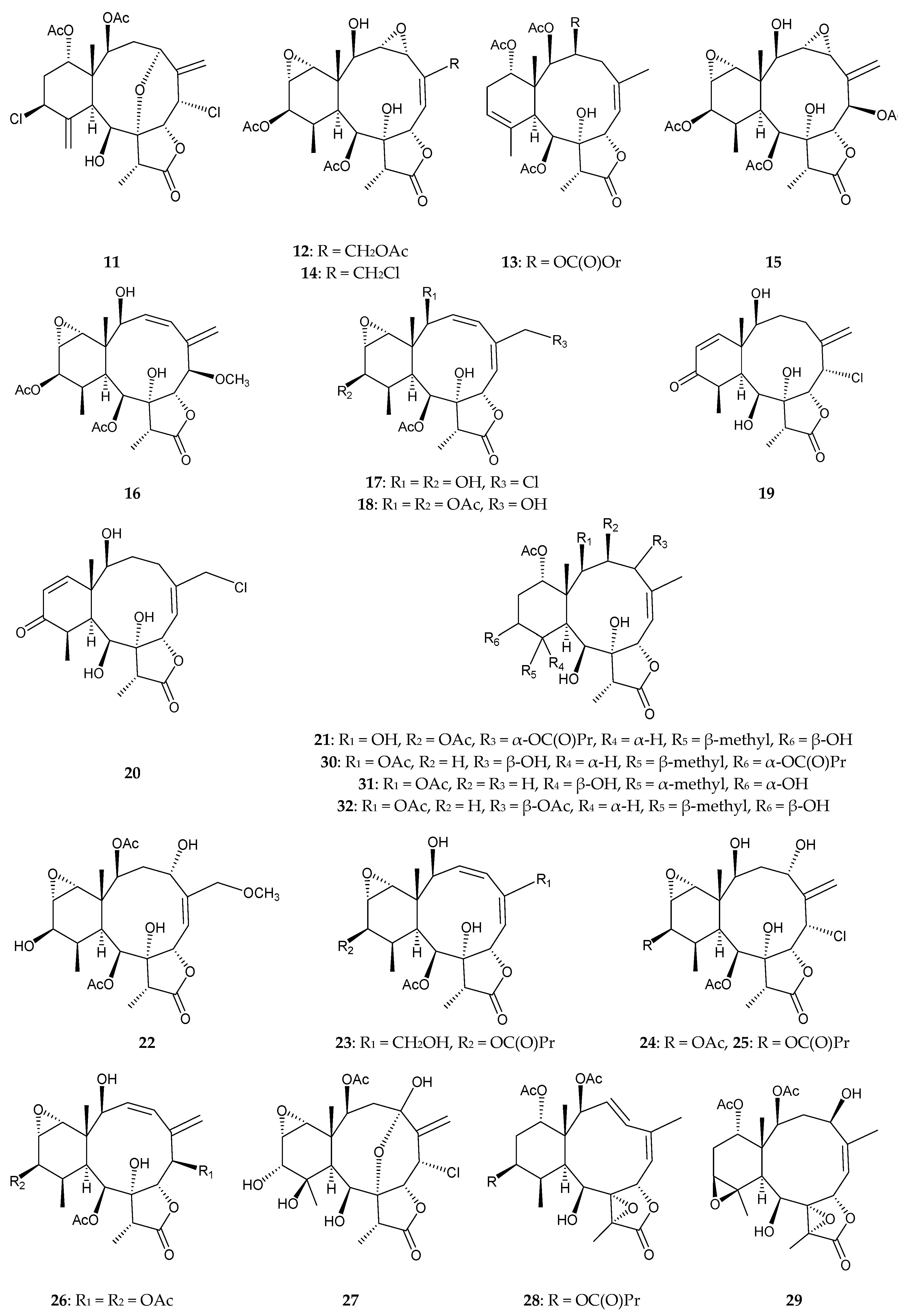
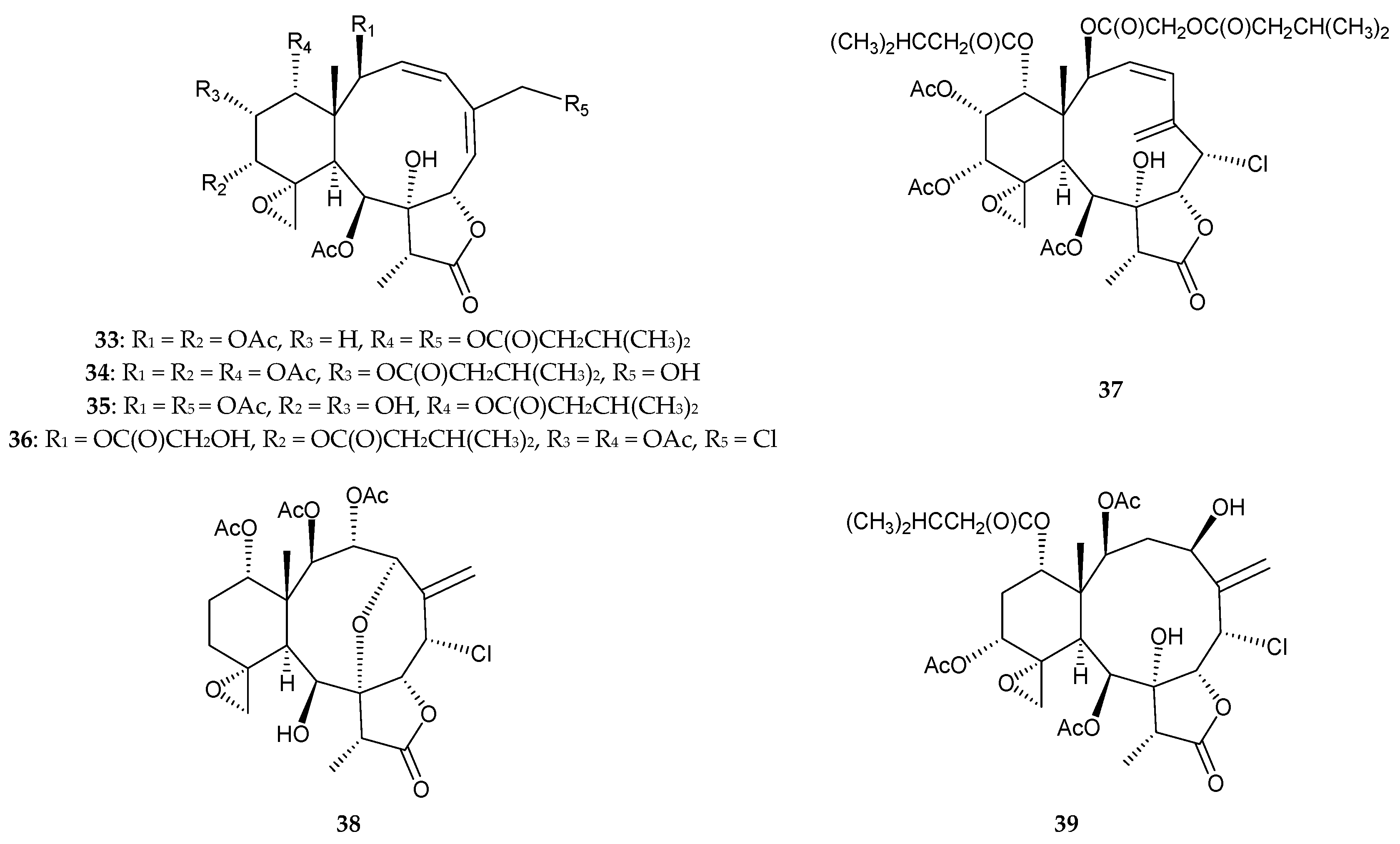
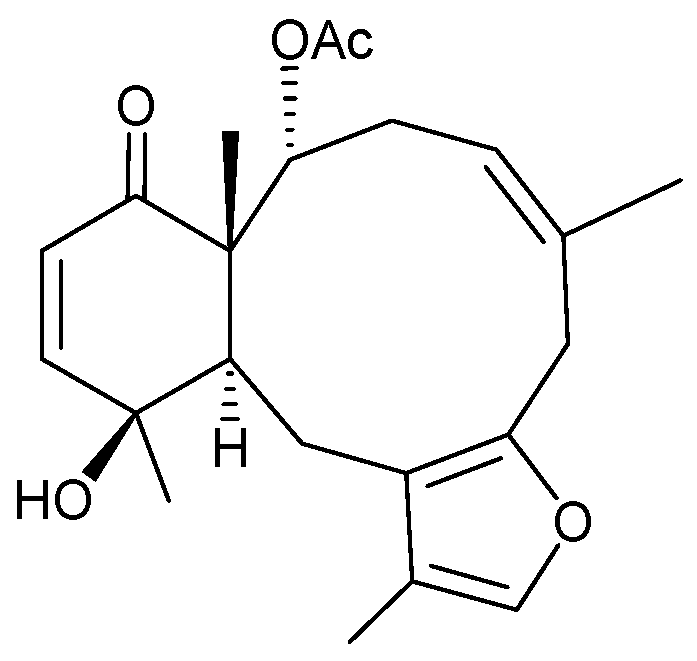
2.2. Briareum sp.
3. Gorgonacea
3.1. Dichotella gemmacea (Family Ellisellidae)
3.2. Ellisella dollfusi (Family Ellisellidae)
3.3. Junceella fragilis (Family Ellisellidae)
3.4. Junceella gemmacea (Family Ellisellidae)
3.5. Junceella sp. (Family Ellisellidae)
4. Pennatulacea
Pennatula aculeata (Family Pennatulidae)
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sung, P.-J.; Sheu, J.-H.; Xu, J.-P. Survey of briarane-type diterpenoids of marine origin. Heterocycles 2002, 57, 535–579. [Google Scholar] [CrossRef]
- Sung, P.-J.; Chang, P.-C.; Fang, L.-S.; Sheu, J.-H.; Chen, W.-C.; Chen, Y.-P.; Lin, M.-R. Survey of briarane-related diterpenoids-part II. Heterocycles 2005, 65, 195–204. [Google Scholar] [CrossRef]
- Sung, P.-J.; Sheu, J.-H.; Wang, W.-H.; Fang, L.-S.; Chung, H.-M.; Pai, C.-H.; Su, Y.-D.; Tsai, W.-T.; Chen, B.-Y.; Lin, M.-R.; et al. Survey of briarane-type diterpenoids–part III. Heterocycles 2008, 75, 2627–2648. [Google Scholar] [CrossRef]
- Sung, P.-J.; Su, J.-H.; Wang, W.-H.; Sheu, J.-H.; Fang, L.-S.; Wu, Y.-C.; Chen, Y.-H.; Chung, H.-M.; Su, Y.-D.; Chang, Y.-C. Survey of briarane-type diterpenoids–part IV. Heterocycles 2011, 83, 1241–1258. [Google Scholar] [CrossRef]
- Sheu, J.-H.; Chen, Y.-H.; Chen, Y.-H.; Su, Y.-D.; Chang, Y.-C.; Su, J.-H.; Weng, C.-F.; Lee, C.-H.; Fang, L.-S.; Wang, W.-H.; et al. Briarane diterpenoids isolated from gorgonian corals between 2011 and 2013. Mar. Drugs 2014, 12, 2164–2181. [Google Scholar] [CrossRef] [PubMed]
- Samimi-Namin, K.; van Ofwegen, L.P. Overview of the genus Briareum (Cnidaria, Octocorallia, Briareidae) in the Indo-Pacific, with the description of a new species. ZooKeys 2016, 557, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Bowden, B.F.; Coll, J.C.; Vasilescu, I.M. Studies of Australian soft corals. XLVI. New diterpenes from a Briareum species (Anthozoa, Octocorallia, Gorgonacea). Aust. J. Chem. 1989, 42, 1705–1726. [Google Scholar] [CrossRef]
- Schmitz, F.J.; Schulz, M.M.; Siripitayananon, J.; Hossain, M.B.; van der Helm, D. New diterpenes from the gorgonian Solenopodium excavatum. J. Nat. Prod. 1993, 56, 1339–1349. [Google Scholar] [CrossRef] [PubMed]
