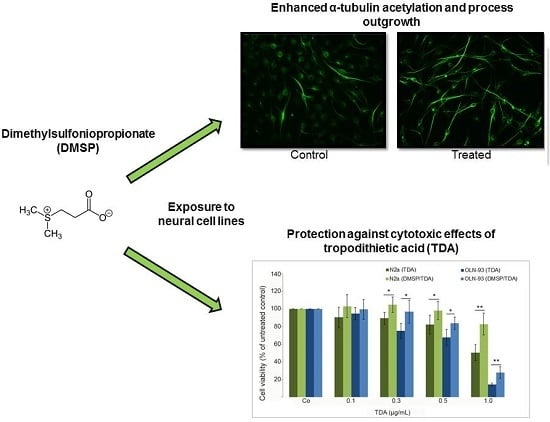
Dimethylsulfoniopropionate Promotes Process Outgrowth in Neural Cells and Exerts Protective Effects against Tropodithietic Acid
Abstract
:1. Introduction
2. Results and Discussion
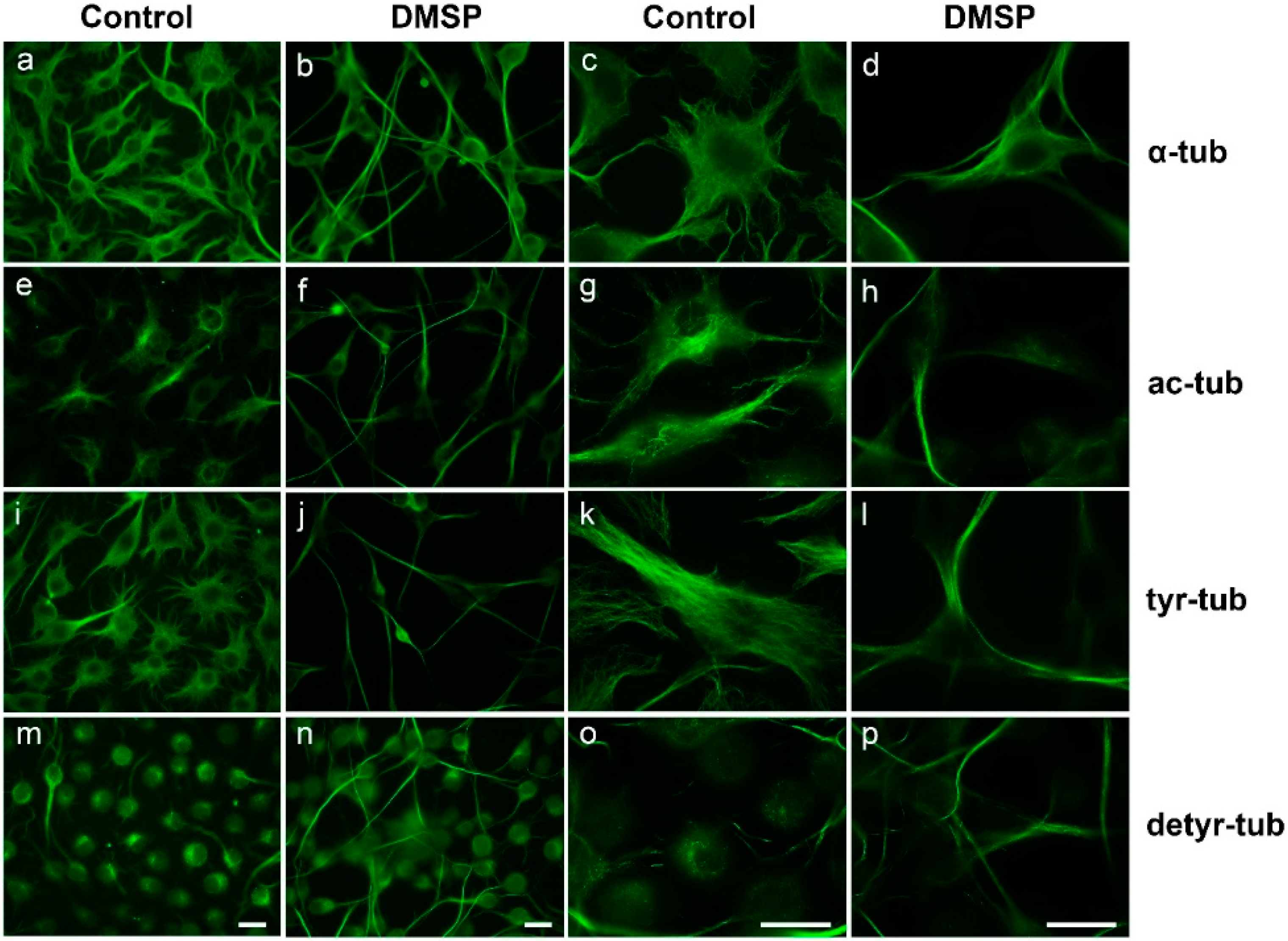
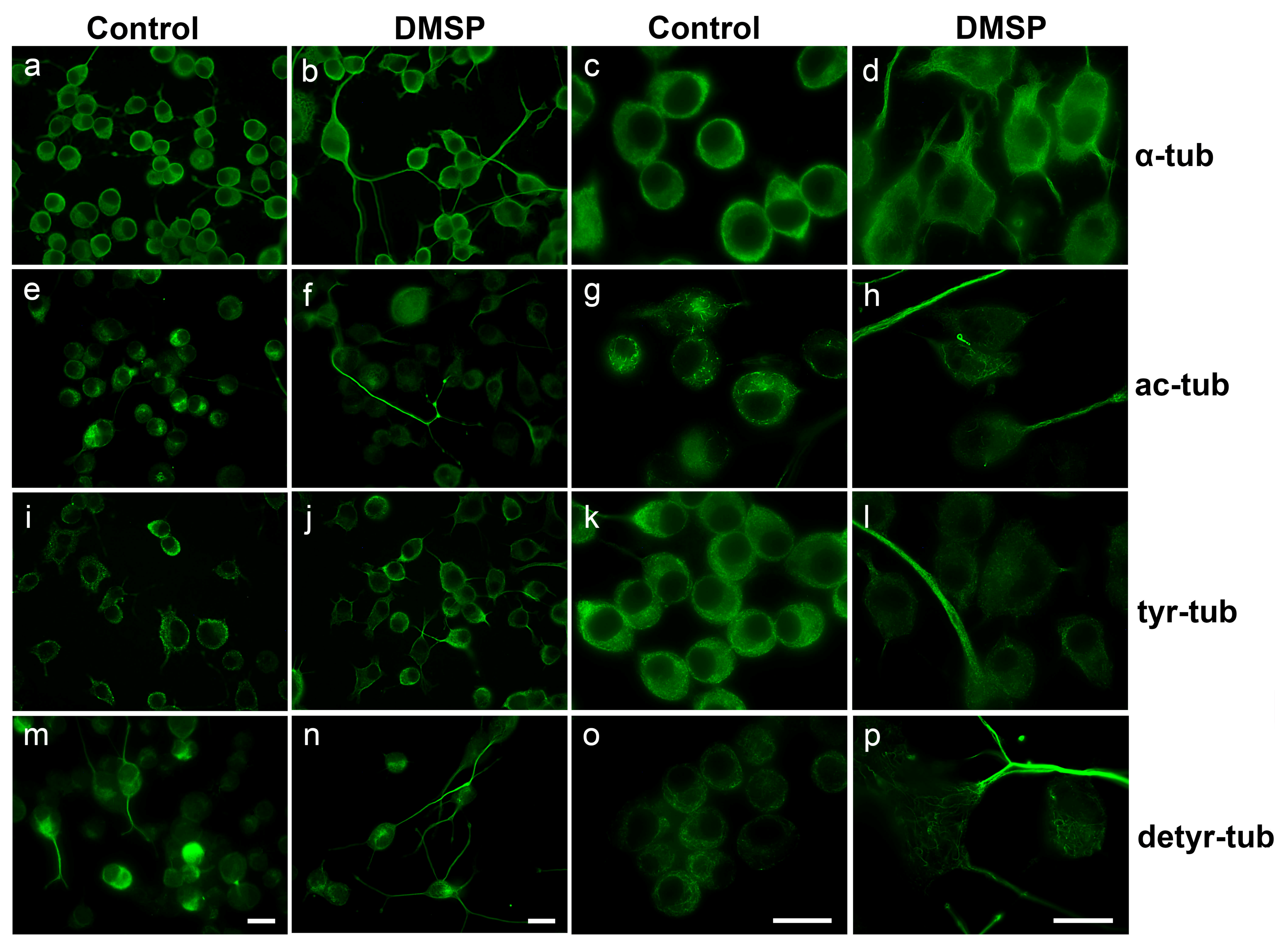
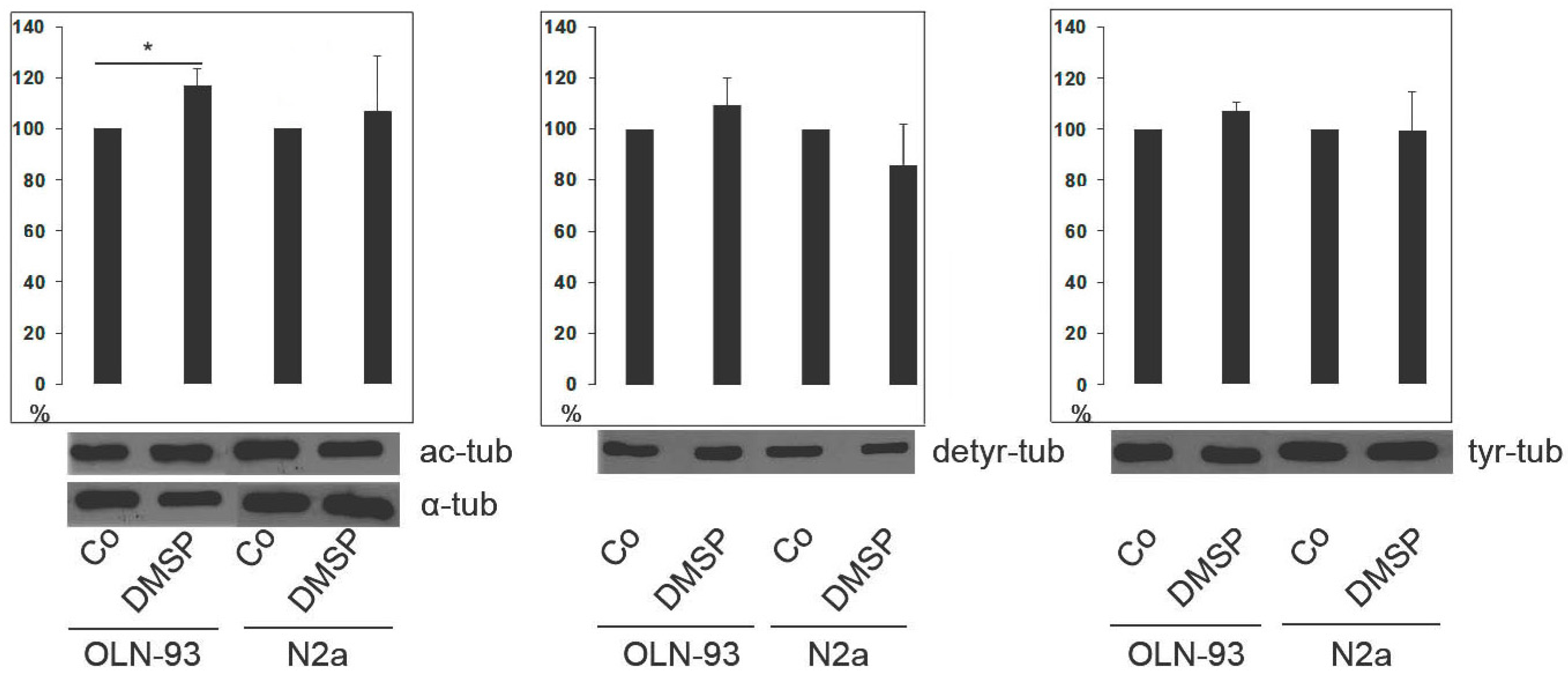
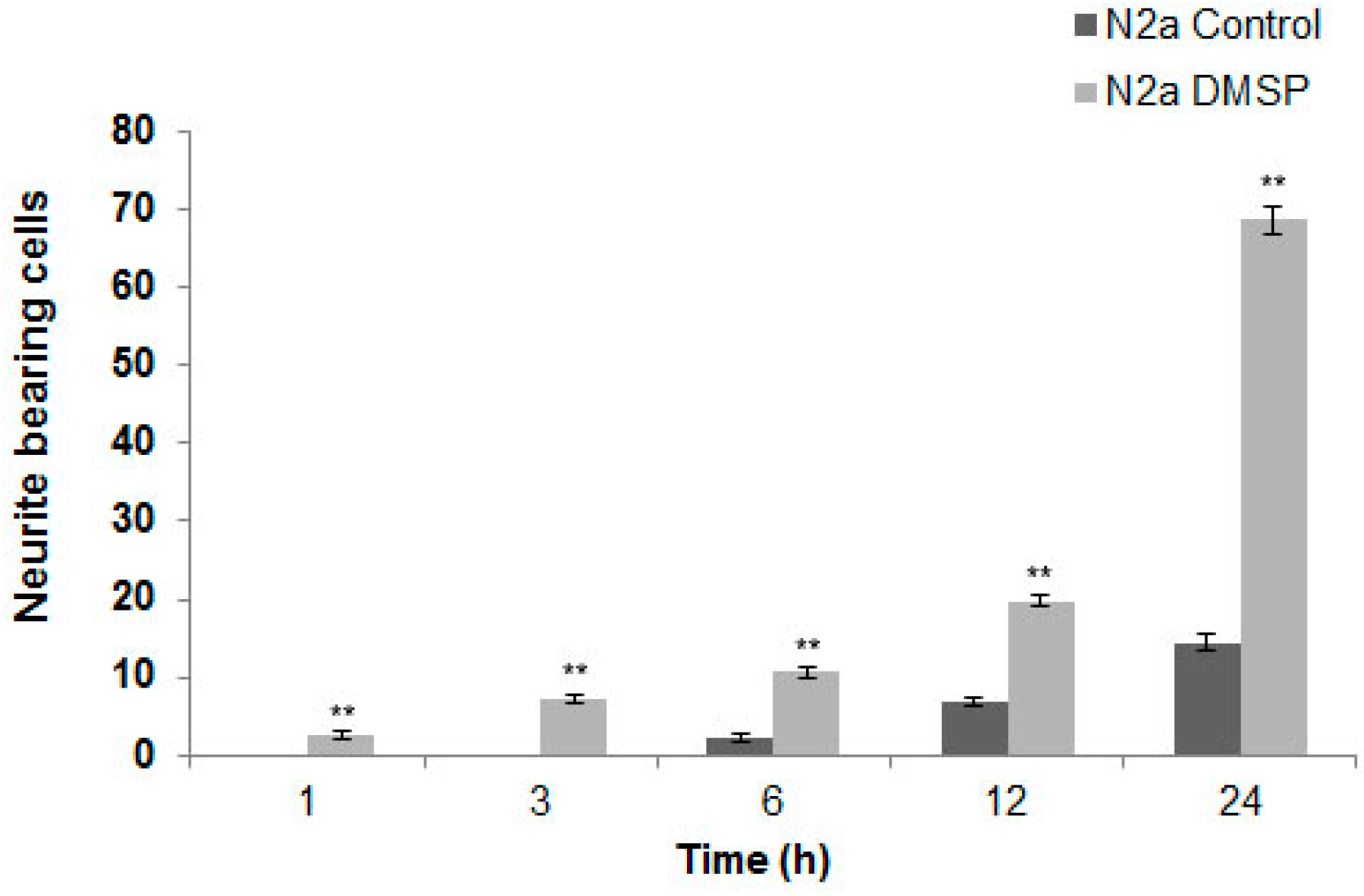
2.1. DMSP Induces Process Outgrowth, Microtubule Reorganization and Bundling
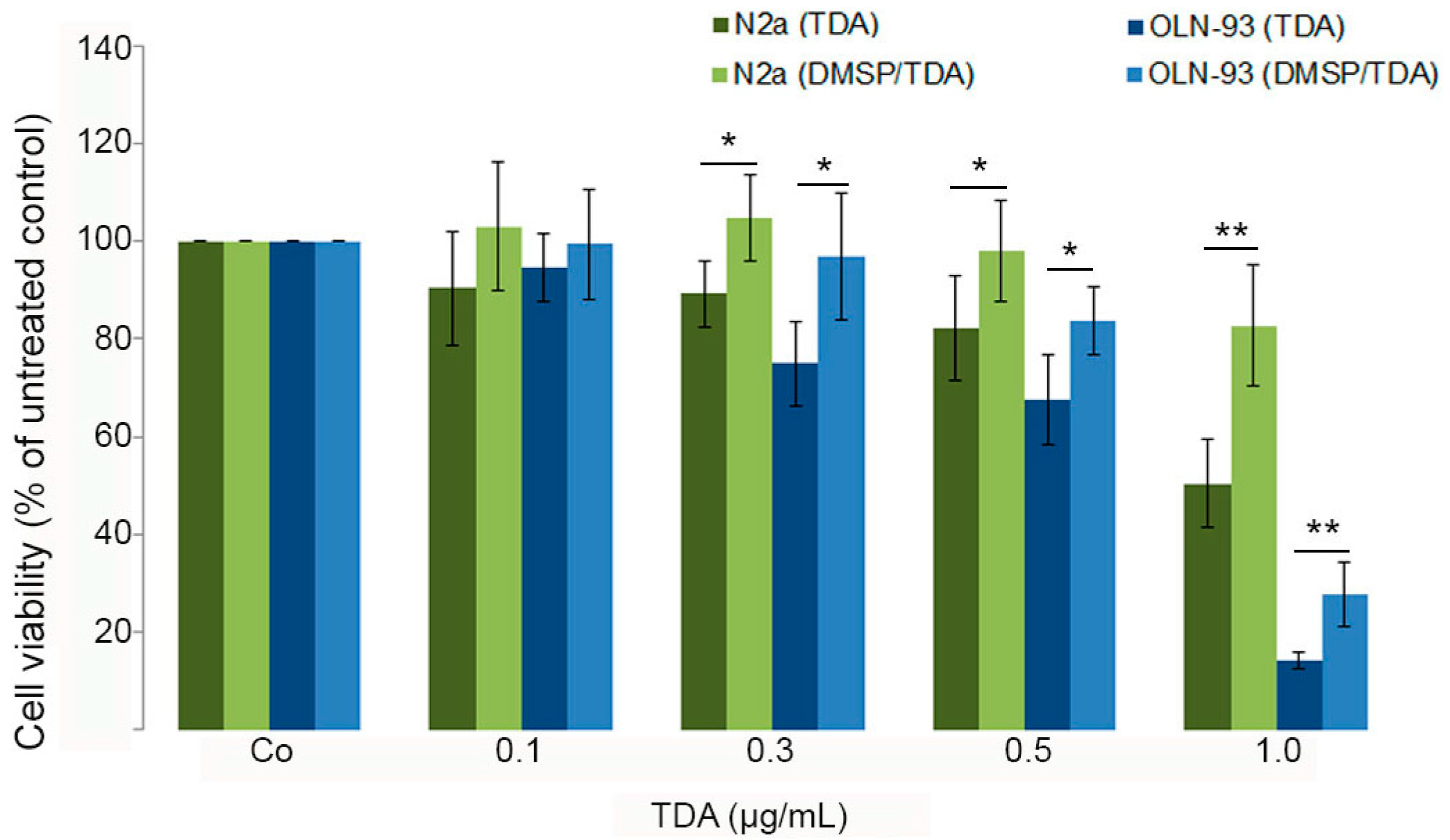
2.2. DMSP Protects Neural Cells against Cytotoxic Effects Exerted by Tropodithietic Acid
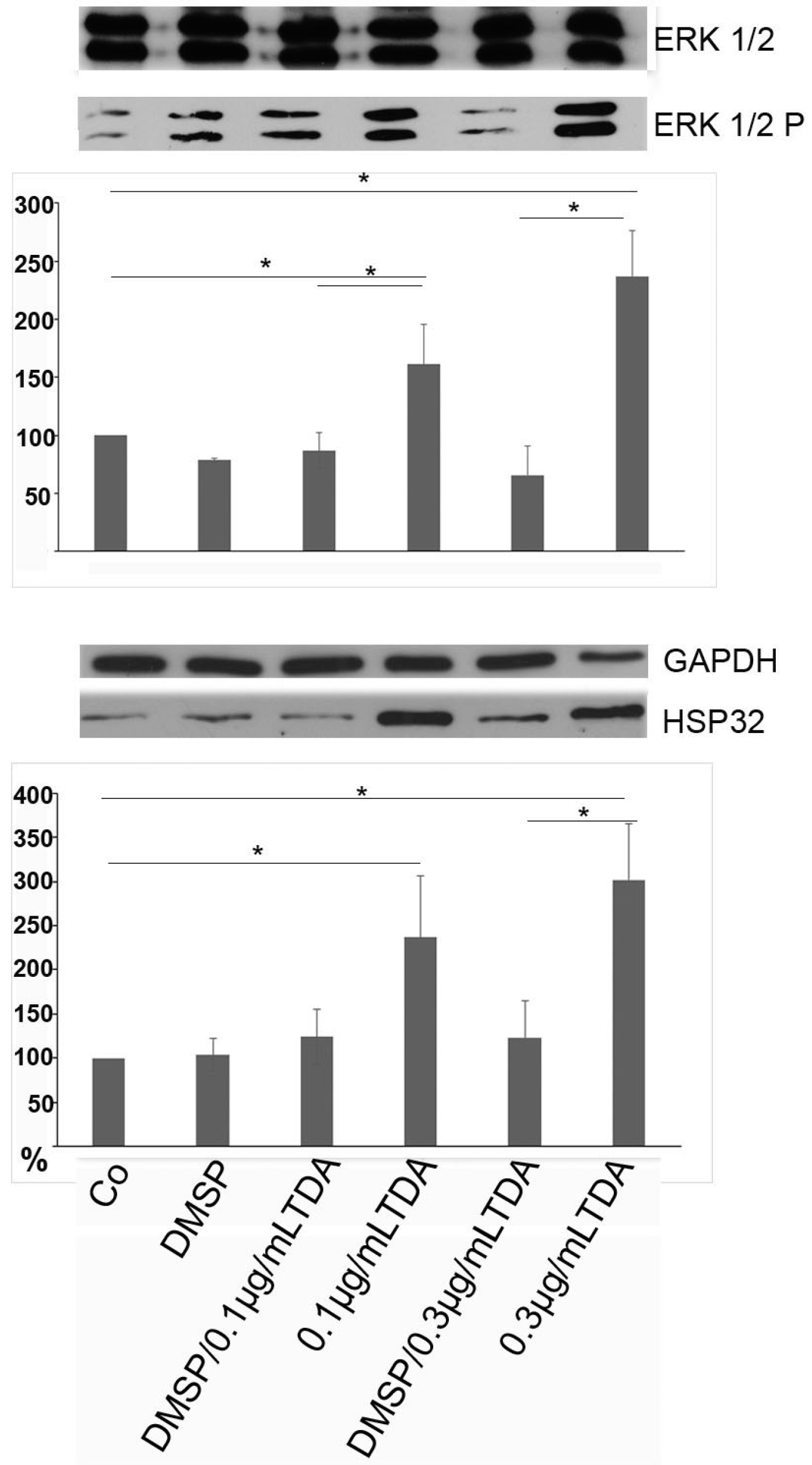
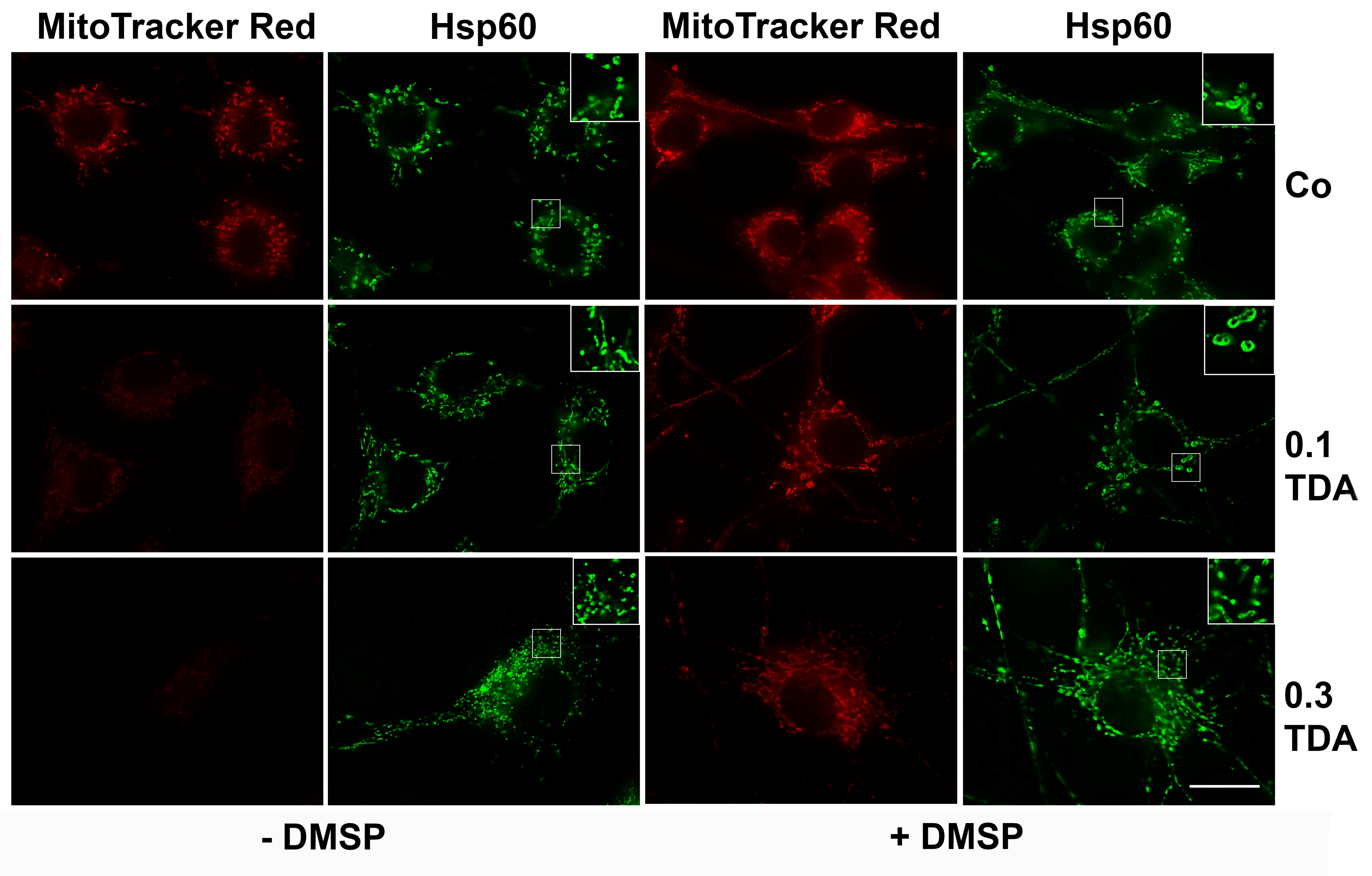
2.3. DMSP Suppresses TDA Induced Stress Responses and Mitochondrial Damage
3. Experimental Section
3.1. Materials and Antibodies
3.2. Cell Culture
3.3. Immunoblot Analysis
3.4. Mitochondrial Staining
3.5. Indirect Immunofluorescence
3.6. MTT-Viability Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sakai, R.; Swanson, G.T. Recent progress in neuroactive marine natural products. Nat. Prod. Rep. 2014, 31, 273–309. [Google Scholar] [CrossRef] [PubMed]
- Gerwick, W.H.; Moore, B.S. Lessons from the past and charting the future of marine natural products drug discovery and chemical biology. Chem. Biol. 2012, 19, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Essa, M.M.; Vijayan, R.K.; Castellano-Gonzalez, G.; Memon, M.A.; Braidy, N.; Guillemin, G.J. Neuroprotective effect of natural products against Alzheimer’s disease. Neurochem. Res. 2012, 37, 1829–1842. [Google Scholar] [CrossRef] [PubMed]
- Grosso, C.; Valentao, P.; Ferreres, F.; Andrade, P.B. Bioactive marine drugs and marine biomaterials for brain diseases. Mar. Drugs 2014, 12, 2539–2589. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.; Valentao, P.; Andrade, P.B. Bioactive compounds from macroalgae in the new millennium: Implications for neurodegenerative diseases. Mar. Drugs 2014, 12, 4934–4972. [Google Scholar] [CrossRef] [PubMed]
