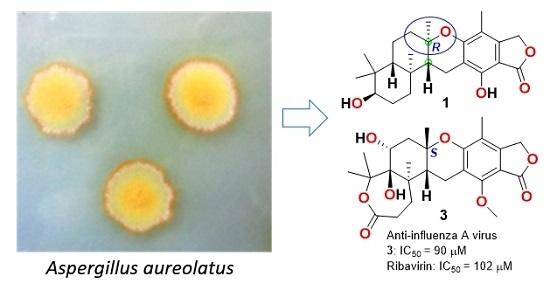
Austalides S-U, New Meroterpenoids from the Sponge-Derived Fungus Aspergillus aureolatus HDN14-107
Abstract
:1. Introduction
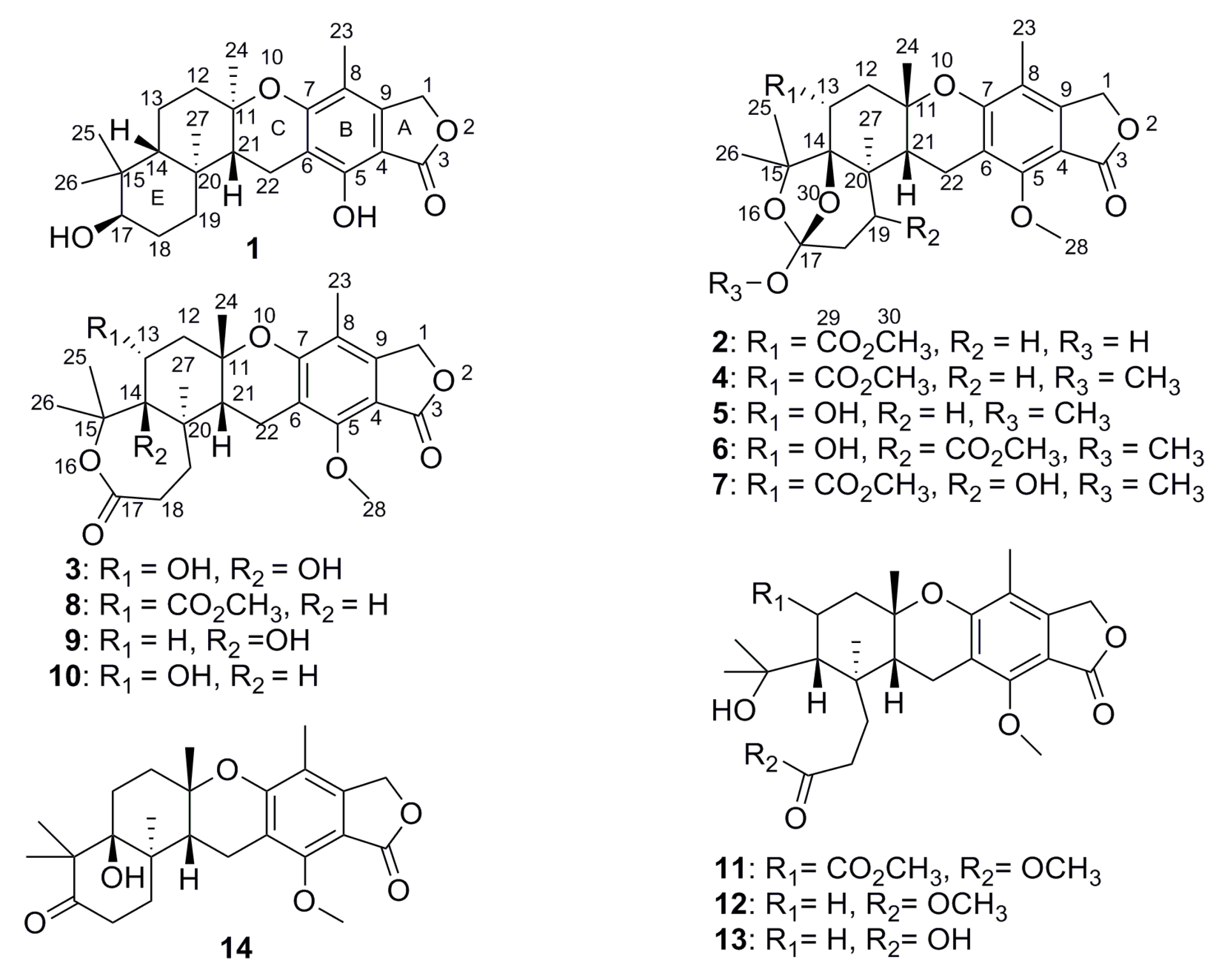
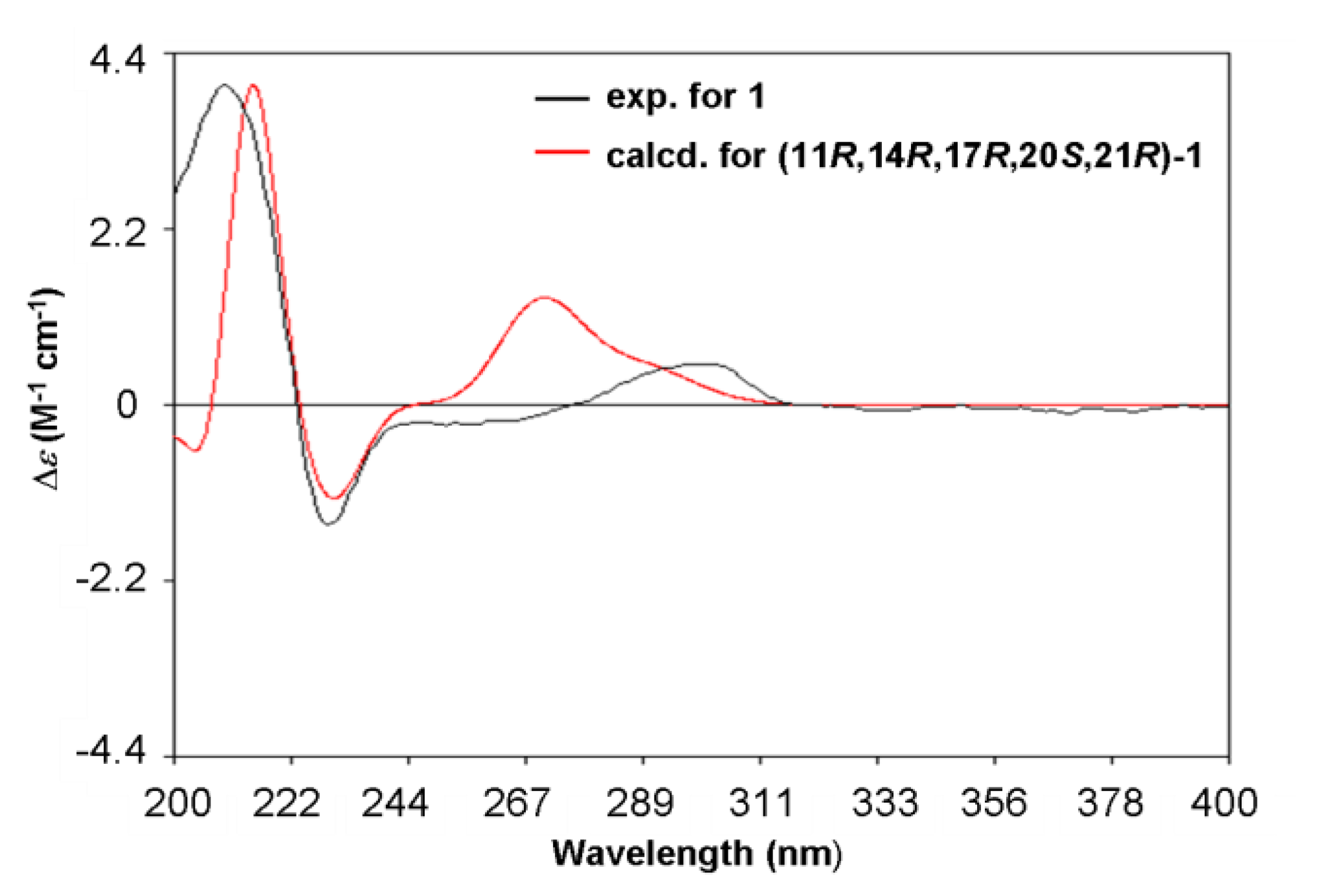
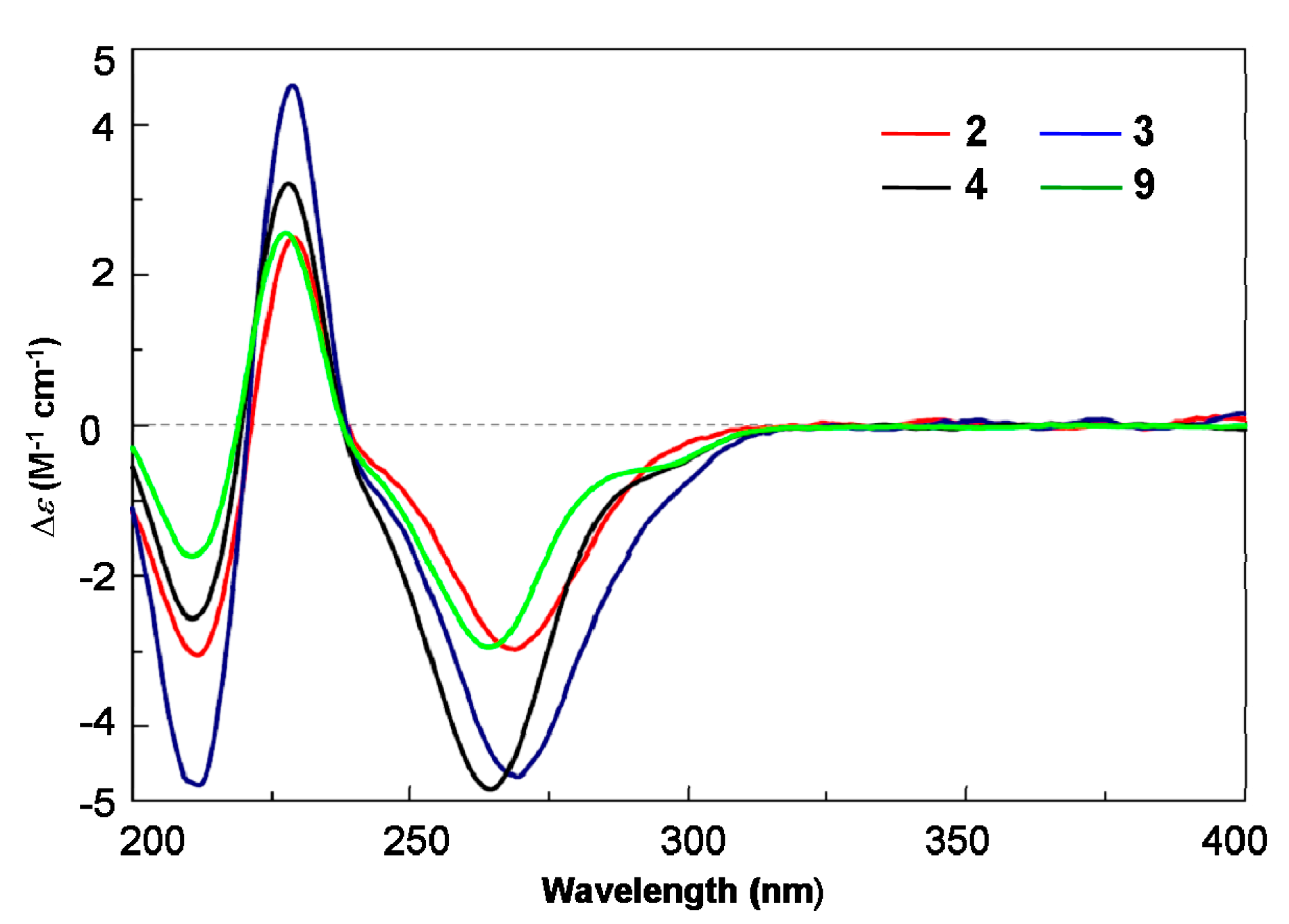
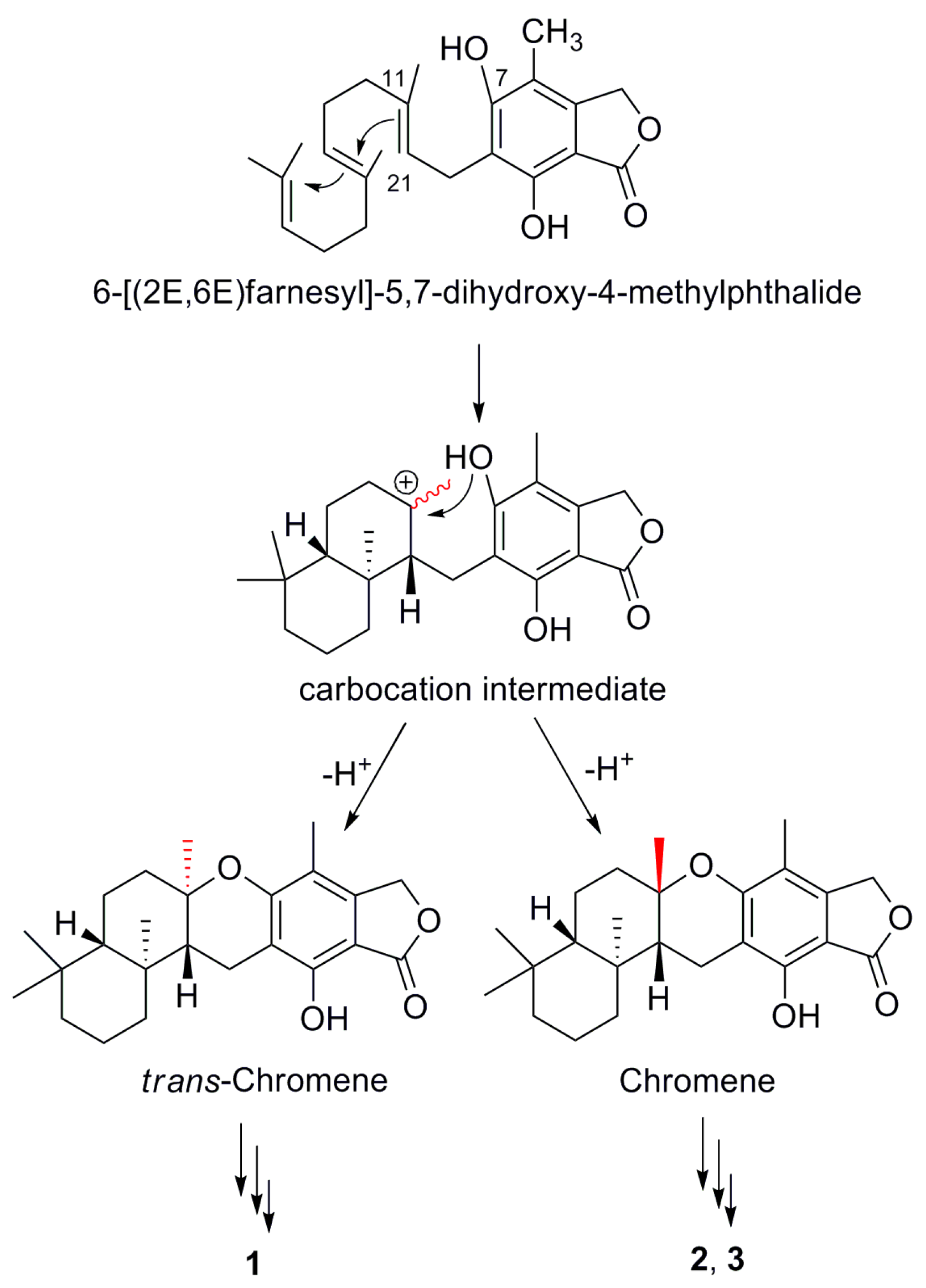
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation and Extraction
3.4. Purification
3.5. Computation Section
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Horak, R.M.; Steyn, P.S.; Rooyen, P.H.V.; Vleggaar, R. Structures of the austalides A–E, five novel toxic metabolites from Aspergillus ustus. J. Chem. Soc. Chem. Commun. 1981, 24, 1265–1267. [Google Scholar] [CrossRef]
- Horak, R.M.; Steyn, P.S.; Vleggaar, R. Metabolites of Aspergillus ustus. Part 3. Structure elucidation of austalides G-L. J. Chem. Soc. Perkin Trans. 1 1985, 2, 363–367. [Google Scholar] [CrossRef]