- Burks, J.E.; van der Helm, D.; Chang, C.Y.; Ciereszko, L.S. The crystal and molecular structure of briarein A, a diterpenoid from the gorgonian Briareum asbestinum. Acta Cryst. 1977, B33, 704–709. [Google Scholar] [CrossRef]
- Groweiss, A.; Look, S.A.; Fenical, W. Solenolides, new antiinflammatory and antiviral diterpenoids from a marine octocoral of the genus Solenopodium. J. Org. Chem. 1988, 53, 2401–2406. [Google Scholar] [CrossRef]
- Cheng, J.-F.; Yamamura, S.; Terada, Y. Stereochemistry of the brianolide acetate (solenolide D) by the molecular mechanics calculations. Tetrahedron Lett. 1992, 33, 101–104. [Google Scholar] [CrossRef]
- Sheu, J.-H.; Sung, P.-J.; Su, J.-H.; Liu, H.-Y.; Duh, C.-Y.; Chiang, M.Y. Briaexcavatolides A–J, new diterpenes from the gorgonian Briareum excavatum. Tetrahedron 1999, 55, 14555–14564. [Google Scholar] [CrossRef]
- Sheu, J.-H.; Sung, P.-J.; Cheng, M.-C.; Liu, H.-Y.; Fang, L.-S.; Duh, C.-Y.; Chiang, M.Y. Novel cytotoxic diterpenes, excavatolides A–E, isolated from the Formosan gorgonian Briareum excavatum. J. Nat. Prod. 1998, 61, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.-H.; Sung, P.-J.; Huang, L.-H.; Lee, S.-F.; Wu, T.; Chang, B.-Y.; Duh, C.-Y.; Fang, L.-S.; Soong, K.; Lee, T.-J. New cytotoxic briaran diterpenes from the Formosan gorgonian Briareum sp. J. Nat. Prod. 1996, 59, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Liaw, C.-C.; Cheng, Y.-B.; Lin, Y.-S.; Kuo, Y.-H.; Hwang, T.-L.; Shen, Y.-C. New briarane diterpenoids from Taiwanese soft coral Briareum violacea. Mar. Drugs 2014, 12, 4677–4692. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-D.; Cheng, C.-H.; Chen, W.-F.; Chang, Y.-C.; Chen, Y.-H.; Hwang, T.-L.; Wen, Z.-H.; Wang, W.-H.; Fang, L.-S.; Chen, J.-J.; et al. Briarenolide J, the first 12-chlorobriarane diterpenoid from an octocoral Briareum sp. (Briareidae). Tetrahedron Lett. 2014, 55, 6065–6067. [Google Scholar] [CrossRef]
- Su, Y.-D.; Su, T.-R.; Wen, Z.-H.; Hwang, T.-L.; Fang, L.-S.; Chen, J.-J.; Wu, Y.-C.; Sheu, J.-H.; Sung, P.-J. Briarenolides K and L, new anti-inflammatory briarane diterpenoids from an octocoral Briareum sp. (Briareidae). Mar. Drugs 2015, 13, 1037–1050. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-D.; Wen, Z.-H.; Wu, Y.-C.; Fang, L.-S.; Chen, Y.-H.; Chang, Y.-C.; Sheu, J.-H.; Sung, P.-J. Briarenolides M–T, new briarane diterpenoids from a Formosan octocoral Briareum sp. Tetrahedron 2016, 72, 944–951. [Google Scholar] [CrossRef]
