Asperlin Inhibits LPS-Evoked Foam Cell Formation and Prevents Atherosclerosis in ApoE−/− Mice
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Characterization of Asperlin
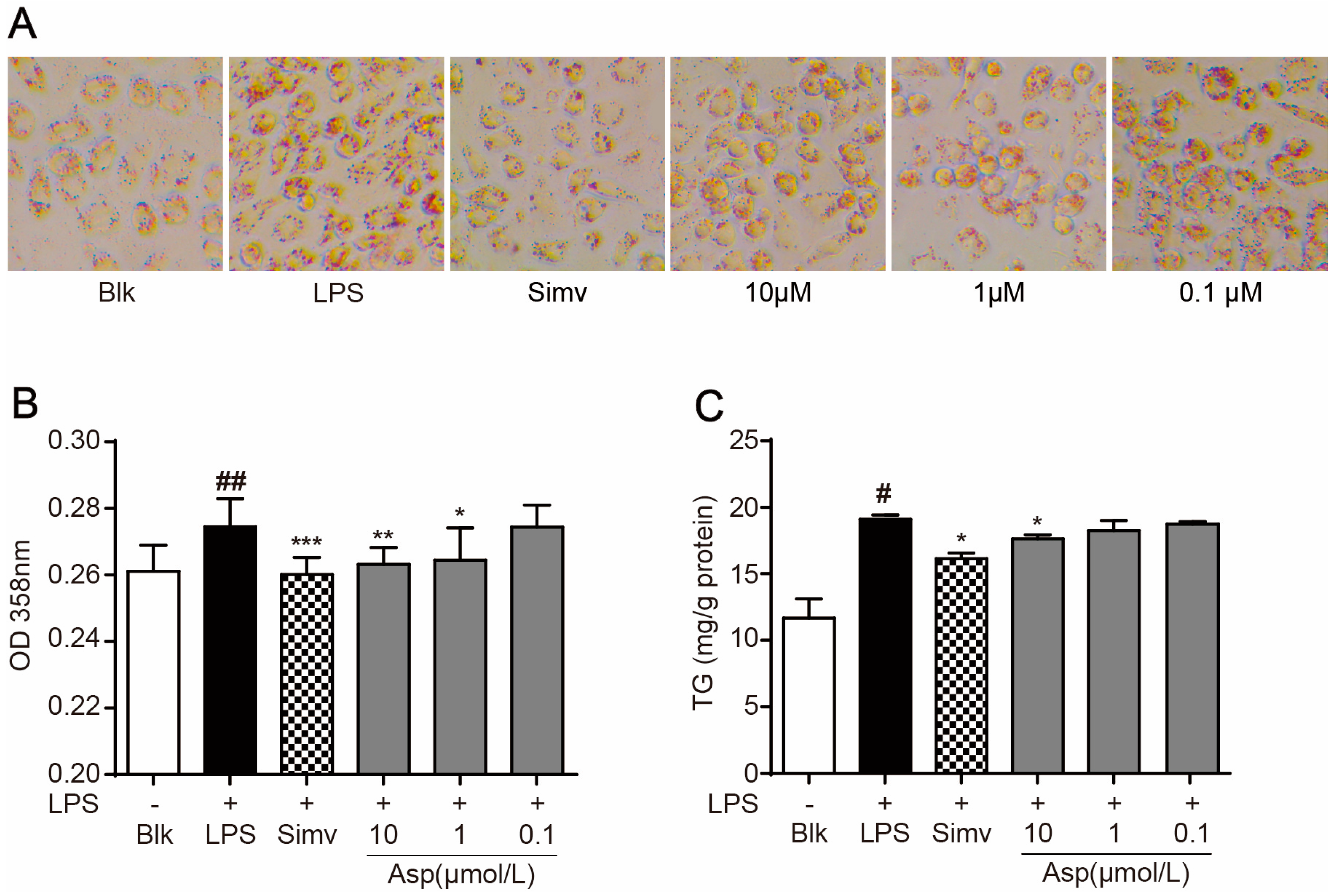
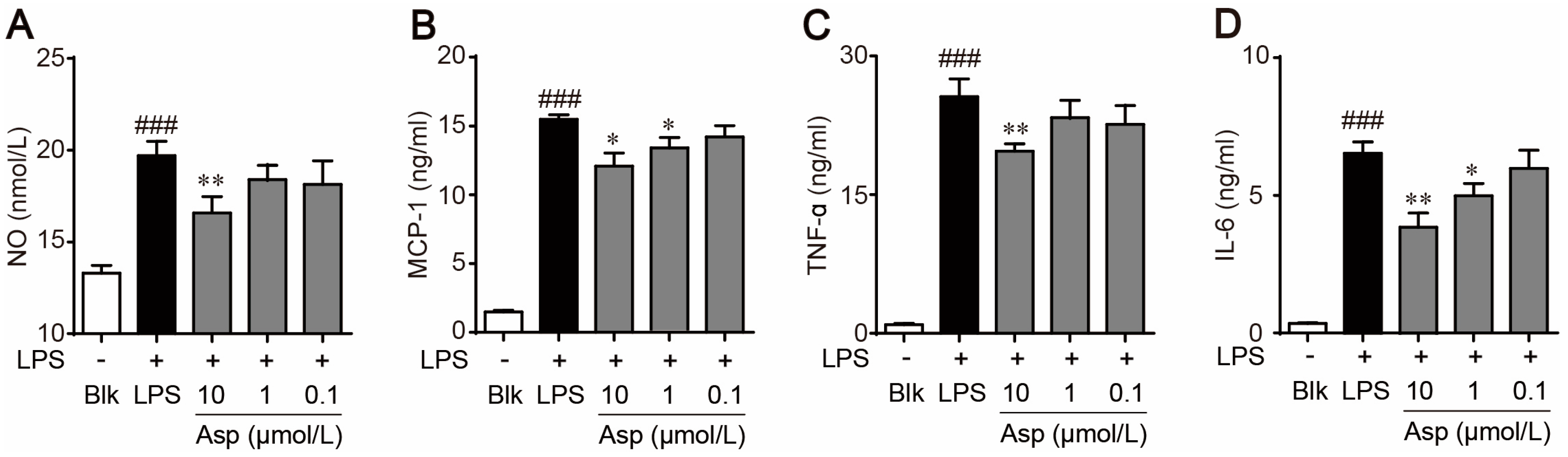
2.2. Asperlin Suppresses LPS-Induced Foam Cell Formation in RAW264.7 Macrophages
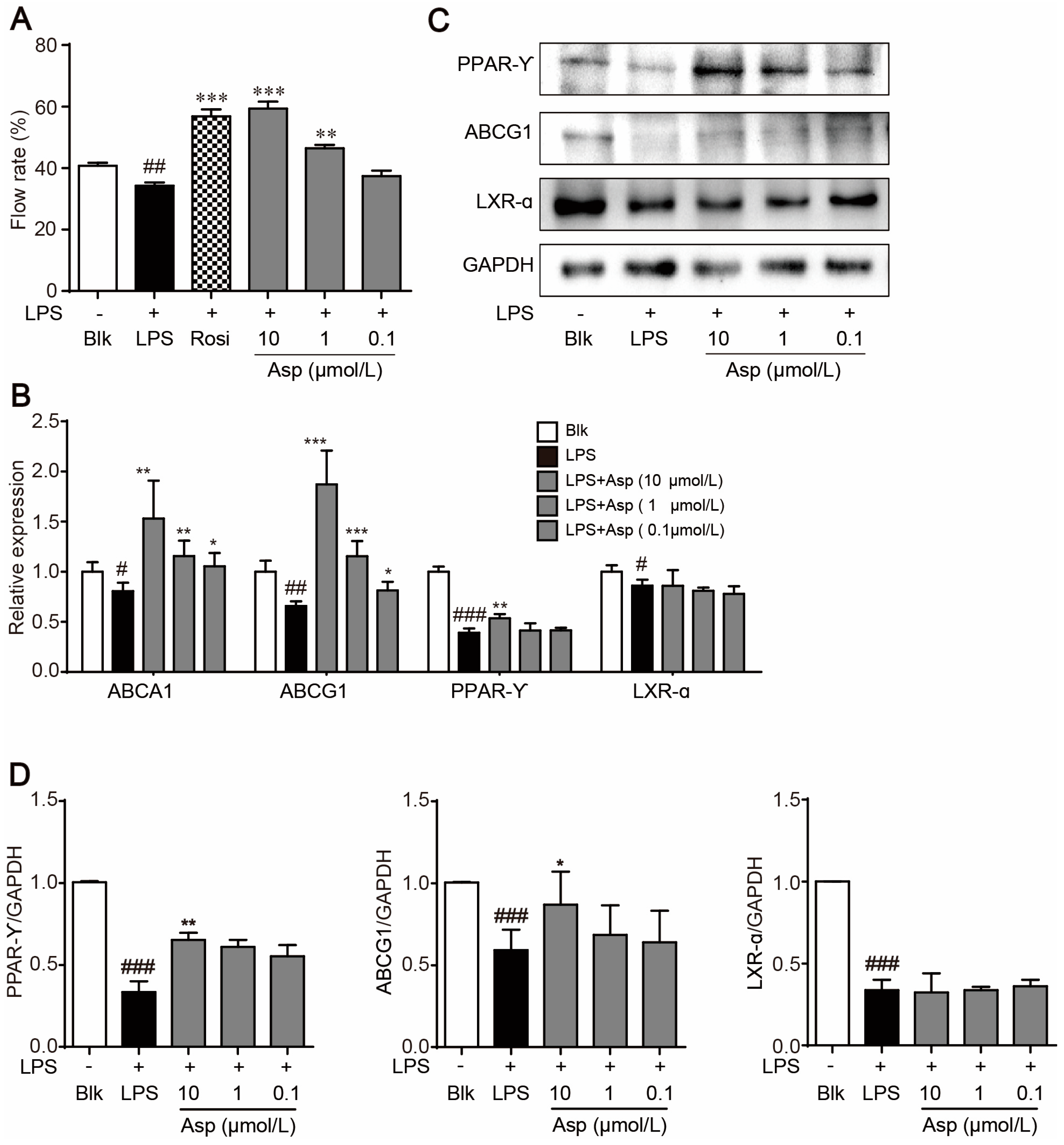
2.3. Asperlin Stimulates Cholesterol Efflux in LPS-Treated RAW264.7 Macrophages
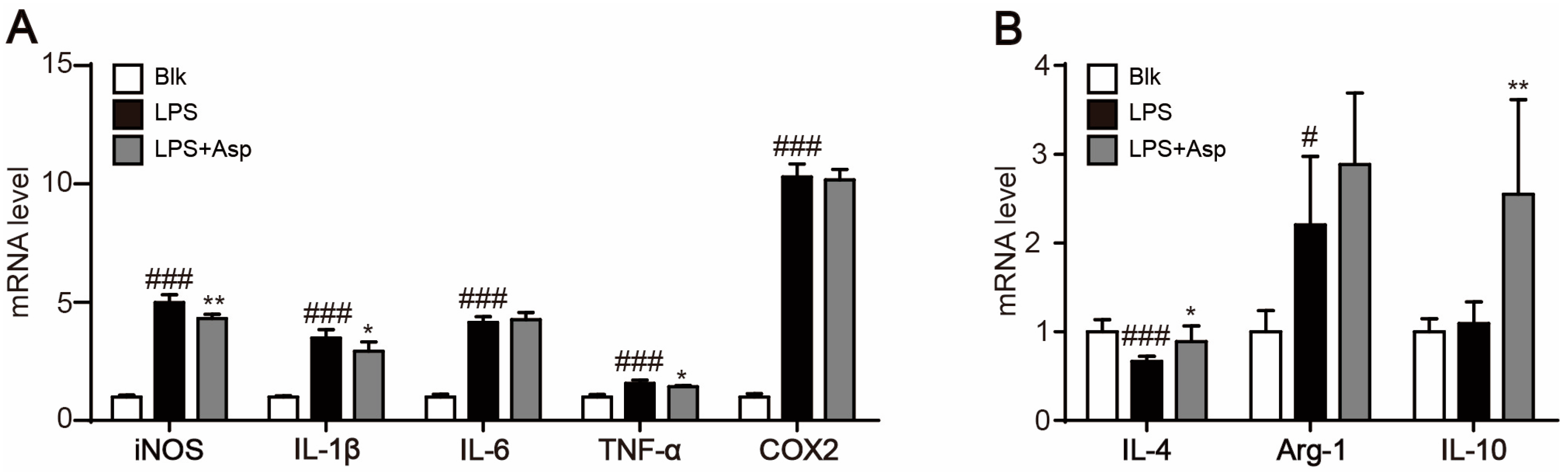
2.4. Asperlin Regulates Macrophage Polarization
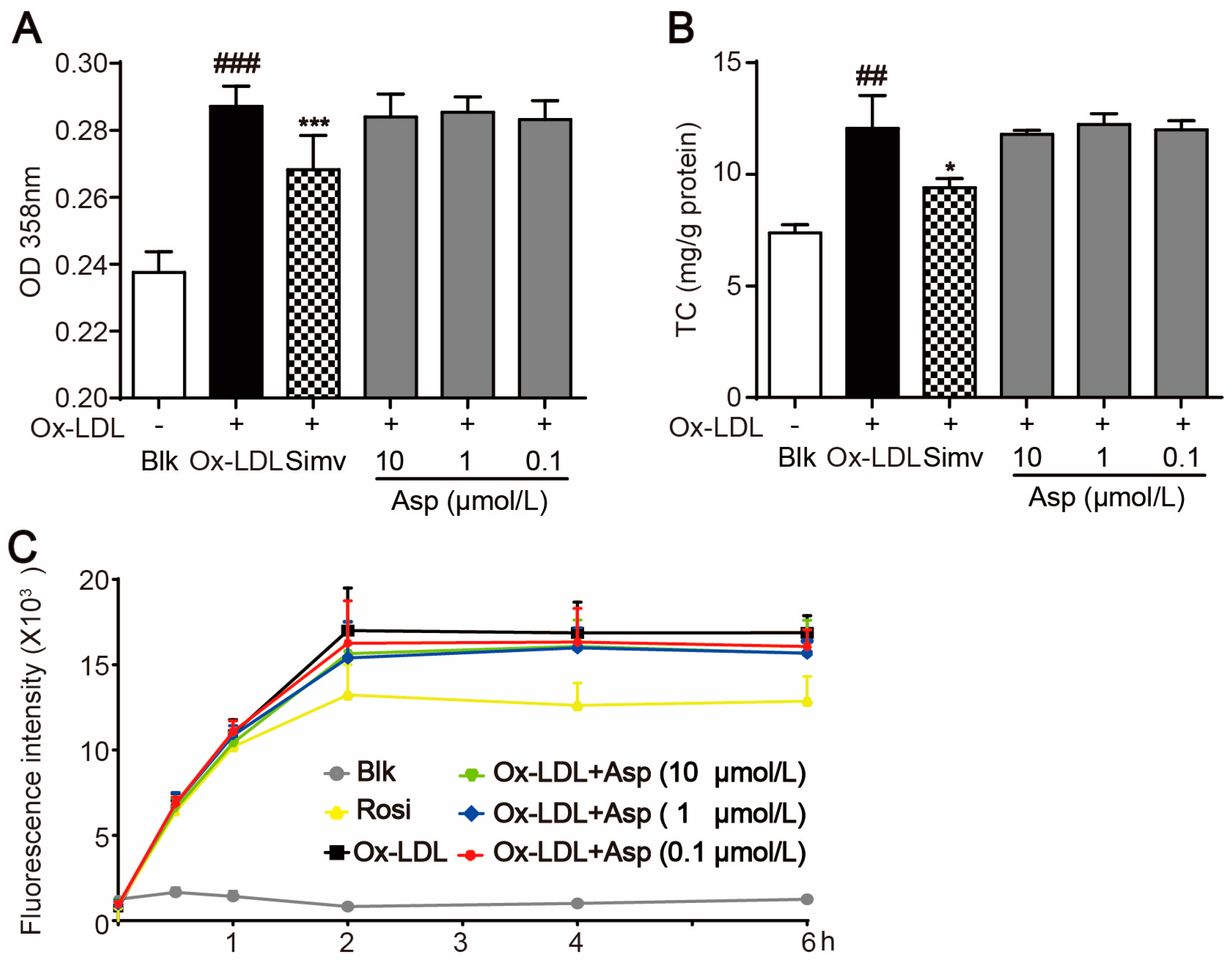
2.5. Asperlin Does Not Inhibit Foam Cell Formation Induced by oxLDL in RAW264.7 Macrophages
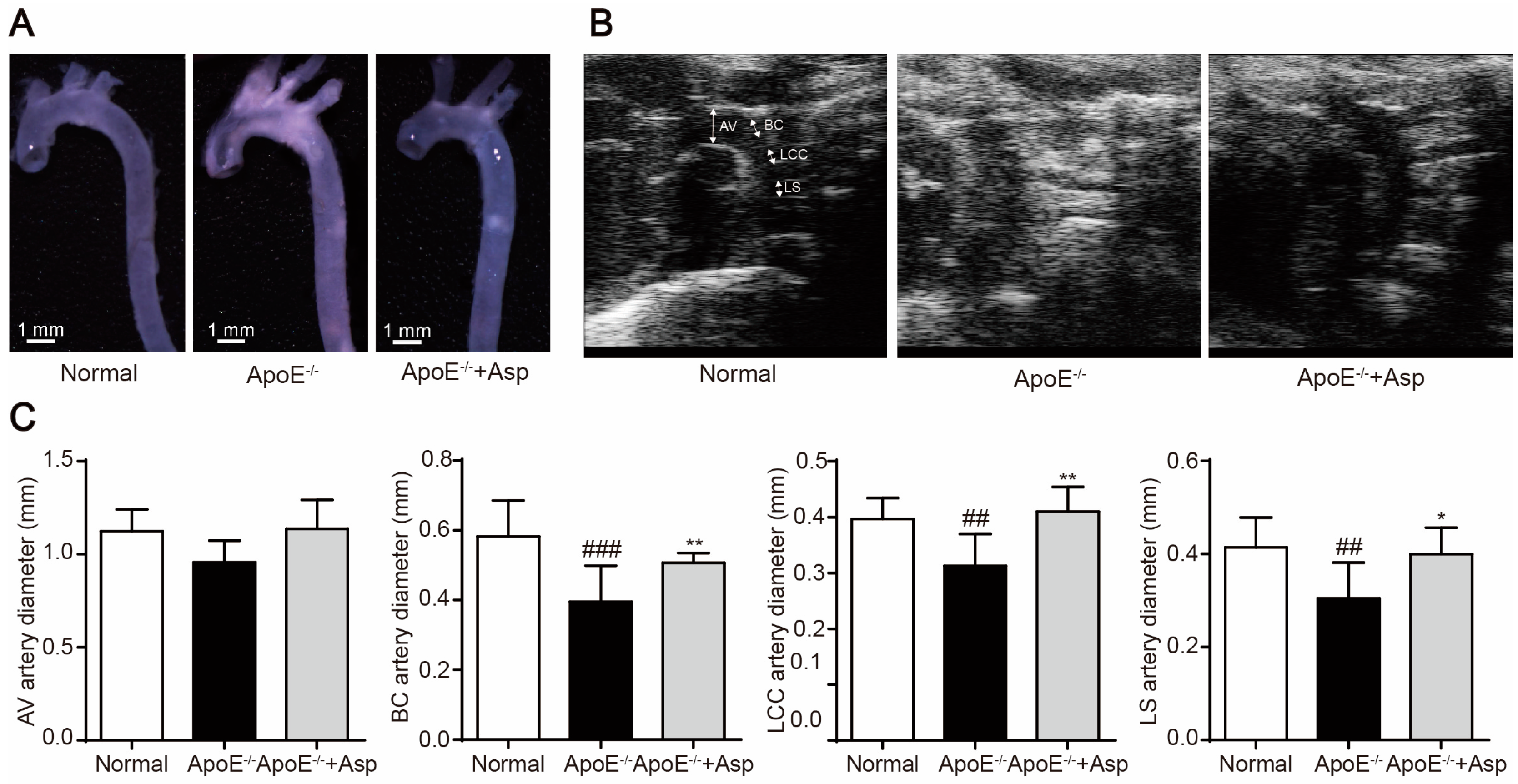
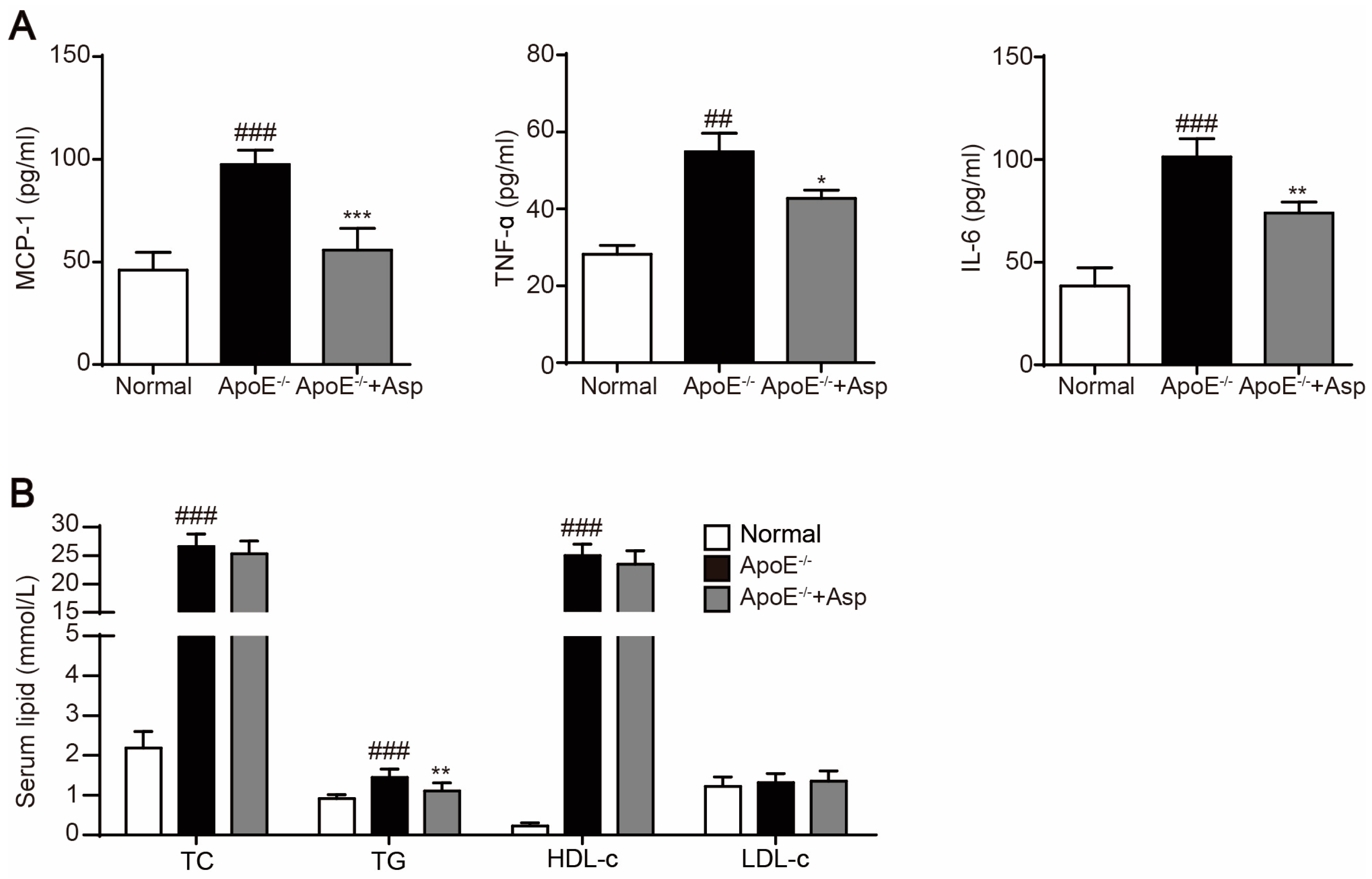
2.6. Asperlin Prevents HFD-Induced Atherosclerosis in ApoE−/− Mice
3. Experimental Section
3.1. Materials and Reagents
3.2. Isolation and Identification of Asperlin
3.2.1. Fungal Material
3.2.2. Fermentation
3.2.3. Extraction and Isolation
3.2.4. Structural Identification
3.3. Cell Culture
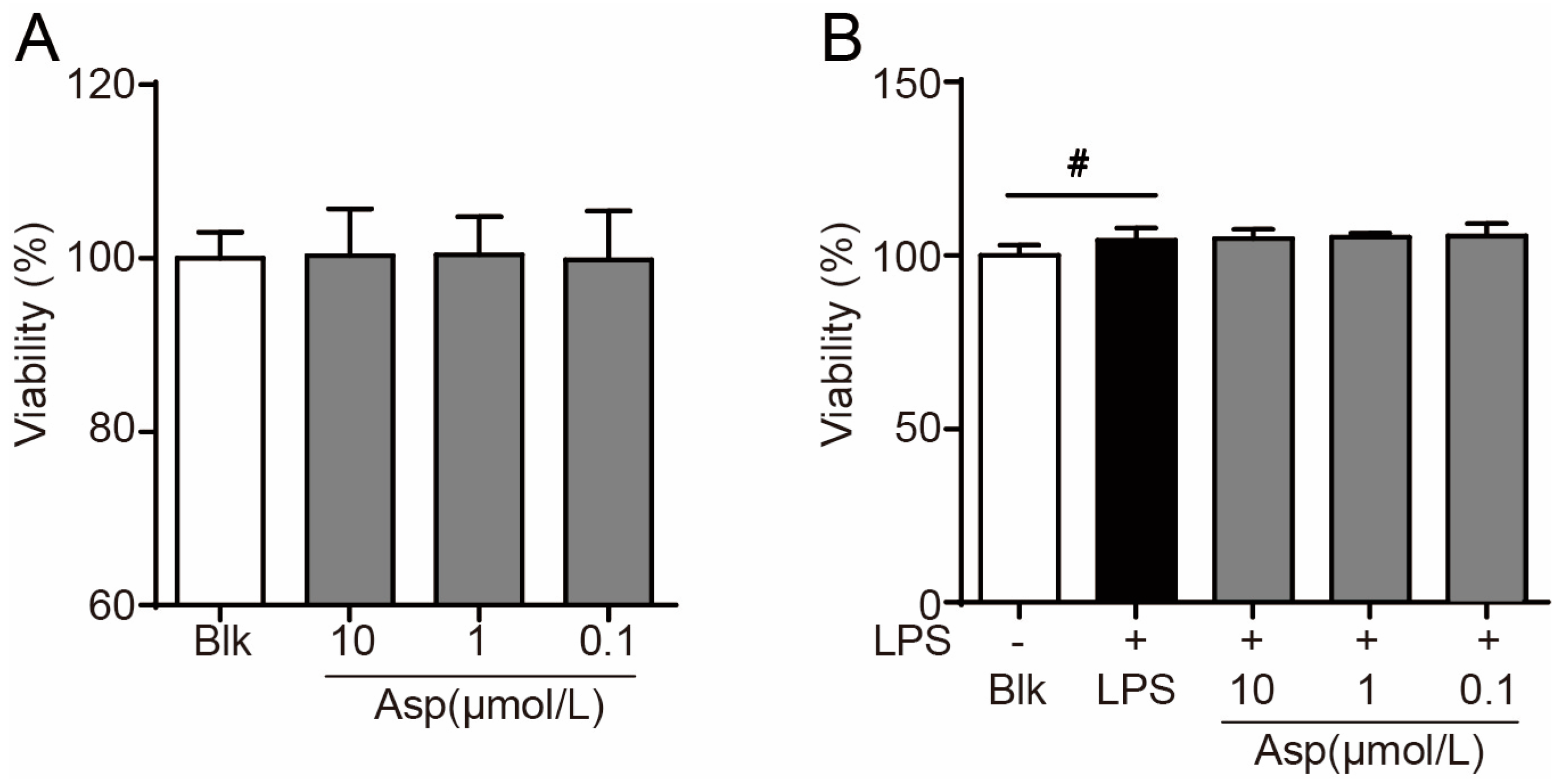
3.4. Cell Viability Assay
3.5. Oil-Red O Staining
3.6. Intracellular TG Quantification
3.7. Cholesterol Efflux Assay
3.8. Realtime Quantitative PCR
3.9. Western Blotting
3.10. Determination of NO Production in RAW264.7 Cells