- Gammone, M.; Riccioni, G.; D’Orazio, N. Marine carotenoids against oxidative stress: Effects on human health. Mar. Drugs 2015, 13, 6226–6246. [Google Scholar] [CrossRef] [PubMed]
- Buchan, A.; González, J.M.; Moran, M.A. Overview of the Marine Roseobacter Lineage. Appl. Environ. Microbiol. 2005, 71, 5665–5677. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Moran, M.A. Evolutionary ecology of the marine Roseobacter clade. Microbiol. Mol. Biol. Rev. 2014, 78, 573–587. [Google Scholar] [CrossRef] [PubMed]
- Raina, J.-B.; Tapiolas, D.M.; Forêt, S.; Lutz, A.; Abrego, D.; Ceh, J.; Seneca, F.O.; Clode, P.L.; Bourne, D.G.; Willis, B.L. DMSP biosynthesis by an animal and its role in coral thermal stress response. Nature 2013, 502, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Alcolombri, U.; Ben-Dor, S.; Feldmesser, E.; Levin, Y.; Tawfik, D.S.; Vardi, A. MARINE SULFUR CYCLE. Identification of the algal dimethyl sulfide-releasing enzyme: A missing link in the marine sulfur cycle. Science 2015, 348, 1466–1469. [Google Scholar] [CrossRef] [PubMed]
- Yoch, D.C. Dimethylsulfoniopropionate: Its sources, role in the marine food web, and biological degradation to dimethylsulfide. Appl. Environ. Microbiol. 2002, 12, 5804–5815. [Google Scholar] [CrossRef]
- Sunda, W.; Kieber, D.J.; Kiene, R.P.; Huntsman, S. An antioxidant function for DMSP and DMS in marine algae. Nature 2002, 418, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K. Effects of DMSP and related compounds on behavior, growth and stress resistance of fish, amphibians and crustaceans. In Biological and Environmental Chemistry of Dmsp and Related Sulphonium Compounds; Kiene, R.P., Visscher, P.T., Keller, M.D., Kirst, G.O., Eds.; Plenum Press: New York, NY, USA, 1996; pp. 165–176. [Google Scholar]