- Zhou, Y.M.; Mandi, A.; Debbab, A.; Wray, V.; Schulz, B.; Mueller, W.E.G.; Lin, W.H.; Proksch, P.; Kurtan, T.; Aly, A.H. New austalides from the sponge-associated fungus Aspergillus sp. Eur. J. Org. Chem. 2011, 30, 6009–6019. [Google Scholar] [CrossRef]
- Zhou, Y.M.; Debbab, A.; Wray, V.; Lin, W.H.; Schulz, B.; Trepos, R.; Pile, C.; Hellio, C.; Proksch, P.; Aly, A.H. Marine bacterial inhibitors from the sponge-derived fungus Aspergillus sp. Tetrahedron Lett. 2014, 55, 2789–2792. [Google Scholar] [CrossRef]
- Zhuravleva, O.I.; Sobolevskaya, M.P.; Leshchenko, E.V.; Kirichuk, N.N.; Denisenko, V.A.; Dmitrenok, P.S.; Dyshlovoy, S.A.; Zakharenko, A.M.; Kim, N.Y.; Afiyatullov, S.S. Meroterpenoids from the alga-derived fungi Penicillium thomii maire and Penicillium lividum westling. J. Nat. Prod. 2014, 77, 1390–1395. [Google Scholar] [CrossRef] [PubMed]