3.11. Measurement of MCP-1, IL-6 and TNFα in RAW264.7 Cells
3.12. Animal Experiment
3.13. In Vivo Ultrasound
3.14. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pothineni, N.V.K.; Subramany, S.; Kuriakose, K.; Shirazi, L.F.; Romeo, F.; Shah, P.K.; Mehta, J.L. Infections, atherosclerosis, and coronary heart disease. Eur. Heart J. 2017. [Google Scholar] [CrossRef] [PubMed]
- Li, A.C.; Glass, C.K. The macrophage foam cell as a target for therapeutic intervention. Nat. Med. 2002, 8, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Li, A.C.; Binder, C.J.; Gutierrez, A.; Brown, K.K.; Plotkin, C.R.; Pattison, J.W.; Valledor, A.F.; Davis, R.A.; Willson, T.M.; Witztum, J.L.; et al. Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARα, β/δ, and γ. J. Clin. Investig. 2004, 114, 1564–1576. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.; Boisvert, W.A.; Lee, C.H.; Laffitte, B.A.; Barak, Y.; Joseph, S.B.; Liao, D.; Nagy, L.; Edwards, P.A.; Curtiss, L.K.; et al. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol. Cell 2001, 7, 161–171. [Google Scholar] [CrossRef]
- Luo, Y.; Duan, H.; Qian, Y.; Feng, L.; Wu, Z.; Wang, F.; Feng, J.; Yang, D.; Qin, Z.; Yan, X. Macrophagic CD146 promotes foam cell formation and retention during atherosclerosis. Cell Res. 2017, 27, 352–372. [Google Scholar] [CrossRef] [PubMed]
