Pharmacokinetics in Mouse and Comparative Effects of Frondosides in Pancreatic Cancer
Abstract
:1. Introduction
2. Results
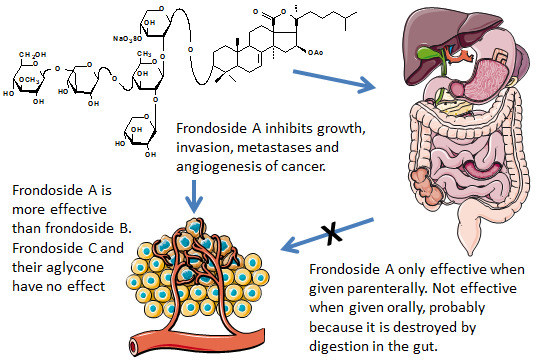
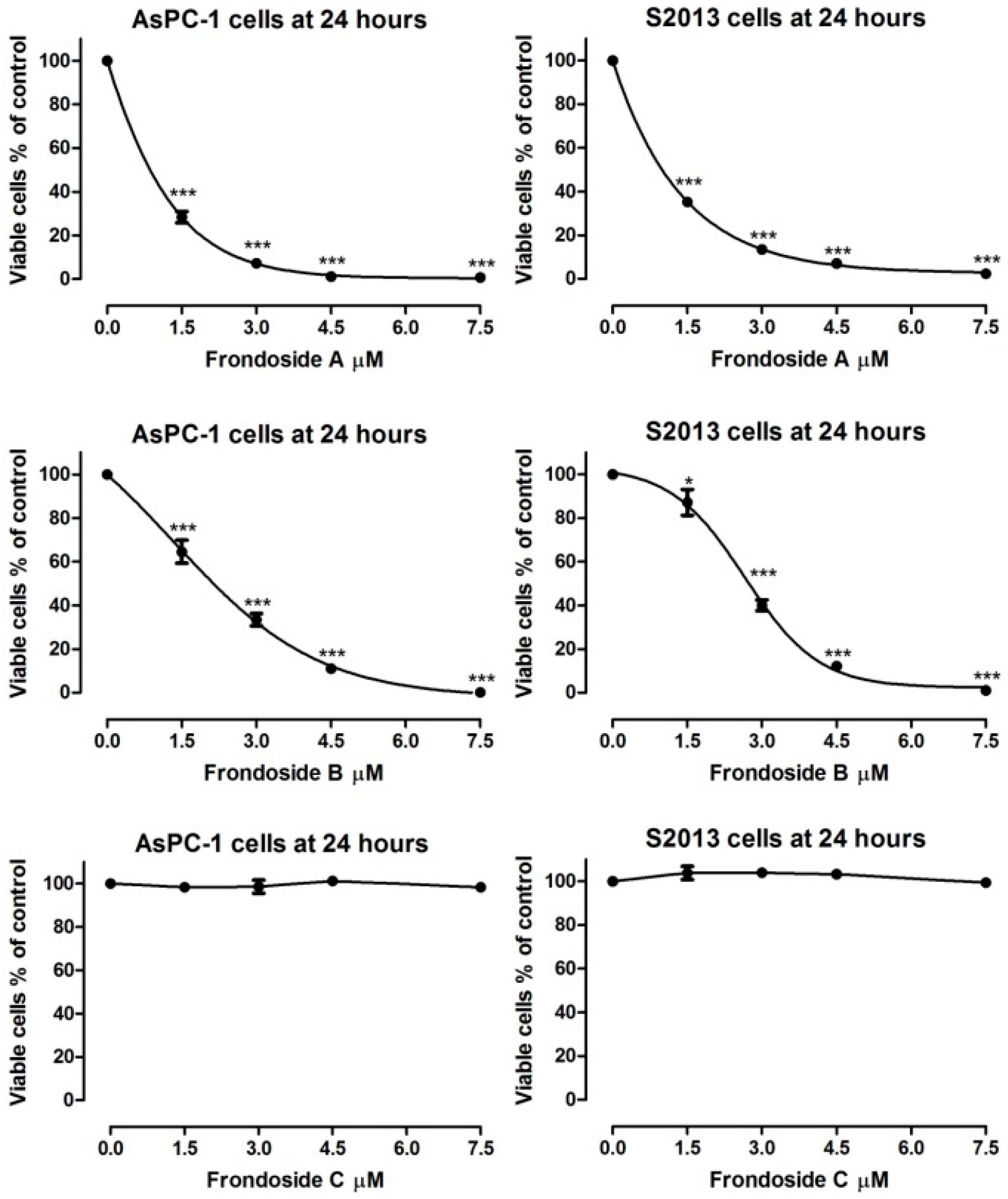
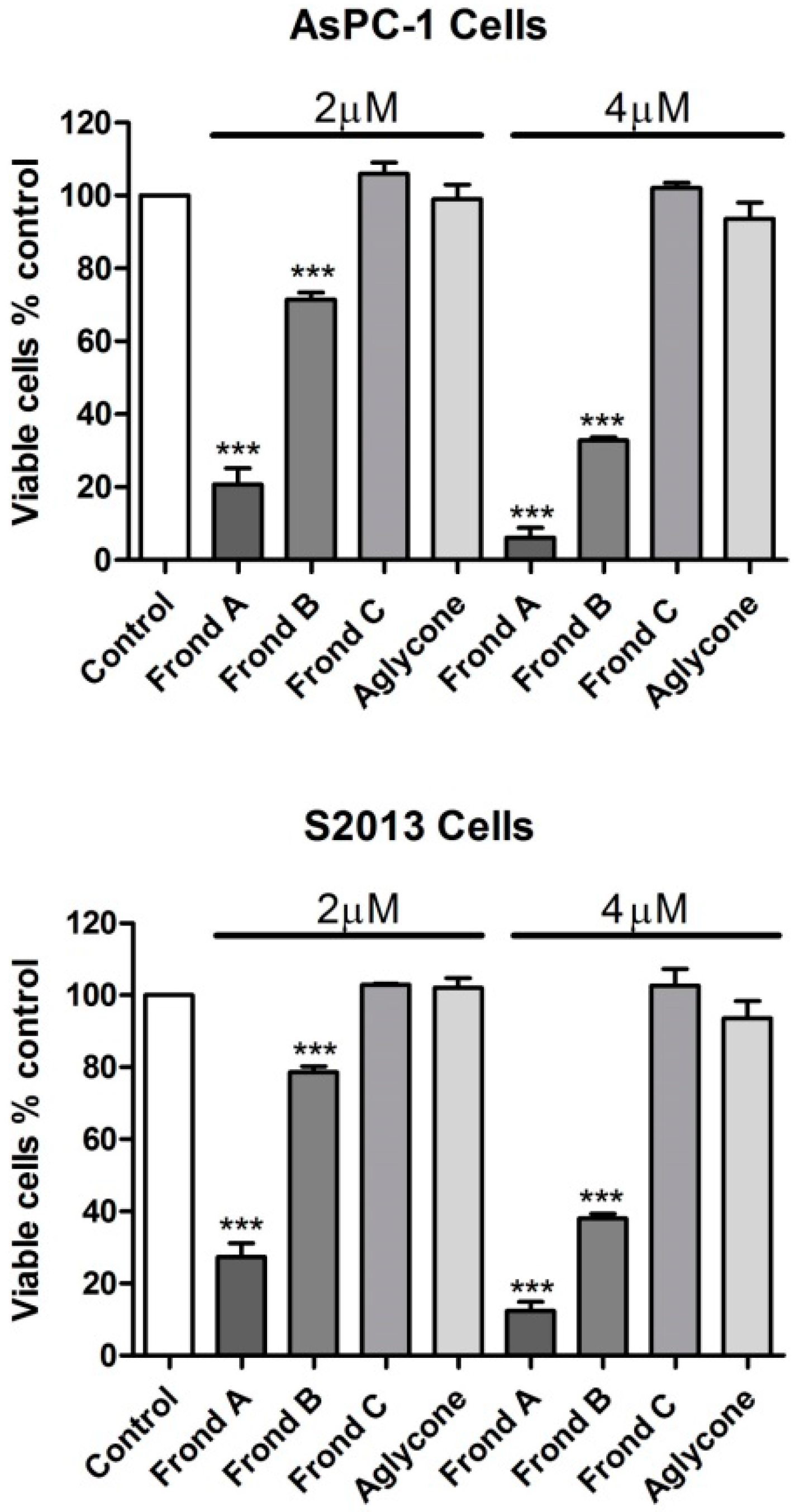
2.1. Effects on Proliferation of AsPC-1 and S2013 Pancreatic Cancer Cells in Vitro
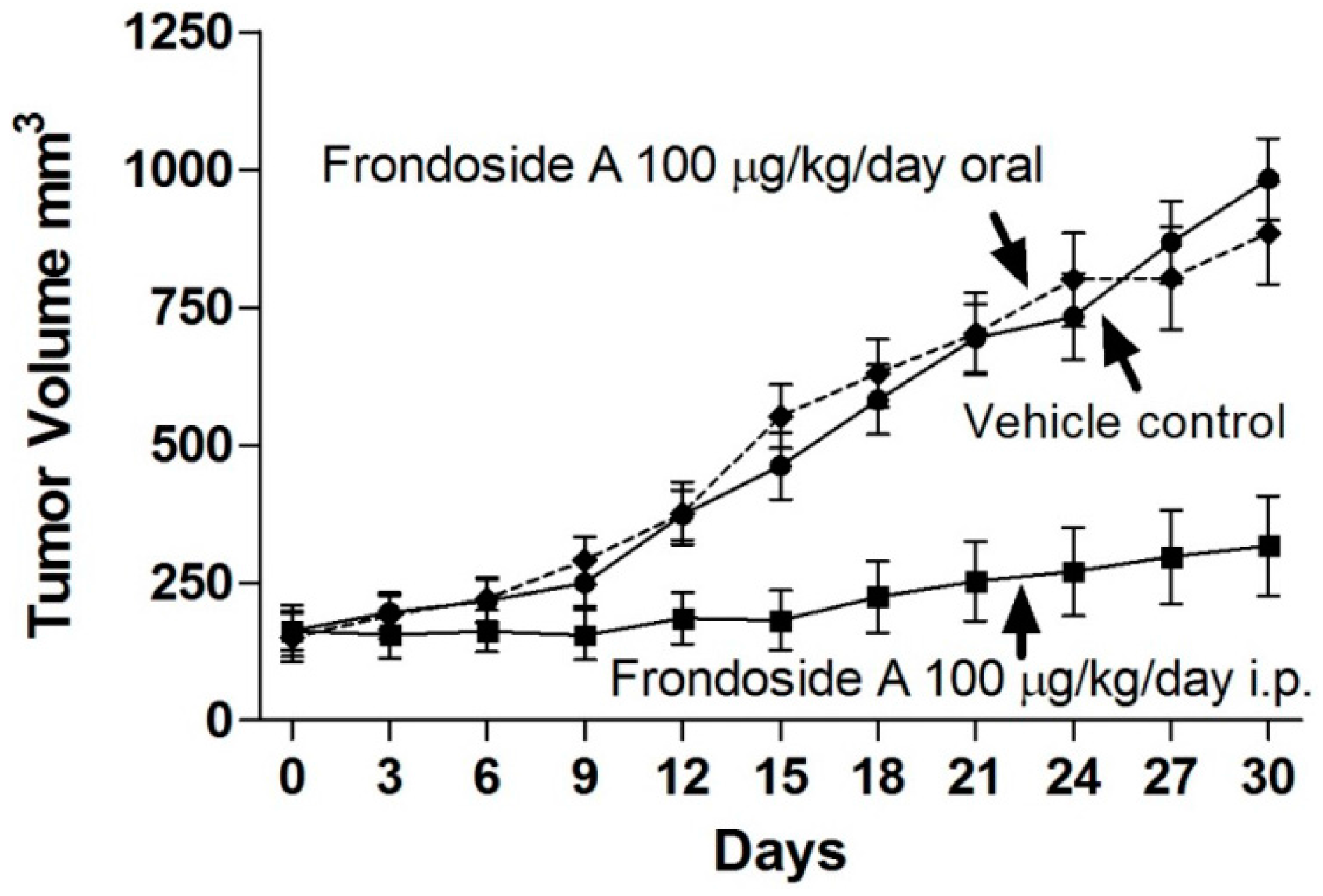
2.2. Comparison of Route of Administration of Frondoside A on Growth of AsPC-1 Xenografts in Athymic Mice
2.3. Pharmacokinetics of Frondoside A
3. Discussion
4. Materials and Methods
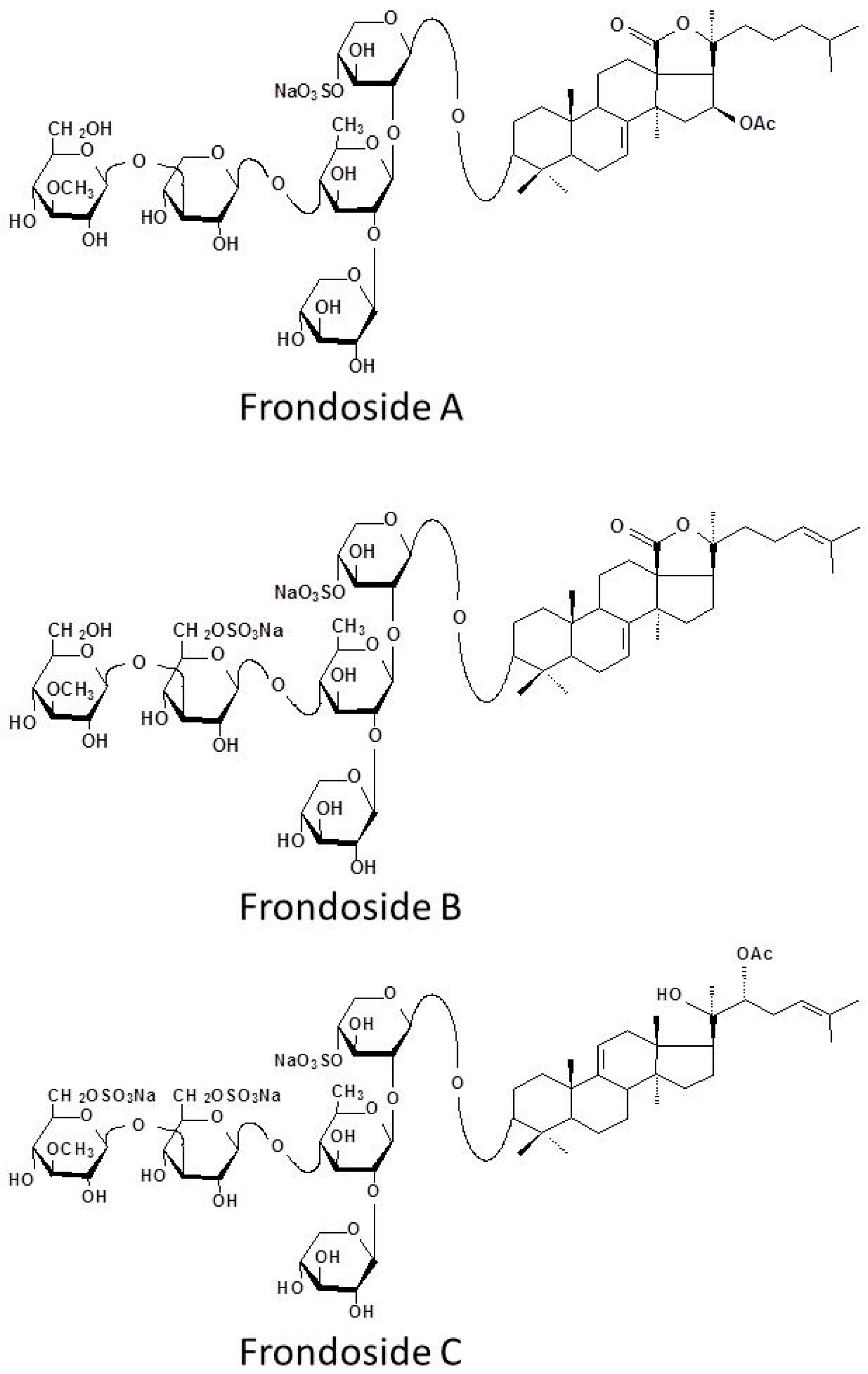
4.1. Isolation and Purification of Frondoside A, B, C and Their Aglycone
4.2. Cell Lines and Cell Cultures
4.3. Cell Proliferation Assays
4.4. Animals and Subcutaneous Tumor Cell Implantation
4.5. Pharmacokinetics of Frondoside A
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AUC | Area under curve |
| Cltb | Total body clearance |
| Cpmax | Maximum plasma concentration |
| CV | Coefficient of variation |