- Xin, Z.H.; Fang, Y.C.; Du, L.; Zhu, T.J.; Duan, L.; Chen, J.; Gu, Q.Q.; Zhu, W.M. Aurantiomides A–C, quinazoline alkaloids from the sponge-derived fungus Penicillium aurantiogriseum SP0-19. J. Nat. Prod. 2007, 70, 853–855. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhu, T.J.; Li, D.L.; Gu, J.Y.; Xia, W.; Fang, Y.C.; Liu, H.B.; Zhu, W.M.; Gu, Q.Q. Two indolocarbazole alkaloids with apoptosis activity from a marine-derived Actinomycete Z2039-2. Arch. Pharm. Res. 2007, 30, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.X.; Jiao, J.J.; Li, J.; Wang, W.; Gu, Q.Q.; Zhu, T.J.; Li, D.H. Pyronepolyene C-glucosides with NF-κB inhibitory and anti-influenza A viral (H1N1) activities from the sponge-associated fungus Epicoccum sp. JJY40. Bioorg. Med. Chem. Lett. 2012, 22, 3188–3190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.J.; Wu, G.W.; Zhu, T.J.; Kurtán, T.; Mándi, A.; Jiao, J.J.; Li, J.; Qi, X.; Gu, Q.Q.; Li, D.H. Meroterpenoids with diverse ring systems from the sponge-associated fungus Alternaria sp. JJY-32. J. Nat. Prod. 2013, 76, 1946–1957. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.H.; Li, L.T.; Zhu, T.J.; Ba, M.Y.; Li, G.Q.; Gu, Q.Q.; Guo, Y.; Li, D.H. Phenylspirodrimanes with anti-HIV activity from the sponge-derived fungus Stachybotrys chartarum MXH-X73. J. Nat. Prod. 2013, 76, 2298–2306. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.H.; Peng, J.X.; Wu, G.W.; Zhu, T.J.; Li, G.Q.; Gu, Q.Q.; Li, D.H. Speradines B–D, oxygenated cyclopiazonic acid alkaloids from the sponge-derived fungus Aspergillus flavus MXH-X104. Tetrahedron 2015, 71, 3522–3527. [Google Scholar] [CrossRef]
