The Cladophora glomerata Enriched by Biosorption Process in Cr(III) Improves Viability, and Reduces Oxidative Stress and Apoptosis in Equine Metabolic Syndrome Derived Adipose Mesenchymal Stromal Stem Cells (ASCs) and Their Extracellular Vesicles (MV’s)
Abstract
:1. Introduction
2. Results
2.1. Algae Content Analysis
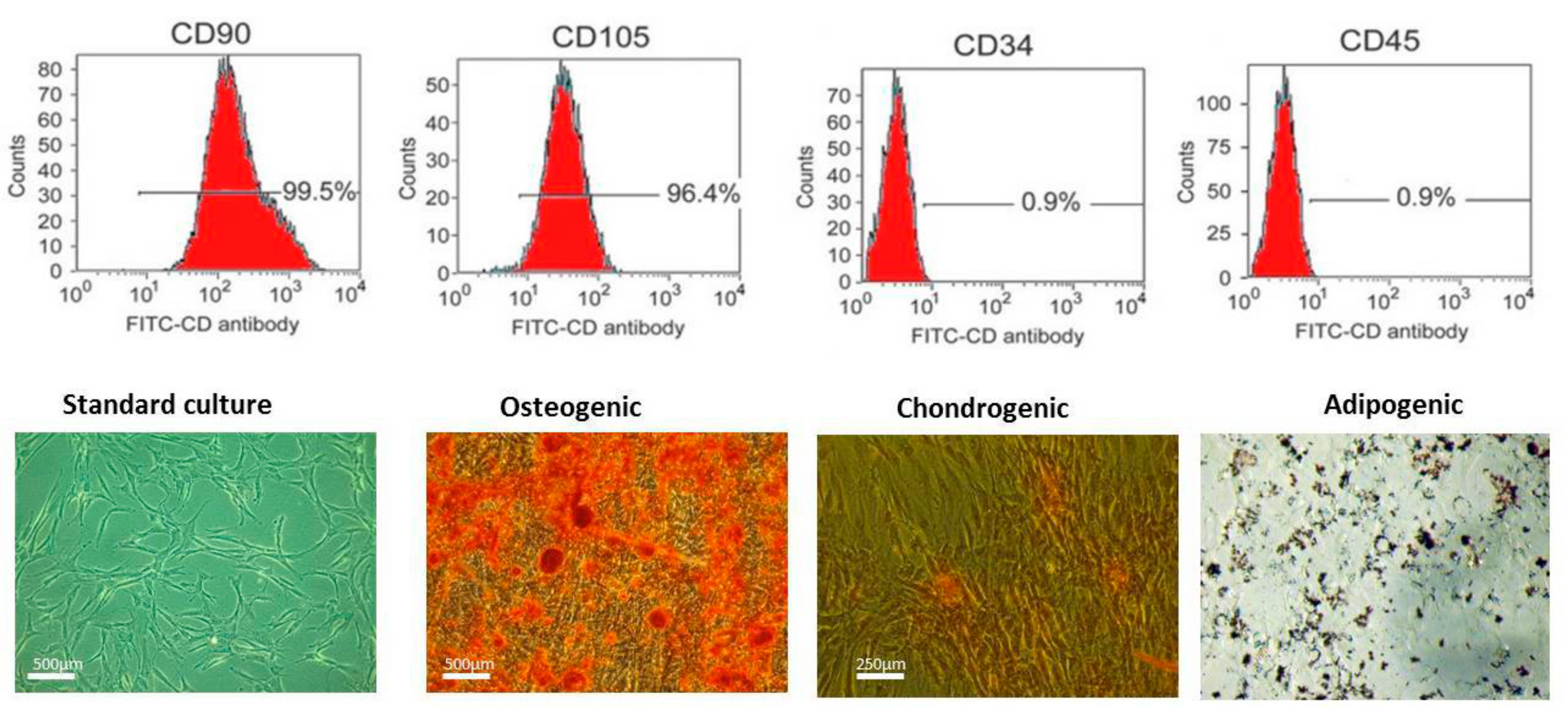
2.2. Immunophenotyping and Multipotency of ASCs
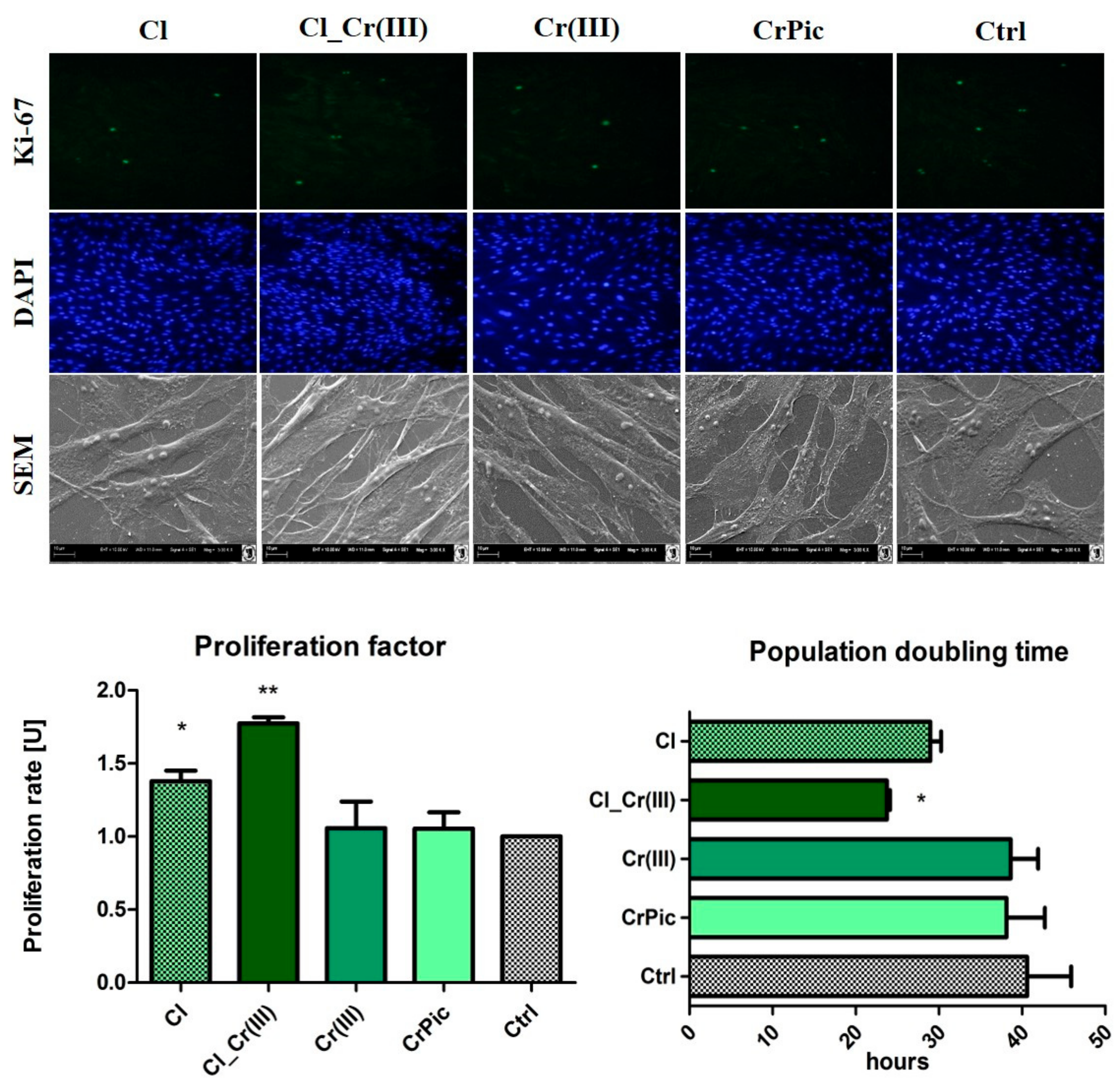
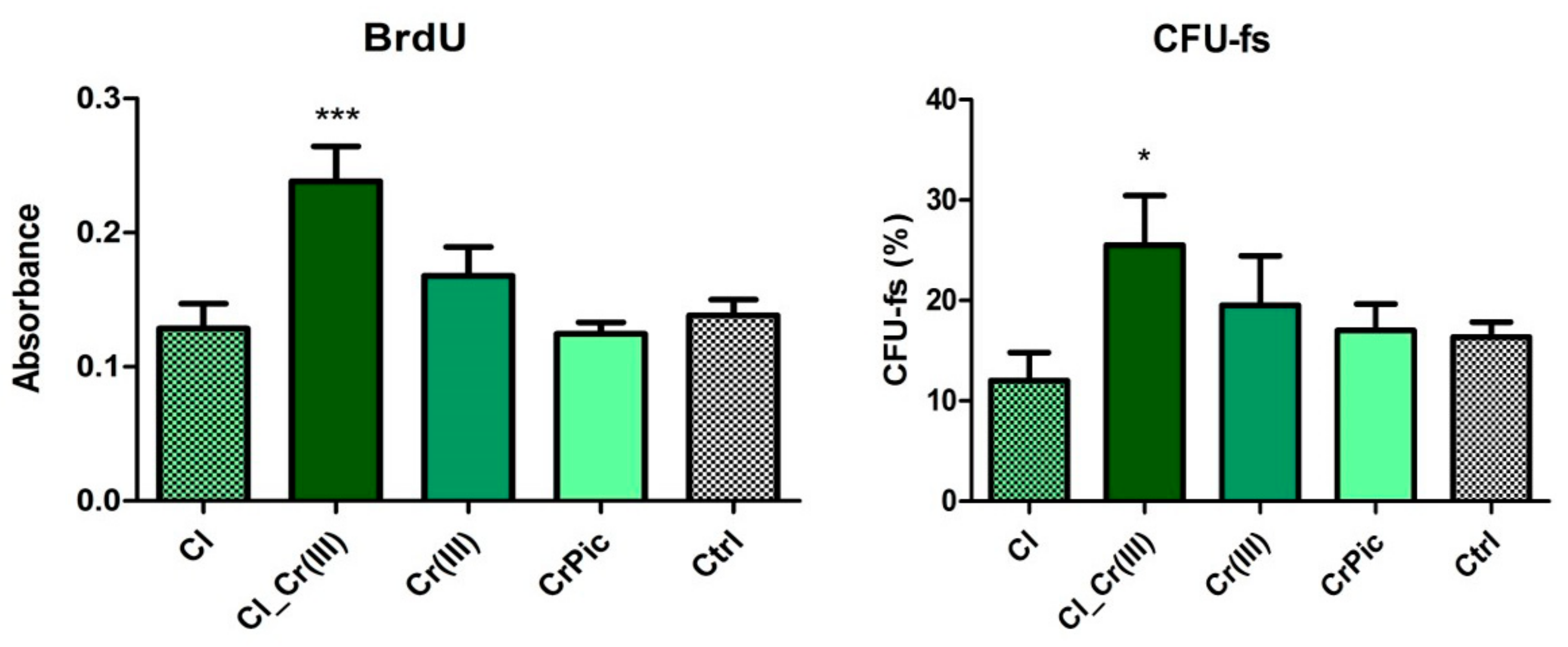
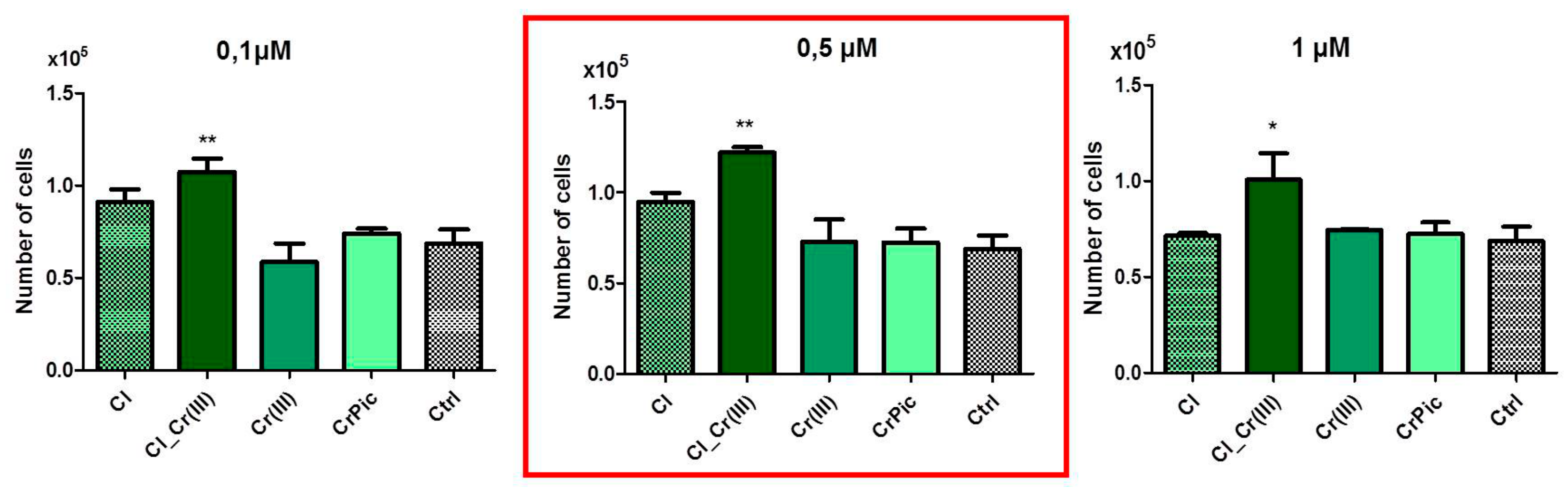
2.3. Cell Viability
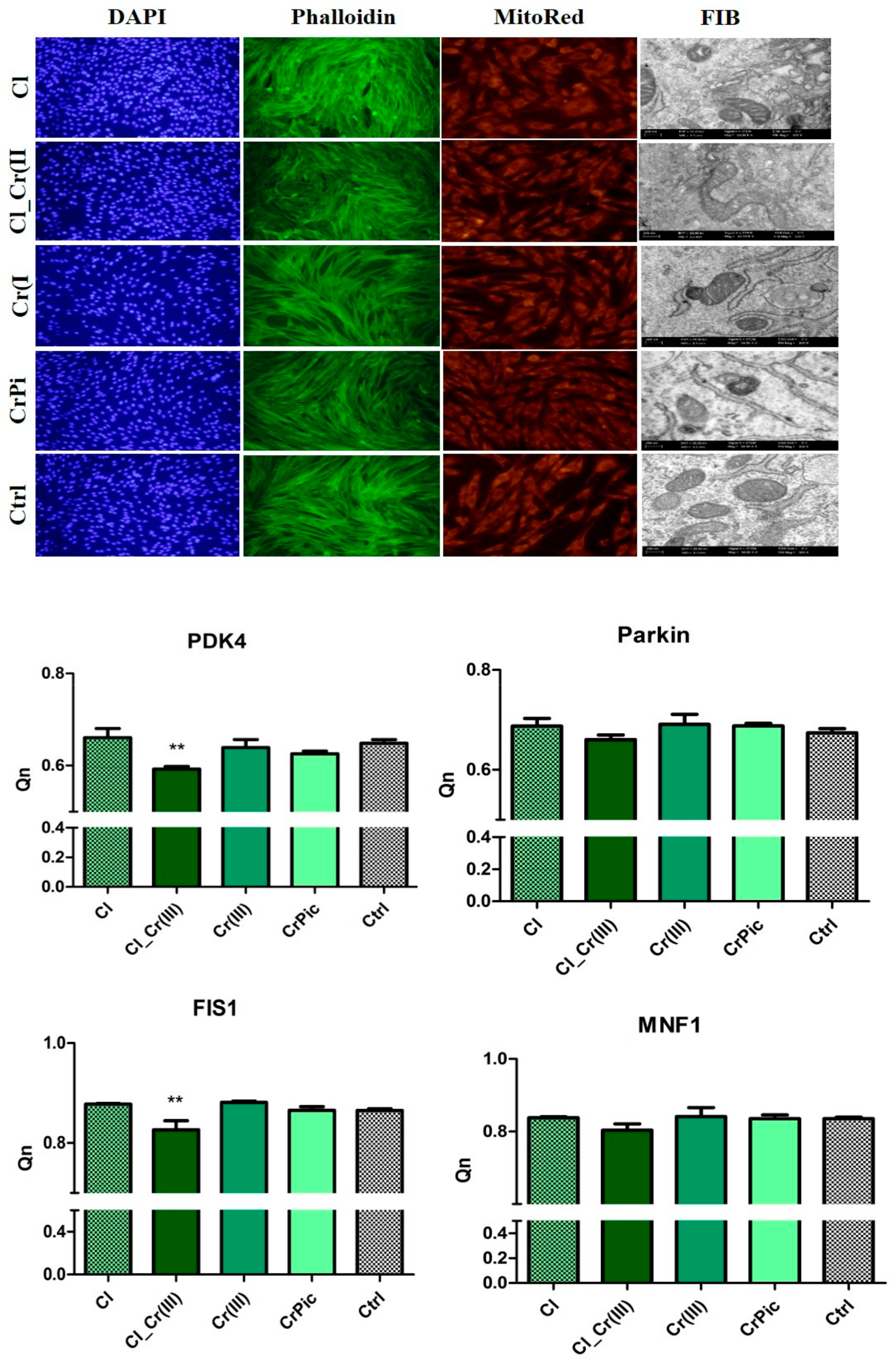
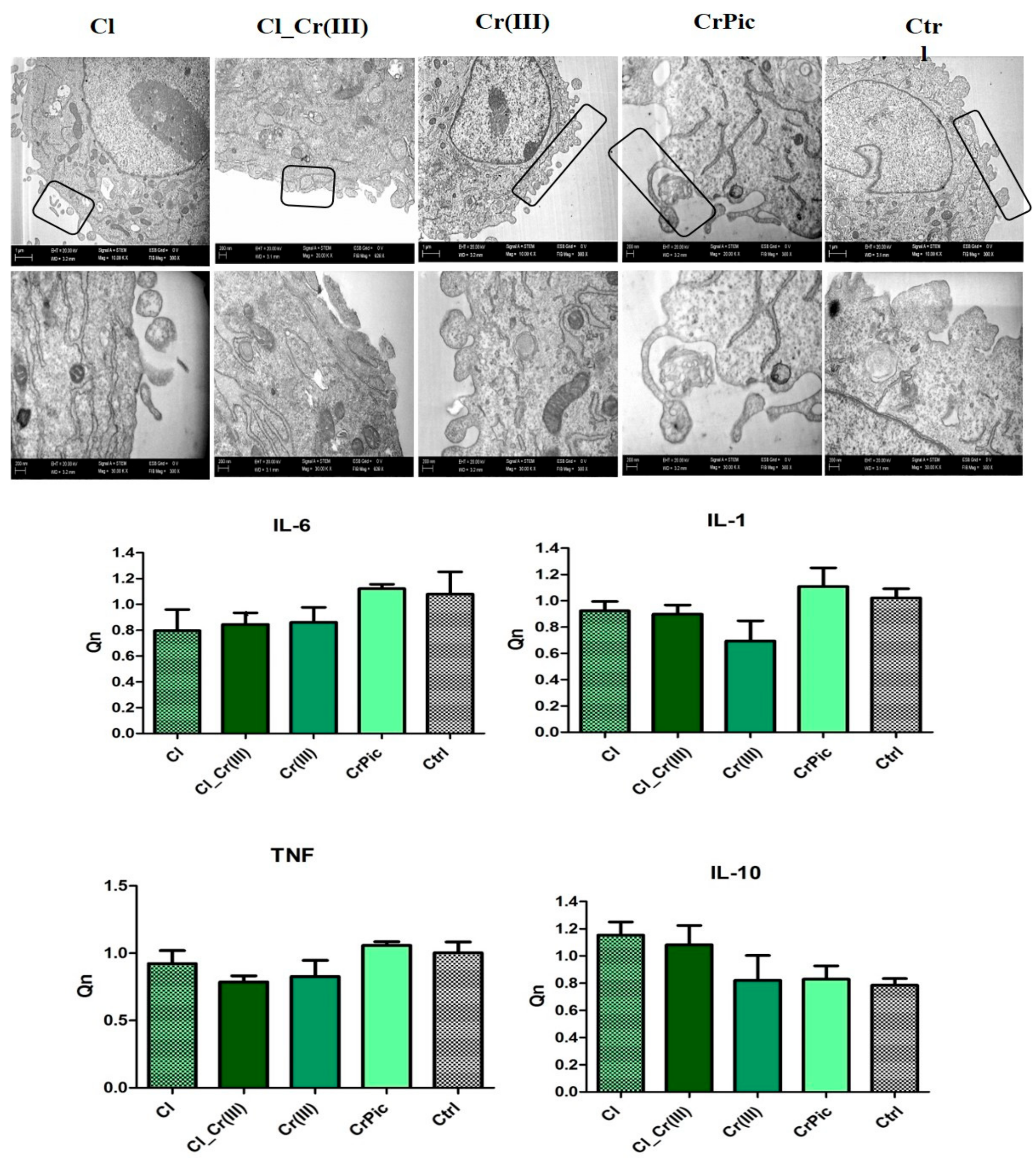
2.4. Morphology of Cells and Mitochondrial Biogenesis
2.5. Endoplasmic Reticulum (ER) Stress and Autophagy
2.6. Secretory Activity
3. Discussion
4. Materials and Methods
4.1. Bioactive Compounds Preparation
4.2. Characteristics of Cladophora glomerata Biomass
4.2.1. Determination of Vitamin C Content in Cladophora glomerata
4.2.2. Gas Chromatographic Assay of Tocopherol Content
4.2.3. Determination of the Total Phenols Content (TPC)
4.2.4. Extraction and Quantification of Fatty Acids
4.2.5. Analysis of Free and Protein-Bound Amino Acids
4.2.6. Determination of Cr(III) in Cladophora glomerata Biomass
4.3. Animals Qualifications
4.4. Isolation and Immunophenotyping of Adipose-Derived Stromal Cells
4.5. Propagation of Equine Adipose Derived Mesenchymal Stromal Cells
4.6. Cell Viability
4.7. Ability of Cells to Form Colonies (CFU-Fs)
4.8. Immunofluorescence Staining
4.9. Morphology Evaluation
4.10. Microvesicles Isolation
4.11. Analysis of mRNA and microRNA Expression—RT qPCR
4.12. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Frank, N.; Geor, R.J.; Bailey, S.R.; Durham, A.E.; Johnson, P.J. Equine Metabolic Syndrome. J. Vet. Intern. Med. 2010, 24, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.; Keen, J.; McGowan, C. Equine metabolic syndrome. Vet. Rec. 2015, 177, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Geor, R.J. Metabolic Predispositions to Laminitis in Horses and Ponies: Obesity, Insulin Resistance and Metabolic Syndromes. J. Equine Vet. Sci. 2008, 28, 753–759. [Google Scholar] [CrossRef]