- Su, Y.-D.; Wu, T.-Y.; Wen, Z.-H.; Su, C.-C.; Chen, Y.-H.; Chang, Y.-C.; Wu, Y.-C.; Sheu, J.-H.; Sung, P.-J. Briarenolides U–Y, new anti-inflammatory briarane diterpenoids from an octocoral Briareum sp. (Briareidae). Mar. Drugs 2015, 13, 7138–7149. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-D.; Sung, C.-S.; Wen, Z.-H.; Chen, Y.-H.; Chang, Y.-C.; Chen, J.-J.; Fang, L.-S.; Wu, Y.-C.; Sheu, J.-H.; Sung, P.-J. New 9-hydroxybriarane diterpenoids from a gorgonian coral Briareum sp. (Briareidae). Int. J. Mol. Sci. 2016, 17, 79. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; La, M.-P.; Li, L.; Li, X.-B.; Tang, H.; Liu, B.-S.; Krohn, K.; Sun, P.; Yi, Y.-H.; Zhang, W. Bioactive 11,20-epoxy-3,5(16)-diene briarane diterpenoids from the South China Sea gorgonian Dichotella gemmacea. J. Nat. Prod. 2011, 74, 1658–1662. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; La, M.-P.; Tang, H.; Pan, W.-H.; Sun, P.; Krohn, K.; Yi, Y.-H.; Li, L.; Zhang, W. Bioactive briarane diterpenoids from the South China Sea gorgonian Dichotella gemmacea. Bioorg. Med. Chem. Lett. 2012, 22, 4368–4372. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-F.; Han, Z.; Zhou, X.-F.; Yang, B.; Lin, X.; Liu, J.; Peng, Y.; Yang, X.-W.; Liu, Y. Antifouling briarane type diterpenoids from South China Sea gorgonians Dichotella gemmacea. Tetrahedron 2013, 69, 871–880. [Google Scholar] [CrossRef]
- Li, C.; Jiang, M.; La, M.-P.; Li, T.-J.; Tang, H.; Sun, P.; Liu, B.-S.; Yi, Y.-H.; Liu, Z.; Zhang, W. Chemistry and tumor cell growth inhibitory activity of 11,20-epoxy-3Z,5(6)E-diene briaranes from the South China Sea gorgonian Dichotella gemmacea. Mar. Drugs 2013, 11, 1565–1582. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Park, M.; Fenical, W. The junceellolides, new anti-inflammatory diterpenoids of the briarane class from the Chinese gorgonian Junceella fragilis. Tetrahedron 1989, 45, 1633–1638. [Google Scholar] [CrossRef]