- Nakajima, K. Amelioration effect of a tertiary sulfonium compound, dimethylsulfoniopropionate, in green sea algae on Ehrlich ascitic-tumor, solid tumor and related diseases. In Handbook for Anticancer Drugs of Marine Origin; Se-Kwon, K., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 205–238. [Google Scholar]
- Nakajima, K.; Miyamoto, Y. Effects of nerve growth factor and dimethylsulfoniopropionate in green sea algae on the outgrowth of neurites from pheochromocytoma cells. J. Nutr. Sci. Vitaminol. 2007, 53, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Minematsu, M.; Miyamoto, Y. Inhibition of the outgrowth and elongation of neurites from pheochromocytoma cells by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and preventive effects of dimethylsulfoniopropionate in the presence of nerve growth factor. J. Nutr. Sci. Vitaminol. 2008, 54, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Palyanova, N.; Pankova, T.; Starostina, M.; Kicha, A.; Ivanchina, N.; Stonik, V. Neuritogenic and neuroprotective effects of polar steroids from the far east starfishes Patiria pectinifera and Distolasterias nipon. Mar. Drugs 2013, 11, 1440–1455. [Google Scholar] [CrossRef] [PubMed]
- More, S.V.; Koppula, S.; Kim, I.-S.; Kumar, H.; Kim, B.-W.; Choi, D.-K. The role of bioactive compounds on the promotion of neurite outgrowth. Molecules 2012, 17, 6728–6753. [Google Scholar] [CrossRef] [PubMed]
- Larpthaveesarp, A.; Ferriero, D.M.; Gonzalez, F.F. Growth factors for the treatment of ischemic brain injury (growth factor treatment). Brain Sci. 2015, 5, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Deister, C.; Schmidt, C.E. Optimizing neurotrophic factor combinations for neurite outgrowth. J. Neural Eng. 2006, 2, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lacoske, M.H.; Theodorakis, E.A. Neurotrophic natural products: Chemistry and Biology. Angew. Chem. Int. Ed. Engl. 2014, 53, 956–987. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, R.G.; Sikorska, M.; Sandhu, J.K.; Lanthier, P.; Ribecco-Lutkiewicz, M.; Bani-Yaghoub, M. Differentiation of mouse Neuro 2A cells into dopamine neurons. J. Neurosci. Methods 2010, 186, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Richter-Landsberg, C.; Heinrich, M. OLN-93: A new permanent oligodendroglia cell line derived from primary rat brain glial cultures. J. Neurosci. Res. 1996, 45, 161–173. [Google Scholar] [CrossRef]
- Brinkhoff, T.; Bach, G.; Heidorn, T.; Liang, L.; Schlingloff, A.; Simon, M. Antibiotic production by a Roseobacter clade-affiliated species from the German Wadden Sea and its antagonistic effects on indigenous isolates. Appl. Environ. Microbiol. 2004, 70, 2560–2565. [Google Scholar] [CrossRef] [PubMed]
- Martens, T.; Gram, L.; Grossart, H.P.; Kessler, D.; Muller, R.; Simon, M.; Wenzel, S.C.; Brinkhoff, T. Bacteria of the Roseobacter clade show potential for secondary metabolite production. Microb. Ecol. 2007, 54, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Neumann, A.; Schulz, S.; Simon, M.; Brinkhoff, T. Tropodithietic acid production in Phaeobacter gallaeciensis is regulated by N-acyl homoserine lactone-mediated quorum sensing. J. Bacteriol. 2011, 193, 6576–6585. [Google Scholar] [CrossRef] [PubMed]
- Seyedsayamdost, M.R.; Case, R.J.; Kolter, R.; Clardy, J. The Jekyll-and-Hyde chemistry of Phaeobacter gallaeciensis. Nat. Chem. 2011, 3, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Harrington, C.; Reen, F.J.; Mooij, M.J.; Stewart, F.A.; Chabot, J.B.; Guerra, A.F.; Glockner, F.O.; Nielsen, K.F.; Gram, L.; Dobson, A.D. Characterisation of non-autoinducing tropodithietic acid (TDA) production from marine sponge Pseudovibrio species. Mar. Drugs 2014, 12, 5960–5978. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.; Belas, R. Expression of tropodithietic acid biosynthesis is controlled by a novel autoinducer. J. Bacteriol. 2010, 192, 4377–4387. [Google Scholar] [CrossRef] [PubMed]