- Geor, R.J.; McCue, M.E.; Schultz, N. Current understanding of the equine metabolic syndrome phenotype. J. Equine Vet. Sci. 2013, 33, 841–844. [Google Scholar] [CrossRef]
- Lopes, H.F.; Corrêa-Giannella, M.L.; Consolim-Colombo, F.M.; Egan, B.M. Visceral adiposity syndrome. Diabetol. Metab. Syndr. 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Hirabara, S.M.; Gorjão, R.; Vinolo, M.A.; Rodrigues, A.C.; Nachbar, R.T.; Curi, R. Molecular Targets Related to Inflammation and Insulin Resistance and Potential Interventions. J. Biomed. Biotechnol. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.R.; Milner, J.J.; Makowski, L. The inflammation highway: Metabolism accelerates inflammatory traffic in obesity. Immunol. Rev. 2012, 249, 218–238. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Grzesiak, J.; Wrzeszcz, K.; Golonka, P. Adipose stem cell combined with plasma-based implant bone tissue differentiation in vitro and in a horse with a phalanx digitalis distalis fracture: A case report. Vet. Med. Czech Repub. 2012, 57, 610–617. [Google Scholar]
- AL-Suhaimi, E.A.; Shehzad, A. Leptin, resistin and visfatin: The missing link between endocrine metabolic disorders and immunity. Eur. J. Med. Res. 2013, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Makki, K.; Froguel, P.; Wolowczuk, I. Adipose Tissue in Obesity-Related Inflammation and Insulin Resistance: Cells, Cytokines, and Chemokines. ISRN Inflamm. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Baraniak, P.R.; McDevitt, T.C. Stem cell paracrine actions and tissue regeneration. Regener. Med. 2010, 5, 121–143. [Google Scholar] [CrossRef] [PubMed]