- Lin, Y.; Long, K. Studies of the chemical constituents of the Chinese gorgonia (IV)-Junceellin, a new chlorine-containing diterpenoid from Junceella squamata. Zhongshan Daxue Xuebao Ziran Kexueban 1983, 22, 46–51. [Google Scholar]
- Yao, J.; Qian, J.; Fan, H.; Shin, K.; Huang, S.; Lin, Y.; Long, K. The structure of crystal and molecule of junceellin. Zhongshan Daxue Xuebao Ziran Kexueban 1984, 1, 83–87. [Google Scholar]
- Lin, Y.; Jin, T.; Long, K.; Wu, Z. The hydrolytic products of junceellin A from Junceella squamata and their activities to inhibit the lung cancer A-549 cells. Zhongguo Haiyang Yaowu 1995, 14, 1–4. [Google Scholar]
- Subrahmanyam, C.; Kulatheeswaran, R.; Ward, R.S. Briarane diterpenes from the Indian Ocean gorgonian Gorgonella umbraculum. J. Nat. Prod. 1998, 61, 1120–1122. [Google Scholar] [CrossRef] [PubMed]
- García, M.; Rodríguez, J.; Jiménez, C. Absolute structures of new briarane diterpenoids from Junceella fragilis. J. Nat. Prod. 1999, 62, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Sung, P.-J.; Fan, T.-Y.; Fang, L.-S.; Wu, S.-L.; Li, J.-J.; Chen, M.-C.; Cheng, Y.-M.; Wang, G.-H. Briarane derivatives from the gorgonian coral Junceella fragilis. Chem. Pharm. Bull. 2003, 51, 1429–1431. [Google Scholar] [CrossRef] [PubMed]
- Sung, P.-J.; Fan, T.-Y.; Chen, M.-C.; Fang, L.-S.; Lin, M.-R.; Chang, P.-C. Junceellin and praelolide, two briaranes from the gorgonian corals Junceella fragilis and Junceella juncea (Ellisellidae). Biochem. Syst. Ecol. 2004, 32, 111–113. [Google Scholar] [CrossRef]
- Liaw, C.-C.; Shen, Y.-C.; Lin, Y.-S.; Hwang, T.-L.; Kuo, Y.-H.; Khalil, A.T. Frajunolides E–K, briarane diterpenes from Junceella fragilis. J. Nat. Prod. 2008, 71, 1551–1556. [Google Scholar] [CrossRef] [PubMed]
- La, M.-P.; Li, J.; Li, C.; Tang, H.; Liu, B.-S.; Sun, P.; Zhuang, C.-L.; Li, T.-J.; Zhang, W. Briarane diterpenoids from the gorgonian Dichotella gemmacea. Mar. Drugs 2014, 12, 6178–6189. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; La, M.-P.; Sun, P.; Kurtan, T.; Mandi, A.; Tang, H.; Liu, B.-S.; Yi, Y.-H.; Li, L.; Zhang, W. Bioactive (3Z,5E)-11,20-epoxybriara-3,5-dien-7,18-olide diterpenoids from the South China Sea gorgonian Dichotella gemmacea. Mar. Drugs 2011, 9, 1403–1418. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-F.; Yang, B.; Zhou, X.