Assignment of the CD Cotton Effect to the Chiral Center in Pseurotins, and the Stereochemical Revision of Pseurotin A2
Abstract
:1. Introduction
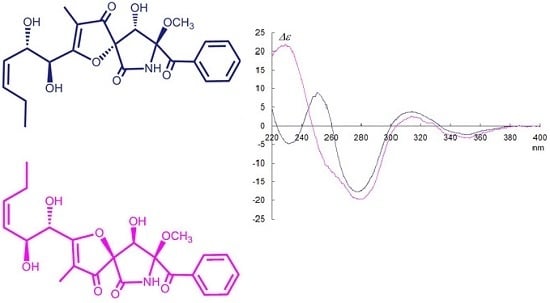
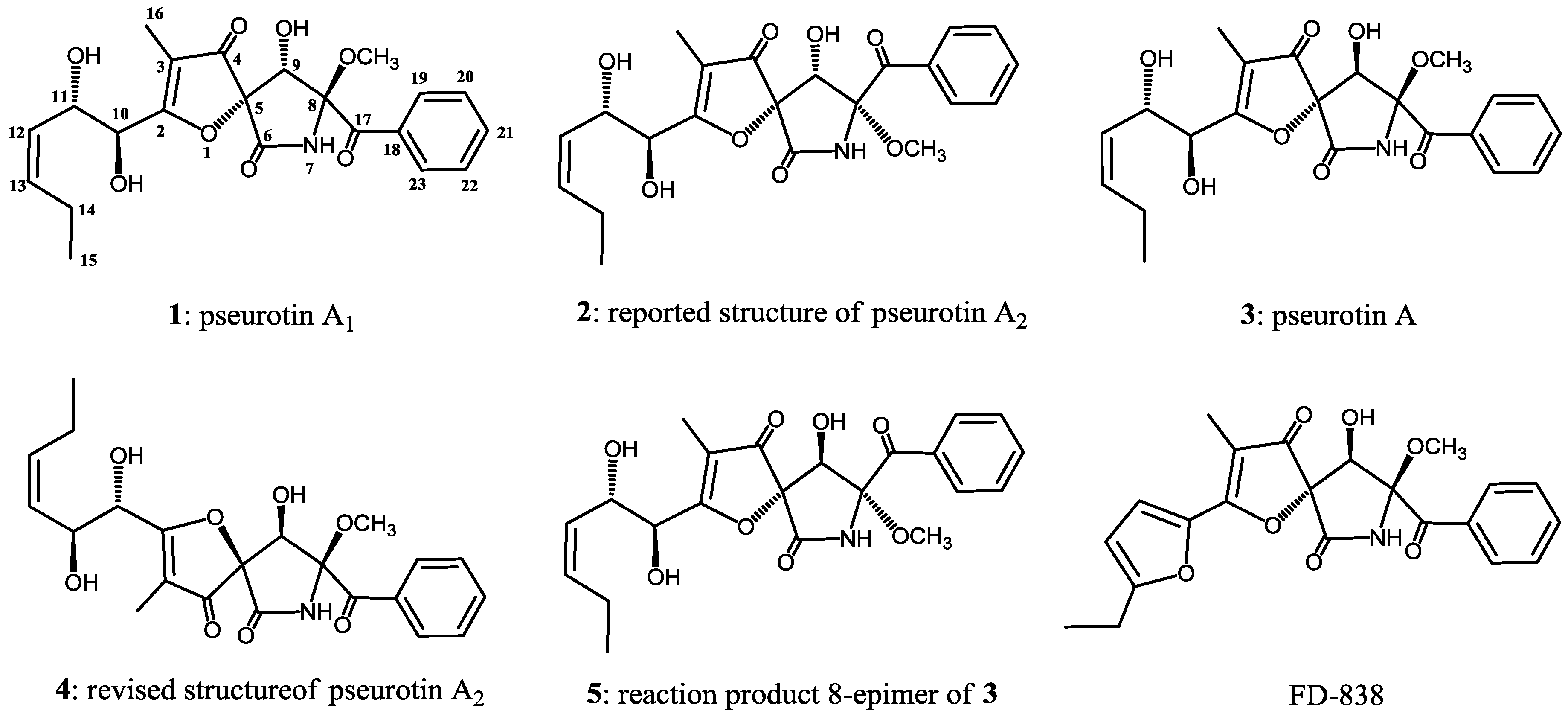
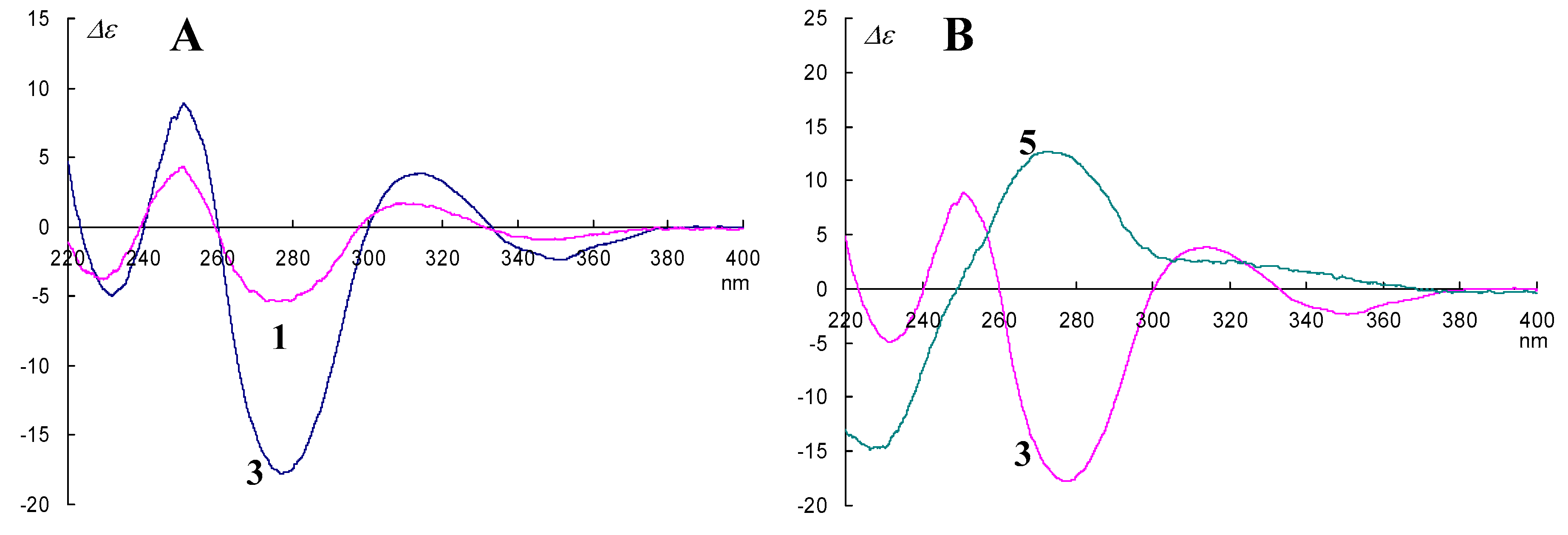
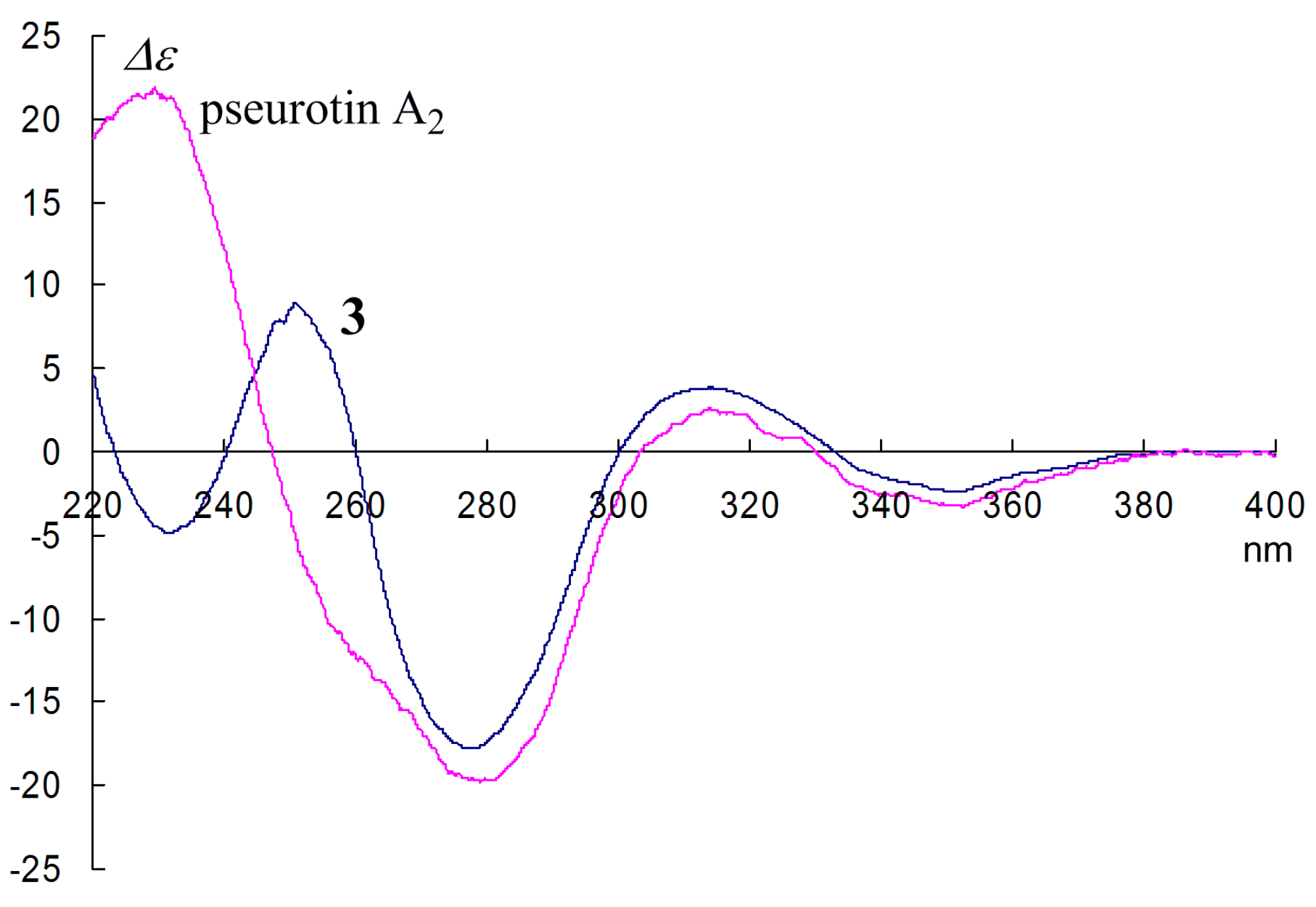
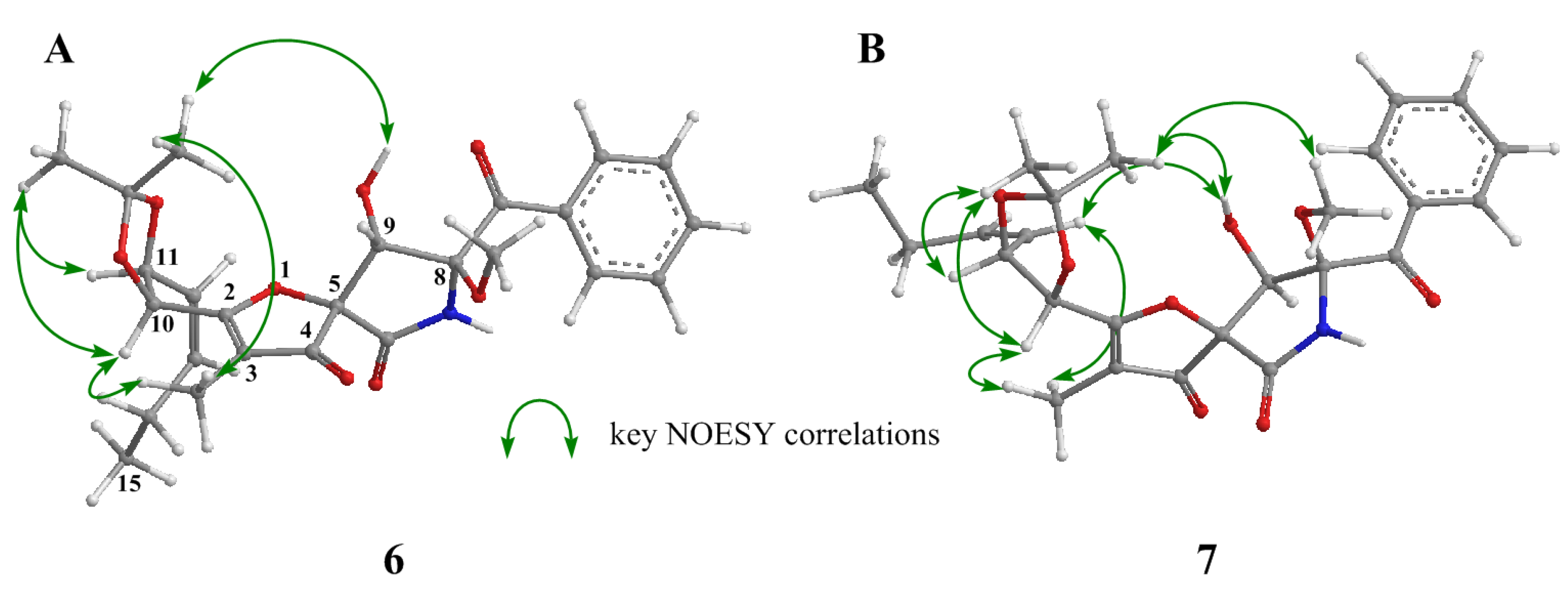
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Culturing and Isolation of Metabolites
3.4. Chemical Transformation
3.4.1. Epimerization of 3