- Ma, X.H.; Wang, H.T.; Li, F.; Zhu, T.J.; Gu, Q.Q.; Li, D.H. Stachybotrin G, a sulfate meroterpenoid from a sponge derived fungus Stachybotrys chartarum MXH-X73. Tetrahedron Lett. 2015, 56, 7053–7055. [Google Scholar] [CrossRef]
- Hung, H.C.; Tseng, C.P.; Yang, J.M.; Ju, Y.W.; Tseng, S.N.; Chen, Y.F.; Chao, Y.S.; Hsieh, H.P.; Shih, S.R.; Hsu, J.T. Aurintricarboxylic acid inhibits influenza virus neuraminidase. Antivir. Res. 2009, 81, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.J. Two rings in them all: The labdane-related diterpenoids. Nat. Prod. Rep. 2010, 27, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Dillen, J.L.M.; Horak, R.M.; Maharaj, V.J.; Marais, S.F.; Vleggaarb, R. Absolute configuration and biosynthesis of the austalides, meroterpenoid metabolites of Aspergillus ustus: Mode of cyclisation of the farnesyl moiety. J. Chem. Soc. Chem. Commun. 1989, 7, 393–394. [Google Scholar] [CrossRef]
- Spartan’14; Wavefunction Inc.: Irvine, CA, USA, 2013.