- Liang, L. Investigation of Secondary Metabolites of North Sea Bacteria: Fermentation, Isolation, Structure Elucidation and Bioactivity. Ph.D. Thesis, University of Göttingen, Göttingen, Germany, 2003. [Google Scholar]
- Wilson, M.Z.; Wang, R.; Gitai, Z.; Seyedsayamdost, M.R. Mode of action and resistance studies unveil new roles for tropodithietic acid as an anticancer agent and the gamma-glutamyl cycle as a proton sink. Proc. Natl. Acad. Sci. USA 2016, 6, 1630–1635. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, H.; Vocke, F.; Brinkhoff, T.; Simon, M.; Richter-Landsberg, C. Cytotoxic effects of tropodithietic acid on mammalian clonal cell lines of neuronal and glial origin. Mar. Drugs 2015, 13, 7113–7123. [Google Scholar] [CrossRef] [PubMed]
- Stahnke, T.; Stadelmann, C.; Netzler, A.; Bruck, W.; Richter-Landsberg, C. Differential upregulation of heme oxygenase-1 (HSP32) in glial cells after oxidative stress and in demyelinating disorders. J. Mol. Neurosci. 2007, 32, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Alstyne, K.V. Ecological and physiological roles of dimethylsulfoniopropionate (DMSP) and its DMSP cleavage in marine macroalgae. In Algal Chemical Ecology; Springer: Berlin, Germany, 2008; pp. 173–194. [Google Scholar]
- Richter-Landsberg, C.; Vollgraf, U. Mode of cell injury and death after hydrogen exposure in cultured oligodendroglia cells. Exp. Cell Res. 1998, 244, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Vollgraf, U.; Wegner, M.; Richter-Landsberg, C. Activation of AP-1 and nuclear factor-kappaB transcription factors is involved in hydrogen peroxide-induced apoptotic cell death of oligodendrocytes. J. Neurochem. 1999, 73, 2501–2509. [Google Scholar] [CrossRef] [PubMed]