- Back, M.; Hansson, G.K. Anti-inflammatory therapies for atherosclerosis. Nat. Rev. Cardiol. 2015, 12, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Ip, W.K.E.; Hoshi, N.; Shouval, D.S.; Snapper, S.; Medzhitov, R. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science 2017, 356, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.L.; Wang, Y.J.; Yan, J.W.; Wan, Y.N.; Chen, B.; Li, B.Z.; Yang, G.J.; Wang, J. Role of anti-inflammatory cytokines IL-4 and IL-13 in systemic sclerosis. Inflamm. Res. 2015, 64, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.; Rastogi, R.; Shelke, J.; Amiji, M.M. Modulation of Macrophage Functional Polarity towards Anti-Inflammatory Phenotype with Plasmid DNA Delivery in CD44 Targeting Hyaluronic Acid Nanoparticles. Sci. Rep. 2015, 5, 16632. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Ishigami, M.; Matsushita, Y.; Hirata, M.; Matsubara, K.; Ishikawa, T.; Hibi, H.; Ueda, M.; Hirooka, Y.; Goto, H.; et al. Secreted Ectodomain of SIGLEC-9 and MCP-1 Synergistically Improve Acute Liver Failure in Rats by Altering Macrophage Polarity. Sci. Rep. 2017, 7, 44043. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Gao, J.; Ishigaki, Y.; Kondo, K.; Sawada, S.; Izumi, T.; Uno, K.; Kaneko, K.; Tsukita, S.; Takahashi, K.; et al. ER Stress Protein CHOP Mediates Insulin Resistance by Modulating Adipose Tissue Macrophage Polarity. Cell Rep. 2017, 18, 2045–2057. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Garlanda, C.; Locati, M. Macrophage diversity and polarization in atherosclerosis: A question of balance. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1419–1423. [Google Scholar] [CrossRef] [PubMed]
- Prieur, X.; Mok, C.Y.; Velagapudi, V.R.; Nunez, V.; Fuentes, L.; Montaner, D.; Ishikawa, K.; Camacho, A.; Barbarroja, N.; O’Rahilly, S.; et al. Differential lipid partitioning between adipocytes and tissue macrophages modulates macrophage lipotoxicity and M2/M1 polarization in obese mice. Diabetes 2011, 60, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Finn, A.V.; Nakano, M.; Polavarapu, R.; Karmali, V.; Saeed, O.; Zhao, X.; Yazdani, S.; Otsuka, F.; Davis, T.; Habib, A.; et al. Hemoglobin directs macrophage differentiation and prevents foam cell formation in human atherosclerotic plaques. J. Am. Coll. Cardiol. 2012, 59, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Hoeksema, M.A.; Stoger, J.L.; de Winther, M.P. Molecular pathways regulating macrophage polarization: Implications for atherosclerosis. Curr. Atheroscler. Rep. 2012, 14, 254–263. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Nan, M.H.; Oh, H.C.; Kim, Y.H.; Jang, J.H.; Erikson, R.L.; Ahn, J.S.; Kim, B.Y. Asperlin induces G(2)/M arrest through ROS generation and ATM pathway in human cervical carcinoma cells. Biochem. Biophys. Res. Commun. 