| DMSO | Dimethyl sulphoxide |
| LOD | Limit of detection |
| VD | Volume of distribution |
References
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer Statistics. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Saif, M.W. Pancreatic Neoplasm in 2011: An Update. JOP J. Pancreas 2011, 12, 316–321. [Google Scholar]
- Al Haddad, A.H.; Adrian, T.E. Challenges and future directions in therapeutics for pancreatic ductal adenocarcinoma. Expert Opin. Investig. Drugs 2014, 23, 1499–1515. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Roginsky, A.B.; Ding, X.Z.; Woodward, C.; Collin, P.; Newman, R.A.; Bell, R.H., Jr.; Adrian, T.E. Review of the apoptosis pathways in pancreatic cancer and the anti-apoptotic effects of the novel sea cucumber compound, Frondoside A. Ann. N. Y. Acad. Sci. 2008, 1138, 181–198. [Google Scholar] [CrossRef] [PubMed]
- Al Marzouqi, N.; Iratni, R.; Nemmar, A.; Arafat, K.; Al Sultan, A.M.; Yasin, J.; Collin, P.; Mester, J.; Adrian, T.E.; Attoub, S. Frondoside A inhibits human breast cancer cell survival, migration, invasion and the growth of breast tumor xenografts. Eur. J. Pharmacol. 2011, 668, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Attoub, S.; Arafat, K.; Gélaude, A.; Al Sultan, M.A.; Bracke, M.; Collin, P.L.; Takahashi, T.; Adrian, T.E.; De Wever, O. Frondoside a suppressive effects on lung cancer survival, tumor growth, angiogenesis, invasion, and metastasis. PLoS ONE 2013, 8, e53087. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Kundu, N.; Collin, P.D.; Goloubeva, O.; Fulton, A.M. Frondoside A inhibits breast cancer metastasis and antagonizes prostaglandin E receptors EP4 and EP2. Breast Cancer Res. Treat. 2011, 132, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.O.; Shastina, V.V.; Shin, S.W.; Xu, Q.; Park, J.I.; Rasskazov, V.A.; Avilov, S.A.; Fedorov, S.N.; Stonik, V.A.; Kwak, J.Y. Differential effects of triterpene glycosides, frondoside A and cucumarioside A2-2 isolated from sea cucumbers on