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision A.1; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Bruhn, T.; Hemberger, Y.; Schaumlöffel, A.; Bringmann, G. SpecDis, version 1.53; University of Wuerzburg: Würzburg, Germany, 2011. [Google Scholar]
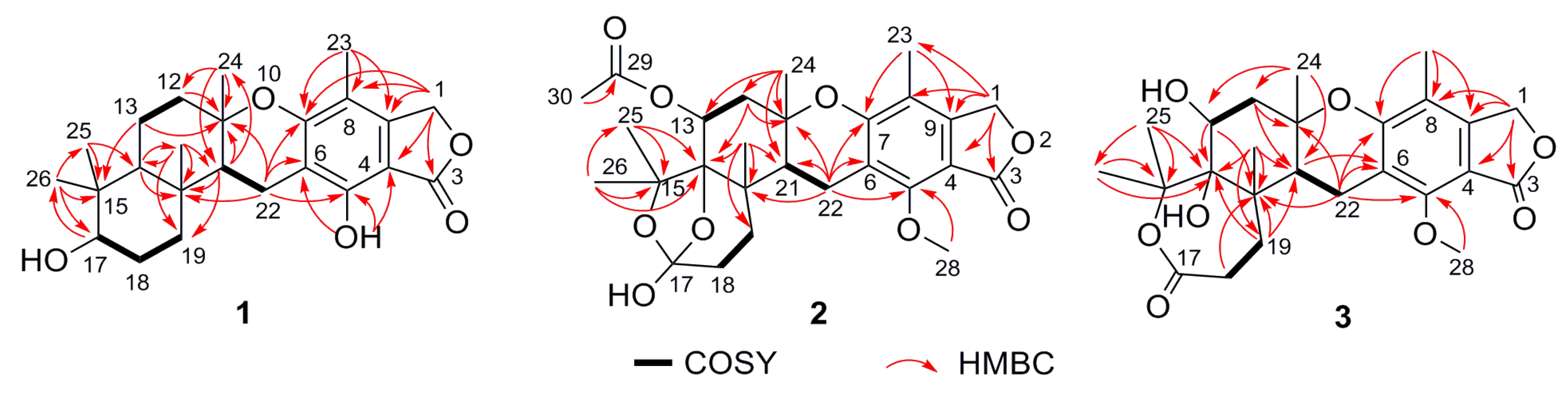
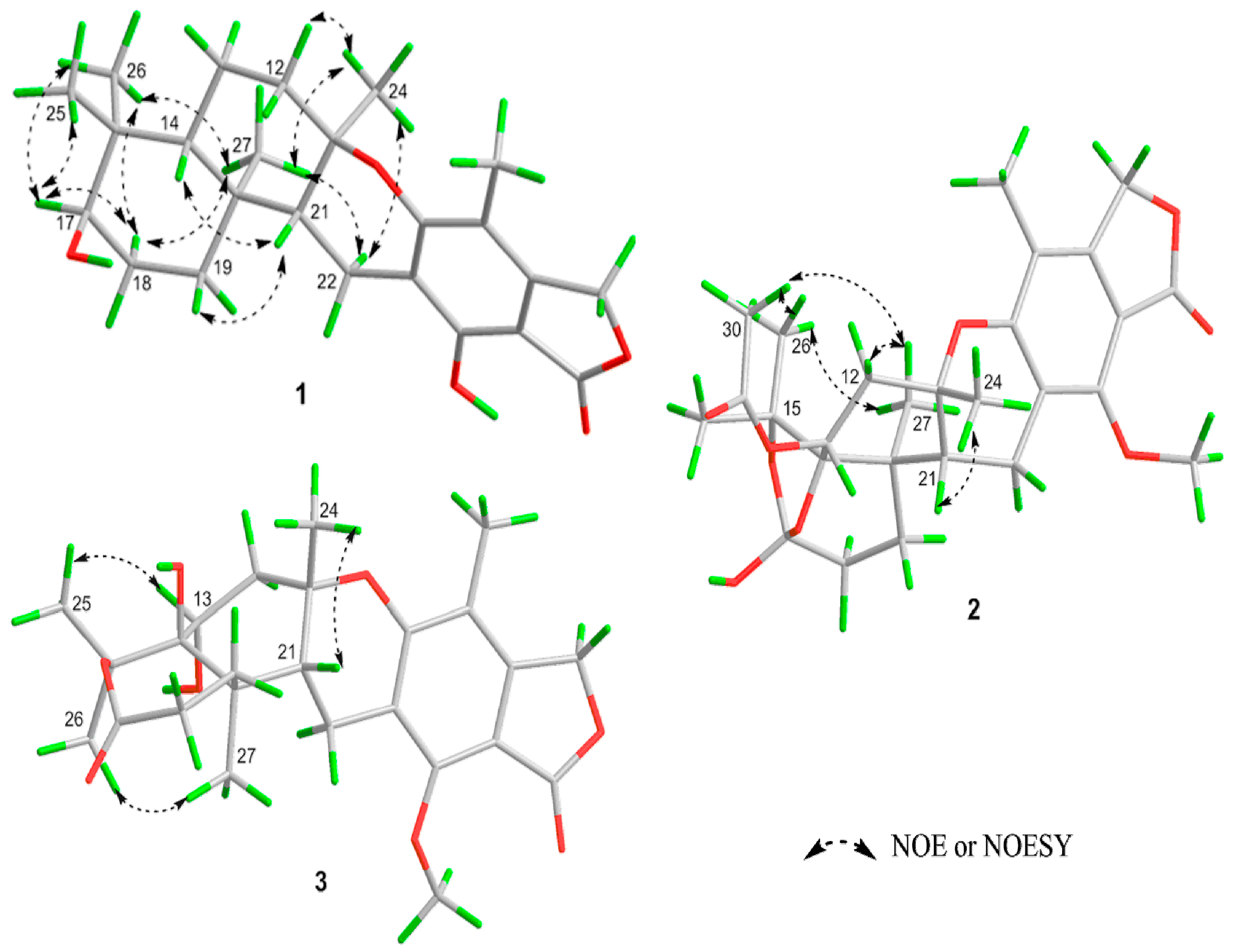
| Position | 1 a | 2 a | 3 b |
|---|---|---|---|
| 1 | 5.18, s | 5.12, s | 5.16, s |
| 12 | 1.93, d (10.8); | 2.56, d (16.2); | 2.30, dd (15.9, 2.1); |
| 1.67, m | 2.17, d (17.9) | 2.18, dd (15.6, 4.4) | |
| 13 | 1.70, m | 5.10, brs | 4.08, m |
| 14 | 0.90, d (12.2) | ||
| 17 | 3.08, m | ||
| 18 | 1.51, m | 1.11, m | 1.87, m; 1.60, m |
| 19 | 1.99, d (12.6); | 1.94, m | 1.91, m |
| 0.68, t (13.3) | |||
| 21 | 1.59, d (7.6) | 2.43, d (8.0) | 2.46, dd (5.8, 3.2) |
| 22 | 2.45, d (15.4) | 2.87, dd (18.8, 8.4) | 2.91, d (6.3) |
| 3.05, d (15.4) | 2.97, d (18.8) | ||
| 23 | 2.05, s | 1.99, s | 2.05, s |
| 24 | 1.34, s | 1.23, s | 1.24, s |
| 25 | 0.96, s | 1.54, s | 1.40, s |
| 26 | 0.78, s | 1.32, s | 1.54, s |