- Cislo-Pakuluk, A.; Marycz, K. A Promising Tool in Retina Regeneration: Current Perspectives and Challenges When Using Mesenchymal Progenitor Stem Cells in Veterinary and Human Ophthalmological Applications. Stem Cell Rev. 2017, 13, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Basinska, K.; Marycz, K.; Śmieszek, A.; Nicpoń, J. The production and distribution of IL-6 and TNF-α in subcutaneous adipose tissue and their correlation with serum concentrations in Welsh ponies with equine metabolic syndrome. J. Vet. Sci. 2015, 16, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Nicpoń, J.; Marycz, K.; Grzesiak, J. Therapeutic effect of adipose-derived mesenchymal stem cell injection in horses suffering from bone spavin. Pol. J. Vet. Sci. 2013, 16, 753–754. [Google Scholar] [CrossRef] [PubMed]
- Marędziak, M.; Marycz, K.; Lewandowski, D.; Siudzińska, A.; Śmieszek, A. Static magnetic field enhances synthesis and secretion of membrane-derived microvesicles (MVs) rich in VEGF and BMP-2 in equine adipose-derived stromal cells (EqASCs)—A new approach in veterinary regenerative medicine. In Vitro Cell. Dev. Biol. Anim. 2015, 51, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Kornicka, K.; Grzesiak, J.; Śmieszek, A.; Szłapka, J. Macroautophagy and Selective Mitophagy Ameliorate Chondrogenic Differentiation Potential in Adipose Stem Cells of Equine Metabolic Syndrome: New Findings in the Field of Progenitor Cells Differentiation. Oxid. Med. Cell. Longev. 2016, 2016, e3718468. [Google Scholar] [CrossRef]
- Marycz, K.; Kornicka, K.; Basinska, K.; Czyrek, A. Equine Metabolic Syndrome Affects Viability, Senescence, and Stress Factors of Equine Adipose-Derived Mesenchymal Stromal Stem Cells: New Insight into EqASCs Isolated from EMS Horses in the Context of Their Aging. Oxid. Med. Cell. Longev. 2016, 2016, e4710326. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Clark, S.; Ren, J.; Sreejayan, N. Molecular Mechanisms of Chromium in Alleviating Insulin Resistance. J. Nutr. Biochem. 