2011, 409, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Jeong, G.S.; Li, B.; Lee, S.U.; Oh, H.; Kim, Y.C. Asperlin from the marine-derived fungus Aspergillus sp. SF-5044 exerts anti-inflammatory effects through heme oxygenase-1 expression in murine macrophages. J. Pharmacol. Sci. 2011, 116, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Majdalawieh, A.; Ro, H.S. LPS-induced suppression of macrophage cholesterol efflux is mediated by adipocyte enhancer-binding protein 1. Int. J. Biochem. Cell Biol. 2009, 41, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.J.; Tang, S.L.; Lv, Y.C.; Ouyang, X.P.; He, P.P.; Yao, F.; Chen, W.J.; Lu, Q.; Tang, Y.Y.; Zhang, M.; et al. Antagonism of betulinic acid on LPS-mediated inhibition of ABCA1 and cholesterol efflux through inhibiting nuclear factor-kappaB signaling pathway and miR-33 expression. PLoS ONE 2013, 8, e74782. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, X.; Liu, M.; Luan, H.; Ji, Y.; Guo, P.; Wu, C. Chrysin inhibits foam cell formation through promoting cholesterol efflux from RAW264.7 macrophages. Pharm. Biol. 2015, 53, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, R.; Liu, M.; Liu, D.; Li, X.; Wang, S.; Niu, S.; Guo, P.; Lin, W. Spiromastixones Inhibit Foam Cell Formation via Regulation of Cholesterol Efflux and Uptake in RAW264.7 Macrophages. Mar. Drugs 2015, 13, 6352–6365. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, M.; Crean, D.; Barry, M.; Belton, O. M1- and M2-Type Macrophage Responses Are Predictive of Adverse Outcomes in Human Atherosclerosis. Front. Immunol. 2016, 7, 275. [Google Scholar] [CrossRef] [PubMed]
- Eigenbrodt, M.L.; Bursac, Z.; Tracy, R.E.; Mehta, J.L.; Rose, K.M.; Couper, D.J. B-mode ultrasound common carotid artery intima-media thickness and external diameter: Cross-sectional and longitudinal associations with carotid atherosclerosis in a large population sample. Cardiovasc. Ultrasound 2008, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Wikstrand, J. Methodological considerations of ultrasound measurement of carotid artery intima-media thickness and lumen diameter. Clin. Physiol. Funct. Imaging 2007, 27, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Luan, H.; Zhang, X.; Wang, S.; Zhang, X.; Sun, X.; Guo, P. Chlorogenic acid protects against atherosclerosis in ApoE−/− mice and promotes cholesterol efflux from RAW264.7 macrophages. PLoS ONE 2014, 9, e95452. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Guo, Y.; Su, Y.; Zhang, X.; Luan, H.; Zhang, X.; Zhu, H.; He, H.; Wang, X.; Sun, G.; et al. Cordycepin activates AMP-activated protein kinase (AMPK) via interaction with the gamma1 subunit. J. Cell. Mol. Med. 2014, 18, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhang, X.; Zhang, X.; Luan, H.; Sun, G.; Sun, X.; Wang, X.; Guo, P.; Xu, X. The caffeoylquinic acid-rich Pandanus tectorius fruit extract increases insulin sensitivity and regulates hepatic glucose and lipid metabolism in diabetic db/db mice. J. Nutr. Biochem. 2014, 25, 412–419. [Google Scholar] [CrossRef] [PubMed]
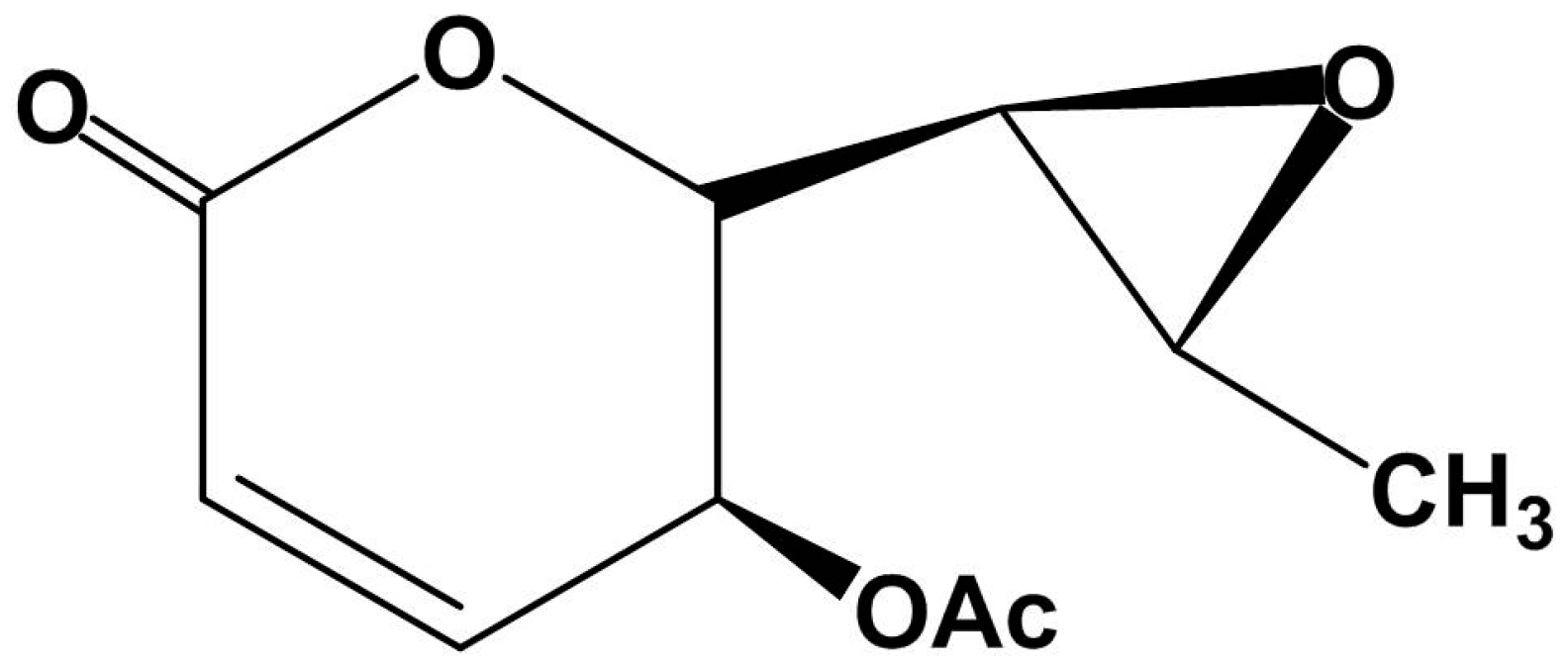
| Position | δH (mult., J in Hz) | δC | Position | δH (mult., J in Hz) | δC |
|---|---|---|---|---|---|
| 1 | 7 | 3.02, dd (6.5, 2.0) | 55.4 | ||
| 2 | 162.2 | 8 | 3.11, dd (6.5, 2.0) | 52.8 | |
| 3 | 6.25, d (12.0) | 124.9 | 9 | 1.28, d (6.5) | 17.3 |
| 4 | 7.09, dd (12.0, 7.5) | 141.5 | 10 | 170.0 | |
| 5 | 5.39, dd (7.5, 3.5) | 62.0 | 11 | 2.10, s | 20.8 |
| 6 | 4.55, dd (6.5, 3.5) | 77.5 |
| Name | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| ABCA1 | GCGGACCTCCTGGGTGTT | CAAGAATCTCCGGGCTTTAGG |
| ABCG1 | AAGGCCTACTACCTGGCAAAGA | GCAGTAGGCCACAGGGAACA |
| PPARγ | CATTCTGGCCCACCAACTTC | TCAAAGGAATGCGAGTGGTCTT |
| LXRα | CCTTCCTCAAGGACTTCAGTTACAA | CATGGCTCTGGAGAACTCAAAGAT |
| iNOS | GCATCCCAAGTACGAGTGGT | CCATGATGGTCACATTCTGC |
| IL-1β | GGAGAAGCTGTGGCAGCTA | GCTGATGTACCAGTTGGGGA |