| 27 | 0.99, s | 1.00, s | 0.97, s |
| 28 | 4.13, s | 4.03, s | |
| 30 | 2.06, s | ||
| 5-OH | 7.84, s |
| Position | 1 a | 2 a | 3 b |
|---|---|---|---|
| 1 | 69.9, CH2 | 68.2, CH2 | 68.2, CH2 |
| 3 | 173.8, C | 169.4, C | 169.0, C |
| 4 | 101.7, C | 113.9, C | 107.6, C |
| 5 | 152.2, C | 155.4, C | 155.0, C |
| 6 | 115.0, C | 115.6, C | 116.6, C |
| 7 | 161.4, C | 157.9, C | 157.9, C |
| 8 | 110.9, C | 107.4, C | 114.6, C |
| 9 | 143.9, C | 145.6, C | 146.0, C |
| 11 | 77.2, C | 75.6, C | 77.0, C |
| 12 | 43.6, CH2 | 38.0, CH2 | 41.8, CH2 |
| 13 | 20.1, CH2 | 70.9, CH | 69.1, CH |
| 14 | 54.9, CH | 86.2, C | 86.6, C |
| 15 | 38.8, C | 85.0, C | 84.5, C |
| 17 | 78.5, CH | 117.8, C | 171.0, C |
| 18 | 26.9, CH2 | 29.7, CH2 | 30.1, CH2 |
| 19 | 35.6, CH2 | 31.0, CH2 | 31.2, CH2 |
| 20 | 38.9, C | 40.2, C | 39.4, C |
| 21 | 59.1, CH | 36.0, CH | 36.1, CH |
| 22 | 17.6, CH2 | 18.0, CH2 | 17.9, CH2 |
| 23 | 11.3, CH3 | 10.6, CH3 | 9.9, CH3 |
| 24 | 24.7, CH3 | 27.5, CH3 | 27.0, CH3 |
| 25 | 28.1, CH3 | 25.5, CH3 | 28.7, CH3 |
| 26 | 15.4, CH3 | 29.4, CH3 | 25.2, CH3 |