2012, 23, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Chameroy, K.A.; Frank, N.; Elliott, S.B.; Boston, R.C. Effects of a supplement containing chromium and magnesium on morphometric measurements, resting glucose, insulin concentrations and insulin sensitivity in laminitic obese horses. Equine Vet. J. 2011, 43, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Shi, X.; Li, H.; Xie, P.; Zhang, H.; Deng, J.; Liang, Y. Effects of lead on tolerance, bioaccumulation, and antioxidative defense system of green algae, Cladophora. Ecotoxicol. Environ. Saf. 2015, 112, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Berdalet, E.; Fleming, L.E.; Gowen, R.; Davidson, K.; Hess, P.; Backer, L.C.; Moore, S.K.; Hoagland, P.; Enevoldsen, H. Marine harmful algal blooms, human health and wellbeing: Challenges and opportunities in the 21st century. J. Mar. Biol. Assoc. UK Mar. Biol. Assoc. UK 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, E.A. Algae as promising organisms for environment and health. Plant Signal. Behav. 2011, 6, 1338–1350. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Chojnacka, K.; Korniewicz, D. New feed supplement from macroalgae as the dietary source of microelements for pigs. Open Chem. 2016, 13. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K.; Dobrzański, Z.; Górecki, H.; Zielińska, A.; Korczyński, M.; Opaliński, S. Effect of macroalgae enriched with microelements on egg quality parameters and mineral content of eggs, eggshell, blood, feathers and droppings. J. Anim. Physiol. Anim. Nutr. 2011, 95, 374–387. [Google Scholar] [CrossRef] [PubMed]
- Lindåse, S.; Nostell, K.; Bröjer, J. A modified oral sugar test for evaluation of insulin and glucose dynamics in horses. Acta Vet. Scand. 2016, 58. [Google Scholar] [CrossRef] [PubMed]
- Durham, A.E. Therapeutics for Equine Endocrine Disorders. Vet. Clin. N. Am. Equine Pract. 2017, 33, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Chojnacka, K.; Saeid, A. Plant Growth Biostimulants, Dietary Feed Supplements and Cosmetics Formulated with Supercritical CO2 Algal Extracts. Molecules 2017, 22, 66. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Miller, U.; Tuhy, Ł.; Sówka, I.; Chojnacka, K. Characterisation of biological properties of co-composted Baltic seaweeds in germination tests. Eng. Life Sci. 2017, 17, 153–164. [Google Scholar] [CrossRef]