| IL-6 | CCGGAGAGGAGACTTCACAG | TGGTCTTGGTCCTTAGCCAC |
| TNF-α | GACCCTCACACTCAGATCAT | TTGAAGAGAACCTGGGAGTA |
| COX-2 | GCTGTACAAGCAGTGGCAAA | GTCTGGAGTGGGAGGCACT |
| IL-4 | GGTCTCAACCCCCAGCTAGT | GCCGATGATCTCTCTCAAGTGAT |
| Arg-1 | TGTCCCTAATGACAGCTCCTT | GCATCCACCCAAATGACACAT |
| IL-10 | CTTACTGACTGGCATGAGGATCA | GCAGCTCTAGGAGCATGTGG |
| β-actin | GGCTGTATTCCCCTCCATCG | CCAGTTGGTAACAATGCCATGT |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Chen, R.; Liu, D.; Wu, C.; Guo, P.; Lin, W. Asperlin Inhibits LPS-Evoked Foam Cell Formation and Prevents Atherosclerosis in ApoE−/− Mice. Mar. Drugs 2017, 15, 358. https://doi.org/10.3390/md15110358
Zhou Y, Chen R, Liu D, Wu C, Guo P, Lin W. Asperlin Inhibits LPS-Evoked Foam Cell Formation and Prevents Atherosclerosis in ApoE−/− Mice. Marine Drugs. 2017; 15(11):358. https://doi.org/10.3390/md15110358
Chicago/Turabian StyleZhou, Yue, Ran Chen, Dong Liu, Chongming Wu, Peng Guo, and Wenhan Lin. 2017. "Asperlin Inhibits LPS-Evoked Foam Cell Formation and Prevents Atherosclerosis in ApoE−/− Mice" Marine Drugs 15, no. 11: 358. https://doi.org/10.3390/md15110358
APA StyleZhou, Y., Chen, R., Liu, D., Wu, C., Guo, P., & Lin, W. (2017). Asperlin Inhibits LPS-Evoked Foam Cell Formation and Prevents Atherosclerosis in ApoE−/− Mice. Marine Drugs, 15(11), 358. https://doi.org/10.3390/md15110358