-F.; Yang, X.-W.; Wang, L.; Liu, Y. A new briarane-type diterpenoid from the South China Sea gorgonian Dichotella gemmacea. Nat. Prod. Res. 2015, 29, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.-H.; Chen, Y.-P.; Hwang, T.-L.; Chiang, M.Y.; Fang, L.-S.; Sung, P.-J. Junceellolides J–L, 11,20-epoxybriaranes from the gorgonian coral Junceella fragilis. J. Nat. Prod. 2006, 69, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Sung, P.-J.; Pai, C.-H.; Su, Y.-D.; Hwang, T.-L.; Kuo, F.-W.; Fan, T.-Y.; Li, J.-J. New 8-hydroxybriarane diterpenoids from the gorgonians Junceella juncea and Junceella fragilis (Ellisellidae). Tetrahedron 2008, 64, 4224–4232. [Google Scholar] [CrossRef]
- Anjaneyulu, A.S.R.; Rao, N.S.K. Juncins G and H: New briarane diterpenoids of the Indian Ocean gorgonian Junceella juncea Pallas. J. Chem. Soc. Perkin Trans. 1 1997, 6, 959–962. [Google Scholar] [CrossRef]
- Anjaneyulu, A.S.R.; Rao, V.L.; Sastry, V.G.; Venugopal, M.J.R.V.; Schmitz, F.J. Juncins I–M, five new briarane diterpenoids from the Indian Ocean gorgonian Junceella juncea Pallas. J. Nat. Prod. 2003, 66, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Long, K.; Fang, Z. Studies of the chemical constituents of the Chinese gorgonia (III)-Isolation and identification of a new polyacetoxy chlorine-containing diterpene lactone (praelolide). Zhongshan Daxue Xuebao Ziran Kexueban 1983, 1, 83–92. [Google Scholar]
- Dai, J.; Wan, Z.; Rao, Z.; Liang, D.; Fang, Z.; Luo, Y.; Long, K. Molecular structure and absolute configuration of the diterpene lactone, praelolide. Sci. Sin. Ser. B 1985, 28, 1132–1142. [Google Scholar]
- Zhang, M.-Q.; Zhao, J.; Liu, H.-Y.; Cao, F.; Wang, C.-Y. Briarane diterpenoids from gorgonian Dichotella gemmacea collected from the South China Sea. Chem. Nat. Comp. 2016, 52, 945–947. [Google Scholar] [CrossRef]
- He, H.-Y.; Faulkner, D.J. New chlorinated diterpenes from the gorgonian Junceella gemmacea. Tetrahedron 1991, 47, 3271–3280. [Google Scholar] [CrossRef]
- Qi, S.-H.; Zhang, S.; Huang, H.; Xiao, Z.-H.; Huang, J.-S.; Li, Q.-X. New briaranes from the South China Sea gorgonian Junceella juncea. J. Nat. Prod. 2004, 67, 1907–1910. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.-H.; Zhang, S.; Qian, P.-Y.; Xiao, Z.-H.; Li, M.-Y. Ten new antifouling briarane diterpenoids from the South China Sea gorgonian Junceella juncea. Tetrahedron 2006, 62, 9123–9130. [Google Scholar] [CrossRef]