- Belay, A.; Ota, Y.; Miyakawa, K.; Shimamatsu, H. Current knowledge on potential health benefits of Spirulina. J. Appl. Phycol. 1993, 5, 235–241. [Google Scholar] [CrossRef]
- Nawrocka, D.; Kornicka, K.; Śmieszek, A.; Marycz, K. Spirulina platensis Improves Mitochondrial Function Impaired by Elevated Oxidative Stress in Adipose-Derived Mesenchymal Stromal Cells (ASCs) and Intestinal Epithelial Cells (IECs), and Enhances Insulin Sensitivity in Equine Metabolic Syndrome (EMS) Horses. Mar. Drugs 2017, 15, 237. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, D.; Zhang, J.; Du, M.; Cheng, Y.; Liu, Y.; Zhang, N.; Wang, D.; Wu, Y. Mitochondria Related Pathway Is Essential for Polysaccharides Purified from Sparassis crispa Mediated Neuro-Protection against Glutamate-Induced Toxicity in Differentiated PC12 Cells. Int. J. Mol. Sci. 2016, 17, 133. [Google Scholar] [CrossRef] [PubMed]
- Subramoniam, A.; Asha, V.V.; Nair, S.A.; Sasidharan, S.P.; Sureshkumar, P.K.; Rajendran, K.N.; Karunagaran, D.; Ramalingam, K. Chlorophyll revisited: Anti-inflammatory activities of chlorophyll a and inhibition of expression of TNF-α gene by the same. Inflammation 2012, 35, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Huang, Y.; Zhang, R.; Cai, T.; Cai, Y. Medical Application of Spirulina platensis Derived C-Phycocyanin. Evid. Based Complement. Altern. Med. 2016, 2016, e7803846. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, H.; Huang, K. Organoselenium Small Molecules and Chromium(III) Complexes for Intervention in Chronic Low-grade Inflammation and Type 2 Diabetes. Curr. Top. Med. Chem. 2016, 16, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Patlolla, A.K.; Barnes, C.; Yedjou, C.; Velma, V.R.; Tchounwou, P.B. Oxidative stress, DNA damage, and antioxidant enzyme activity induced by hexavalent chromium in Sprague-Dawley rats. Environ. Toxicol. 