- Janke, C.; Bulinski, J.C. Post-translational regulation of the microtubule cytoskeleton: Mechanisms and functions. Nat. Rev. Mol. Cell Biol. 2011, 12, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Janke, C.; Kneussel, M. Tubulin post-translational modifications: Encoding functions on the neuronal microtubule cytoskeleton. Trends Neurosci. 2010, 33, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Howes, S.C.; Alushin, G.M.; Shida, T.; Nachury, M.V.; Nogales, E. Effects of tubulin acetylation and tubulin acetyltransferase binding on microtubule structure. Mol. Biol. Cell 2014, 25, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Brady, S.T. Post-translational modifications of tubulin: Pathways to functional diversity of microtubules. Trends Cell Biol. 2015, 25, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Wienecke, A.; Bacher, G. Indibulin, a novel microtubule inhibitor, discriminates between mature neuronal and nonneuronal tubulin. Cancer Res. 2009, 69, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Black, M.M.; Keyser, P. Acetylation of alpha-tubulin in cultured neurons and the induction of alpha-tubulin acetylation in PC12 cells by treatment with nerve growth factor. J. Neurosci. 1987, 7, 1833–1842. [Google Scholar] [PubMed]
- Cappello, F.; Conway de Macario, E.; Marasa, L.; Zummo, G.; Macario, A.J. Hsp60 expression, new locations, functions and perspectives for cancer diagnosis and therapy. Cancer Biol. Ther. 2008, 7, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Chandra, D.; Choy, G.; Tang, D.G. Cytosolic accumulation of HSP60 during apoptosis with or without apparent mitochondrial release: Evidence that its pro-apoptotic or pro-survival functions involve differential interactions with caspase-3. J. Biol. Chem. 2007, 282, 31289–31301. [Google Scholar] [CrossRef] [PubMed]