caspase activation and apoptosis of human leukemia cells. FEBS Lett. 2009, 583, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Al Shemaili, J.; Mensah-Brown, E.; Parekh, K.; Thomas, S.A.; Attoub, S.; Hellman, B.; Nyberg, F.; Adem, A.; Collin, P.; Adrian, T.E. Frondoside A enhances the antiproliferative effects of gemcitabine in pancreatic cancer. Eur. J. Cancer 2014, 50, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Aminin, D.L.; Agafonova, I.G.; Kalinin, V.I.; Silchenko, A.S.; Avilov, S.A.; Stonik, V.A.; Collin, P.D.; Woodward, C. Immunomodulatory properties of frondoside A, a major triterpene glycoside from the North Atlantic commercially harvested sea cucumber Cucumaria frondosa. J. Med. Food 2008, 11, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Aminin, D.L.; Koy, C.; Dmitrenok, P.S.; Müller-Hilke, B.; Koczan, D.; Arbogast, B.; Silchenko, A.A.; Kalinin, V.I.; Avilov, S.A.; Stonik, V.A.; et al. Immunomodulatory effects of holothurian triterpene glycosides on mammalian splenocytes determined by mass spectrometric proteome analysis. J. Proteom. 2009, 72, 886–906. [Google Scholar] [CrossRef] [PubMed]