- Li, C.; La, M.-P.; Tang, H.; Sun, P.; Liu, B.-S.; Zhuang, C.-L.; Yi, Y.-H.; Zhang, W. Chemistry and bioactivity of briaranes from the South China Sea gorgonian Dichotella gemmacea. Mar. Drugs 2016, 14, 201. [Google Scholar] [CrossRef] [PubMed]
- Cardellina, J.H., II; James, T.R., Jr.; Chen, M.H.M.; Clardy, J. Structure of brianthein W, from the soft coral Briareum polyanthes. J. Org. Chem. 1984, 49, 3398–3399. [Google Scholar] [CrossRef]
- Guerriero, A.; D’Ambrosio, M.; Pietra, F. Bis-allylic reactivity of the funicolides, 5,8(17)-diunsaturated briarane diterpenes of the sea pen Funiculina quadrangularis from the tuscan archipelago, leading to 16-nortaxane derivatives. Helv. Chim. Acta 1995, 78, 1465–1478. [Google Scholar] [CrossRef]
- Bowden, B.F.; Coll, J.C.; König, G.M. Studies of Australian soft corals. XLVIII. New briaran diterpenoids from the gorgonian coral Junceella gemmacea. Aust. J. Chem. 1990, 43, 151–159. [Google Scholar] [CrossRef]
- Pordesimo, E.O.; Schmitz, F.J.; Ciereszko, L.S.; Hossain, M.B.; van der Helm, D. New briarein diterpenes from the Caribbean gorgonians Erythropodium caribaeorum and Briareum sp. J. Org. Chem. 1991, 56, 2344–2357. [Google Scholar] [CrossRef]
- Subrahmanyam, C.; Ratnakumar, S.; Ward, R.S. Umbraculolides B–D, further briarane diterpenes from the gorgonian Gorgonella umbraculum. Tetrahedron 2000, 56, 4585–4588. [Google Scholar] [CrossRef]
- Zhou, Y.-M.; Shao, C.-L.; Huang, H.; Zhang, X.-L.; Wang, C.-Y. New briarane-type diterpenoids from gorgonian Ellisella dollfusi from the South China Sea. Nat. Prod. Res. 2014, 28, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Sung, P.-J.; Gwo, H.-H.; Fan, T.-Y.; Li, J.-J.; Dong, J.; Han, C.-C.; Wu, S.-L.; Fang, L.-S. Natural product chemistry of gorgonian corals of the genus Junceella. Biochem. Syst. Ecol. 2004, 32, 185–196. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Su, J.-H.; Chou, T.-T.; Cheng, Y.-P.; Weng, C.-F.; Lee, C.-H.; Fang, L.-S.; Wang, W.-H.; Li, J.-J.; Lu, M.-C.; et al. Natural product chemistry of gorgonian corals of genus Junceella-part II. Mar. Drugs 2011, 9, 2773–2792. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Sun, J.-F.; Han, Z.; Zhou, X.-F.; Yang, B.; Liu, Y. Fragilisinins A–L, new briarane-type diterpenoids from gorgonian Junceella fragilis. RSC Adv. 2014, 4, 5261–5271. [Google Scholar] [CrossRef]