- Doughman, S.D.; Krupanidhi, S.; Sanjeevi, C.B. Omega-3 fatty acids for nutrition and medicine: Considering microalgae oil as a vegetarian source of EPA and DHA. Curr. Diabetes Rev. 2007, 3, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Xue, R.; Xu, J.; Liu, Z. Effects of docosahexaenoic acid on the survival and neurite outgrowth of rat cortical neurons in primary cultures. J. Nutr. Biochem. 2005, 16, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, J. Docosahexaenoic acid (DHA): An ancient nutrient for the modern human brain. Nutrients 2011, 3, 529–554. [Google Scholar] [CrossRef] [PubMed]
- Seidl, S.E.; Santiago, J.A.; Bilyk, H.; Potashkin, J.A. The emerging role of nutrition in Parkinson’s disease. Front. Aging Neurosci. 2014, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Yu, J.T.; Tan, L.; Wang, Y.L.; Sun, L. Nutrition and the risk of Alzheimer’s disease. Biomed Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Vina, J.; Lloret, A.; Giraldo, E.; Badia, M.C.; Alonso, M.D. Antioxidant pathways in Alzheimer’s disease: Possibilities of intervention. Curr. Pharm. Des. 2011, 17, 3861–3864. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ichikawa, S.; Tani, C.; Zhu, B.; Tada, M.; Shimoishi, Y.; Murata, Y.; Nakamura, Y. Docosahexaenoic acid induces ERK1/2 activation and neuritogenesis via intracellular reactive oxygen species production in human neuroblastoma SH-SY5Y cells. Biochim. Biophys. Acta 2009, 1791, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Bai, L.; Zhu, L.; Yang, L.; Zhang, X. Marine algae-derived bioactive peptides for human nutrition and health. J. Agric. Food Chem. 2014, 62, 9211–9222. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Kim, S.K. Neuroprotective effects of marine algae. Mar. Drugs 2011, 9, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Neuhoff, V.; Philipp, K.; Zimmer, H.G.; Mesecke, S. A simple, versatile, sensitive and volume-independent method for quantitative protein determination which is independent of other external influences. Physiol. Chem. 1979, 360, 1657–1670. [Google Scholar] [CrossRef]