2009, 24, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Jeoung, N.H.; Harris, R.A. Pyruvate dehydrogenase kinase-4 deficiency lowers blood glucose and improves glucose tolerance in diet-induced obese mice. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E46–E54. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-K. The Role of Pyruvate Dehydrogenase Kinase in Diabetes and Obesity. Diabetes Metab. J. 2014, 38, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Shenouda, S.M.; Widlansky, M.E.; Chen, K.; Xu, G.; Holbrook, M.; Tabit, C.E.; Hamburg, N.M.; Frame, A.A.; Caiano, T.L.; Kluge, M.A.; et al. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation 2011, 124, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Robotham, J.L.; Yoon, Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc. Natl. Acad. Sci. USA 2006, 103, 2653–2658. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Chojnacka, K. The new application of biosorption properties of Enteromorpha prolifera. Appl. Biochem. Biotechnol. 2010, 160, 1540–1556. [Google Scholar] [CrossRef] [PubMed]
- Davidson, I. Hydrolysis of samples for amino acid analysis. Methods Mol. Biol. 2003, 211, 111–122. [Google Scholar] [PubMed]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]


| Fatty Acid | Content [mg/100 g of Dry Mass] |
|---|---|
| 8:0 | 0.50 ± 0 |
| 10:0 | 0.30 ± 0.10 |
| 12:0 | 0.20 ± 0.060 |
| 14:0 | 83.8 ± 0.50 |
| 14:1 (n-5) | 0.50 ± 0.050 |
| 15:0 | 1.0 ± 0.20 |
| 16:0 | 160 ± 1.0 |
| 16:1 (n-7) | 30 ± 0.20 |
| 18:0 | 4.8 ± 0.55 |
| 18:1 (n-12) | 34 ± 0.26 |
| 18:2 (n-6) | 14.23 ± 0.35 |
| 18:3 (n-3) | 23 ± 1.1 |
| 18:3 (n-6) | n.d. |
| 18:4 (n-3) | 30 ± 0.55 |
| 20:0 | 0.80 ± 0.10 |
| 20:2 (n-6) | n.d. |
| 22:0 | 4.0 ± 0.68 |
| Amino Acid | Free | Bound |
|---|---|---|
| Ala | 27.3 ± 2.5 | 1523.3 ± 70.2 |
| Glc | 9.3 ± 2.0 | 1313.3 ± 73.7 |
| Val | 0 | 1163.3 ± 55.0 |
| Leu | 4.3 ± 2.0 | 1503.3 ± 35.1 |
| Ile | 5.0 ± 1.7 | 826.6 ± 35.1 |
| Asn | 0 | 1496.6 ± 159.5 |
| Asp | 6.0 ± 1.7 | 1496.6 ± 159.5 |