- Qi, S.-H.; Zhang, S.; Wen, Y.-M.; Xiao, Z.-H.; Li, Q.-X. New briaranes from the South China Sea gorgonian Junceella fragilis. Helv. Chim. Acta 2005, 88, 2349–2354. [Google Scholar] [CrossRef]
- Sung, P.-J.; Wang, S.-H.; Chiang, M.Y.; Su, Y.-D.; Chang, Y.-C.; Hu, W.-P.; Tai, C.-Y.; Liu, C.-Y. Discovery of new chlorinated briaranes from Junceella fragilis. Bull. Chem. Soc. Jpn. 2009, 82, 1426–1432. [Google Scholar] [CrossRef]
- Sung, P.-J.; Lin, M.-R.; Su, Y.-D.; Chiang, M.Y.; Hu, W.-P.; Su, J.-H.; Cheng, M.-C.; Hwang, T.-L.; Sheu, J.-H. New briaranes from the octocorals Briareum excavatum (Briareidae) and Junceella fragilis (Ellisellidae). Tetrahedron 2008, 64, 2596–2604. [Google Scholar] [CrossRef]
- Shen, Y.-C.; Chen, Y.-H.; Hwang, T.-L.; Guh, J.-H.; Khalil, A.T. Four new briarane diterpenoids from the gorgonian coral Junceella fragilis. Helv. Chim. Acta 2007, 90, 1391–1398. [Google Scholar] [CrossRef]
- Zhou, W.; Li, J.; E, H.-C.; Liu, B.-S.; Tang, H.; Gerwick, W.H.; Hua, H.-M.; Zhang, W. Briarane diterpenes from the South China Sea gorgonian coral, Junceella gemmacea. Mar. Drugs 2014, 12, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Kapustina, I.I.; Kalinovskii, A.I.; Dmitrenok, P.S.; Kuz’mich, A.S.; Nedashkovskaya, O.I.; Grebnev, B.B. Diterpenoids and other metabolites from the Vietnamese gorgonians Lophogorgia sp. and Junceella sp. Chem. Nat. Comp. 2014, 50, 1140–1142. [Google Scholar] [CrossRef]
- Bahl, A.; Jachak, S.M.; Palaniveloo, K.; Ramachandram, T.; Vairappan, C.S.; Chopra, H.K. 2-Acetoxy-verecynarmin C, a new briarane COX inhibitory diterpenoid from Pennatula aculeata. Nat. Prod. Commun. 2014, 9, 1139–1141. [Google Scholar] [PubMed]