- Rose, S.E.; Chalk, J.B.; Galloway, G.J.; Doddrell, D.M. Detection of dimethyl sulfone in the human brain by in vivo proton magnetic resonance spectroscopy. Magn. Reson. Imaging 2000, 18, 95–98. [Google Scholar] [CrossRef]
- Ziesche, L.; Bruns, H.; Dogs, M.; Wolter, L.; Mann, F.; Wagner-Döbler, I.; Brinkhoff, T.; Schulz, S. Homoserine lactones, methyl oligohydroxybutyrates, and other extracellular metabolites of macroalgae-associated bacteria of the Roseobacter Clade: Identification and functions. ChemBioChem 2015, 16, 2094–2107. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wichmann, H.; Brinkhoff, T.; Simon, M.; Richter-Landsberg, C. Dimethylsulfoniopropionate Promotes Process Outgrowth in Neural Cells and Exerts Protective Effects against Tropodithietic Acid. Mar. Drugs 2016, 14, 89. https://doi.org/10.3390/md14050089
Wichmann H, Brinkhoff T, Simon M, Richter-Landsberg C. Dimethylsulfoniopropionate Promotes Process Outgrowth in Neural Cells and Exerts Protective Effects against Tropodithietic Acid. Marine Drugs. 2016; 14(5):89. https://doi.org/10.3390/md14050089
Chicago/Turabian StyleWichmann, Heidi, Thorsten Brinkhoff, Meinhard Simon, and Christiane Richter-Landsberg. 2016. "Dimethylsulfoniopropionate Promotes Process Outgrowth in Neural Cells and Exerts Protective Effects against Tropodithietic Acid" Marine Drugs 14, no. 5: 89. https://doi.org/10.3390/md14050089
APA StyleWichmann, H., Brinkhoff, T., Simon, M., & Richter-Landsberg, C. (2016). Dimethylsulfoniopropionate Promotes Process Outgrowth in Neural Cells and Exerts Protective Effects against Tropodithietic Acid. Marine Drugs, 14(5), 89. https://doi.org/10.3390/md14050089