| Gln | 0 | 1820.0 ± 70.0 |
| Glu | 0 | 1820.0 ± 70.0 |
| Lys | 0 | 560.0 ± 45.8 |
| Arg | 0 | 923.3 ± 15.3 |
| His | 0 | 90.0 ± 10.0 |
| Phe | 2.6 ± 1.2 | 916.6 ± 65.0 |
| Tyr | 0 | 743.3 ± 32.1 |
| Trp | 0 | 91.6 ± 16.0 |
| Ser | 14 ± 0.0 | 730.0 ± 43.6 |
| Thr | 0 | 596.6 ± 32.1 |
| Met | 0 | 230.0 ± 10.0 |
| Cys | 0 | 91.6 ± 7.6 |
| Pro | 6.6 ± 0.6 | 813.3 ± 32.1 |
| Gene | Primer | Sequence 5′-3′ | Amplicon Length | Accession No. |
|---|---|---|---|---|
| GAPDH | F: | GATGCCCCAATGTTTGTGA | 250 | NM_001163856.1 |
| R: | AAGCAGGGATGATGTTCTGG | |||
| p53 | F: | TACTCCCCTGCCCTCAACAA | 252 | U37120.1 |
| R: | AGGAATCAGGGCCTTGAGGA | |||
| p21 | F: | GAAGAGAAACCCCCAGCTCC | 241 | XM_003365840.2 |
| R: | TGACTGCATCAAACCCCACA | |||
| Bcl-2 | F: | TTCTTTGAGTTCGGTGGGGT | 164 | XM_014843802.1 |
| R: | GGGCCGTACAGTTCCACAA | |||
| BAX | F: | GCCAGCAAATTGGTGCTCAA | 94 | XM_005596728.1 |
| R: | AGCAGTCACTTCCATGGCTC | |||
| Casp9 | F: | CACCTTCCCAGGCTTTGTCT | 224 | XM_014836232.1 |
| R: | GGCTCTGGCCTCAGTAAGTT | |||
| PDK4 | F: | GCTGGTTTTGGTTATGGCTTGC | 137 | XM_014853326.1 |
| R: | TCCACAGACTCAGAAGACAAAGCC | |||
| Parkin | F: | TCCCAGTGGAGGTCGATTCT | 218 | XM_005608126.2 |
| R: | CCCTCCAGGTGTGTTCGTTT | |||
| FIS | F: | GGTGCGAAGCAAGTACAACG | 118 | XM_014854003.1 |
| R: | GTTGCCCACAGCCAGATAGA | |||
| MNF | F: | AAGTGGCATTTTTCGGCAGG | 217 | XM_001495170.5 |
| R: | TCCATATGAAGGGCATGGGC | |||
| LC3 | F: | TTACTGCTTTGCTCTGCCAC | 213 | XM_005608485.2 |
| R: | AGCTGCTTCTCCCCCTTGT | |||
| Beclin | F: | GATGCGTTATGCCCAGATGC | 147 | XM_014729146.1 |
| R: | ATCCAGCGAACACTCTTGGG | |||
| Lamp2 | F: | GCACCCCTGGGAAGTTCTTA | 139 | XM_014733098.1 |
| R: | TTCGAGGATCTGTGCCAATCA | |||
| CHOP | F: | AGCCAAAATCAGAGCCGGAA | 272 | XM_014844003.1 |
| R: | GGGGTCAAGAGTGGTGAAGG | |||
| PERK | F: | GTGACTGCAATGGACCAGGA | 283 | XM_014852775.1 |
| R: | TCACGTGCTCACGAGGATATT | |||
| eIF2 alpha | F: | AGCCAGAAGCCACTGCTAAG | 196 | XM_001488848.5 |
| R: | TGCACCTCCACCAGTTTGTT | |||
| IL-6 | F: | CGTCACTCCAGTTGCCTTCT | 225 | XM_014830631.1 |