- Girard, M.; Bélanger, J.; ApSimon, J.W.; Garneau, F.-X.; Harvey, C.; Brisson, J.R. Frondoside A. A novel triterpene glycoside from the holothurian Cucumaria frondosa. Can. J. Chem. 1990, 68, 11–18. [Google Scholar]
- Chen, W.H.; Horoszewicz, J.S.; Leong, S.S.; Shimano, T.; Penetrante, R.; Sanders, W.H.; Berjian, R.; Douglass, H.O.; Martin, E.W.; Chu, T.M. Human pancreatic adenocarcinoma: In vitro and in vivo morphology of a new tumor line established from ascites. In Vitro 1982, 18, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Iwamura, T.; Taniguchi, S.; Kitamura, N.; Yamanari, H.; Kojima, A.; Hidaka, K.; Setoguchi, T.; Katsuki, T. Correlation between CA19-9 production in vitro and histological grades of differentiation in vivo in clones isolated from a human pancreatic cancer cell line (SUIT-2). J. Gastroenterol. Hepatol. 1992, 7, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.G.; Ding, X.-Z.; Hennig, R.; Witt, R.C.; Standop, J.; Pour, P.M.; Adrian, T.E. Leukotriene B4 receptor antagonist LY293111 inhibits proliferation and induces apoptosis in human pancreatic cancer cells. Clin. Cancer Res. 2002, 8, 3232–3242. [Google Scholar] [PubMed]
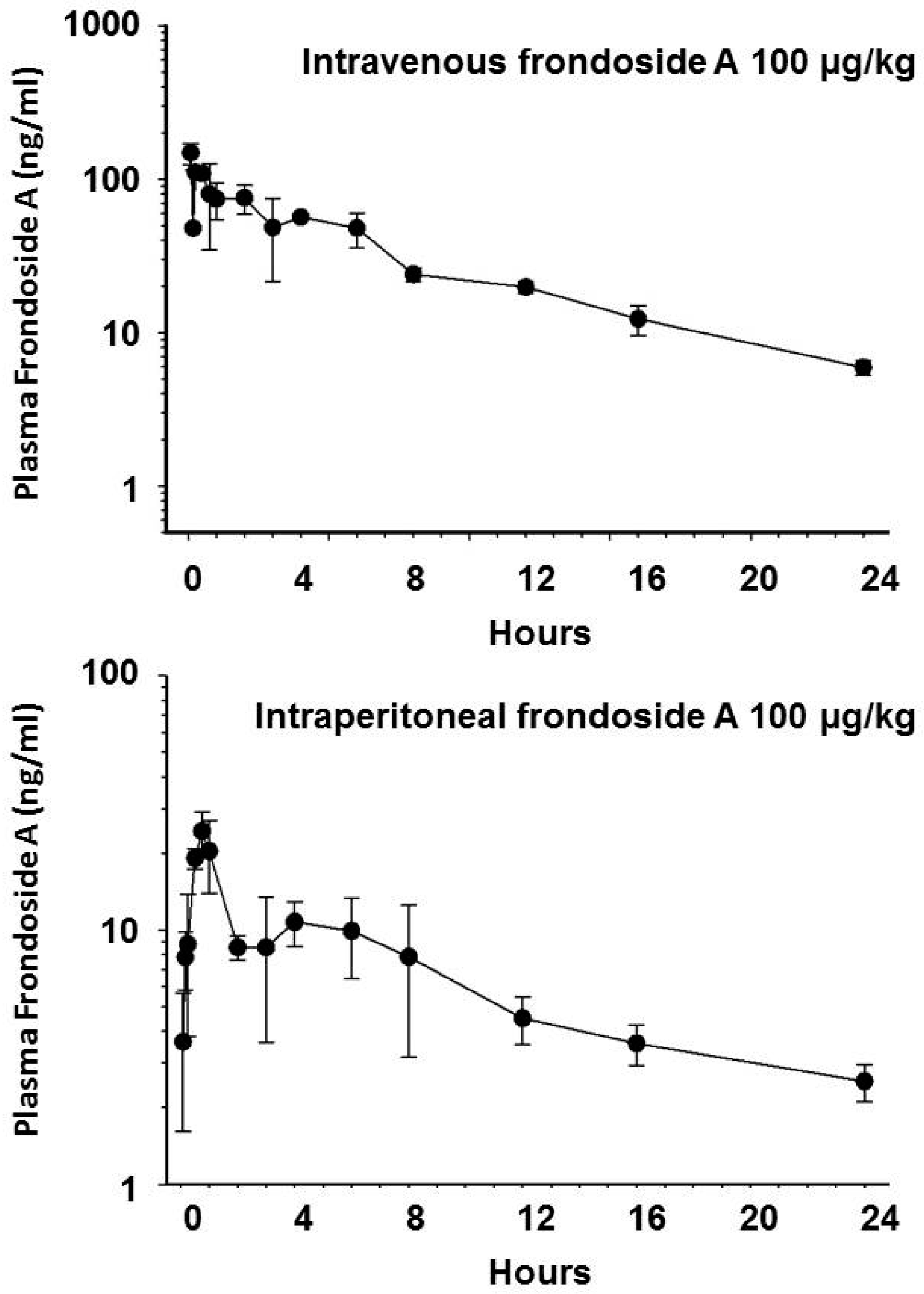
| Parameter | IP Bolus | IV Bolus |
|---|---|---|
| Area under curve (AUC) µg/L × min | 9984 | 47,220 |
| Total body clearance (Cltb) mL/min/m2 | 127 | 6.35 |
| Maximum plasma concentration (Cpmax) nM | 18.3 | 129 |
| Bioavailability (%) | 20 | 100 |
| Apparent volume of distribution (L/m2) | 28 | - |
| Volume of distribution (L/m2) | - | 0.87 |
| Half-life γ (T½ γ) minutes | 840 | 510 |
| Half-life α (T½ α: distribution phase) minutes | - | 2 |
| Half-life β (T½ β elimination phase) minutes | - | 158 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Shemaili, J.; Parekh, K.A.; Newman, R.A.; Hellman, B.; Woodward, C.; Adem, A.; Collin, P.; Adrian, T.E. Pharmacokinetics in Mouse and Comparative Effects of Frondosides in Pancreatic Cancer. Mar. Drugs 2016, 14, 115. https://doi.org/10.3390/md14060115
Al Shemaili J, Parekh KA, Newman RA, Hellman B, Woodward C, Adem A, Collin P, Adrian TE. Pharmacokinetics in Mouse and Comparative Effects of Frondosides in Pancreatic Cancer. Marine Drugs. 2016; 14(6):115. https://doi.org/10.3390/md14060115
Chicago/Turabian StyleAl Shemaili, Jasem, Khatija A. Parekh, Robert A. Newman, Björn Hellman, Carl Woodward, Abdu Adem, Peter Collin, and Thomas E. Adrian. 2016. "Pharmacokinetics in Mouse and Comparative Effects of Frondosides in Pancreatic Cancer" Marine Drugs 14, no. 6: 115. https://doi.org/10.3390/md14060115
APA StyleAl Shemaili, J., Parekh, K. A., Newman, R. A., Hellman, B., Woodward, C., Adem, A., Collin, P., & Adrian, T. E. (2016). Pharmacokinetics in Mouse and Comparative Effects of Frondosides in Pancreatic Cancer. Marine Drugs, 14(6), 115. https://doi.org/10.3390/md14060115