- Lin, Y.-Y.; Lin, S.-C.; Feng, C.-W.; Chen, P.-C.; Su, Y.-D.; Li, C.-M.; Yang, S.-N.; Jean, Y.-H.; Sung, P.-J.; Duh, C.-Y.; et al. Anti-inflammatory and analgesic effects of the marine-derived compound excavatolide B isolated from the culture-type Formosan gorgonian Briareum excavatum. Mar. Drugs 2015, 13, 2559–2579. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-Y.; Jean, Y.-H.; Lee, H.-P.; Lin, S.-C.; Pan, C.-Y.; Chen, W.-F.; Wu, S.-F.; Su, J.-H.; Tsui, K.-H.; Sheu, J.-H.; et al. Excavatolide B attenuates rheumatoid arthritis through the inhibition of osteoclastogenesis. Mar. Drugs 2017, 15, 9. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.-R.; Tai, B.-Y.; Cheng, P.-Y.; Chen, P.-N.; Sung, P.-J.; Wen, Z.-H.; Hsu, C.-H. Excavatolide B modulates the electrophysiological characteristics and calcium homeostasis of atrial myocytes. Mar. Drugs 2017, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, B.K.; Yang, H.-H.; Sung, P.-J.; Weng, C.-F. Excavatolide B inhibits nonsmall cell lung cancer proliferation by altering peroxisome proliferator activated receptor gamma expression and PTEN/AKT/NF-Kβ expression. Environ. Toxicol. 2017, 32, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Crimmins, M.T.; Knight, J.D.; Williams, P.S.; Zhang, Y. Stereoselective synthesis of quaternary carbons via the dianionic Ireland–Claisen rearrangement. Org. Lett. 2014, 16, 2458–2461. [Google Scholar] [CrossRef] [PubMed]
- Moon, N.G.; Harned, A.M. A concise synthetic route to the stereotetrad core of the briarane diterpenoids. Org. Lett. 2015, 17, 2218–2221. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Y.-D.; Su, J.-H.; Hwang, T.-L.; Wen, Z.-H.; Sheu, J.-H.; Wu, Y.-C.; Sung, P.-J. Briarane Diterpenoids Isolated from Octocorals between 2014 and 2016. Mar. Drugs 2017, 15, 44. https://doi.org/10.3390/md15020044
Su Y-D, Su J-H, Hwang T-L, Wen Z-H, Sheu J-H, Wu Y-C, Sung P-J. Briarane Diterpenoids Isolated from Octocorals between 2014 and 2016. Marine Drugs. 2017; 15(2):44. https://doi.org/10.3390/md15020044
Chicago/Turabian StyleSu, Yin-Di, Jui-Hsin Su, Tsong-Long Hwang, Zhi-Hong Wen, Jyh-Horng Sheu, Yang-Chang Wu, and Ping-Jyun Sung. 2017. "Briarane Diterpenoids Isolated from Octocorals between 2014 and 2016" Marine Drugs 15, no. 2: 44. https://doi.org/10.3390/md15020044
APA StyleSu, Y.-D., Su, J.-H., Hwang, T.-L., Wen, Z.-H., Sheu, J.-H., Wu, Y.-C., & Sung, P.-J. (2017). Briarane Diterpenoids Isolated from Octocorals between 2014 and 2016. Marine Drugs, 15(2), 44. https://doi.org/10.3390/md15020044