| R: | GCCAGTACCTCCTTGCTGTT | |||
| IL-1 | F: | TATGTGTGTGATGCAGCTGTG | 352 | XM_014852743.1 |
| R: | ACTCAAATTCCACGTTGCCC | |||
| IL-10 | F: | GCGCTGGAGTTCCATCTCTT | 196 | XM_014739408.1 |
| R: | GCCTAGGTCCAAAGGGACAA | |||
| TNF-alpha | F: | AAGTGACAAGCCTGTAGCCC | 254 | XM_014831605.1 |
| R: | GGTTGACCTTGGACGGGTAG |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marycz, K.; Michalak, I.; Kocherova, I.; Marędziak, M.; Weiss, C. The Cladophora glomerata Enriched by Biosorption Process in Cr(III) Improves Viability, and Reduces Oxidative Stress and Apoptosis in Equine Metabolic Syndrome Derived Adipose Mesenchymal Stromal Stem Cells (ASCs) and Their Extracellular Vesicles (MV’s). Mar. Drugs 2017, 15, 385. https://doi.org/10.3390/md15120385
Marycz K, Michalak I, Kocherova I, Marędziak M, Weiss C. The Cladophora glomerata Enriched by Biosorption Process in Cr(III) Improves Viability, and Reduces Oxidative Stress and Apoptosis in Equine Metabolic Syndrome Derived Adipose Mesenchymal Stromal Stem Cells (ASCs) and Their Extracellular Vesicles (MV’s). Marine Drugs. 2017; 15(12):385. https://doi.org/10.3390/md15120385
Chicago/Turabian StyleMarycz, Krzysztof, Izabela Michalak, Ievgeniia Kocherova, Monika Marędziak, and Christine Weiss. 2017. "The Cladophora glomerata Enriched by Biosorption Process in Cr(III) Improves Viability, and Reduces Oxidative Stress and Apoptosis in Equine Metabolic Syndrome Derived Adipose Mesenchymal Stromal Stem Cells (ASCs) and Their Extracellular Vesicles (MV’s)" Marine Drugs 15, no. 12: 385. https://doi.org/10.3390/md15120385
APA StyleMarycz, K., Michalak, I., Kocherova, I., Marędziak, M., & Weiss, C. (2017). The Cladophora glomerata Enriched by Biosorption Process in Cr(III) Improves Viability, and Reduces Oxidative Stress and Apoptosis in Equine Metabolic Syndrome Derived Adipose Mesenchymal Stromal Stem Cells (ASCs) and Their Extracellular Vesicles (MV’s). Marine Drugs, 15(12), 385. https://doi.org/10.3390/md15120385