| 27 | 15.0, CH3 | 18.0, CH3 | 18.2, CH3 |
| 28 | 62.0, CH3 | 61.3, CH3 | |
| 29 | 169.5, C | ||
| 30 | 21.2, CH3 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, J.; Zhang, X.; Wang, W.; Zhu, T.; Gu, Q.; Li, D. Austalides S-U, New Meroterpenoids from the Sponge-Derived Fungus Aspergillus aureolatus HDN14-107. Mar. Drugs 2016, 14, 131. https://doi.org/10.3390/md14070131
Peng J, Zhang X, Wang W, Zhu T, Gu Q, Li D. Austalides S-U, New Meroterpenoids from the Sponge-Derived Fungus Aspergillus aureolatus HDN14-107. Marine Drugs. 2016; 14(7):131. https://doi.org/10.3390/md14070131
Chicago/Turabian StylePeng, Jixing, Xiaomin Zhang, Wei Wang, Tianjiao Zhu, Qianqun Gu, and Dehai Li. 2016. "Austalides S-U, New Meroterpenoids from the Sponge-Derived Fungus Aspergillus aureolatus HDN14-107" Marine Drugs 14, no. 7: 131. https://doi.org/10.3390/md14070131
APA StylePeng, J., Zhang, X., Wang, W., Zhu, T., Gu, Q., & Li, D. (2016). Austalides S-U, New Meroterpenoids from the Sponge-Derived Fungus Aspergillus aureolatus HDN14-107. Marine Drugs, 14(7), 131. https://doi.org/10.3390/md14070131