3.4.2. Derivatization to Acetonides from Pseurotin A1 (1) and A2 (4)
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bloch, P.; Tamm, C.; Bollinger, P.; Petcher, T.J.; Weber, H.P. Pseurotin, a New Metabolite of Pseudeurotium ovalis STOLK Having an Unusual Hetero-Spirocyclic System. Helv. Chim. Acta 1976, 59, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Weber, H.P.; Petcher, T.J.; Bloch, P.; Tamm, C. The Crystal and Molecular Structure of 12, 13-Dibromopseurotin. Helv. Chim. Acta 1976, 59, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Bloch, P.; Tamm, C. Isolation and Structure of Pseurotin A, a Microbial Metabolite of Pseudeurotium ovalis STOLK with an Unusual Heterospirocyclic System. Helv. Chim. Acta 1981, 64, 304–315. [Google Scholar] [CrossRef]
- Ishikawa, M.; Ninomiya, T.; Akabane, H.; Kushida, N.; Tsujiuchi, G.; Ohyama, M.; Gomi, S.; Shito, K.; Murata, T. Pseurotin A and its analogues as inhibitors of immunoglobuline E production. Bioorg. Med. Chem. Lett. 2009, 19, 1457–1460. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Shoji, M.; Yamaguchi, J.; Sato, K.; Yamaguchi, S.; Mukaiyama, T.; Sakai, K.; Asami, Y.; Kakeya, H.; Osada, H. Asymmetric Total Synthesis of (−)-Azaspirene, a Novel Angiogenesis Inhibitor. J. Am. Chem. Soc. 2002, 124, 12078–12079. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.; Oi, T.; Shimizu, K.; Shiraki, R.; Takao, K.; Tadano, K. Total Syntheses of Natural Pseurotins A, F2, and Azaspirene. Bull. Chem. Soc. Jpn. 2004, 77, 1703–1716. [Google Scholar] [CrossRef]
- Hayashi, Y.; Shoji, M.; Yamaguchi, S.; Mukaiyama, T.; Yamaguchi, J.; Kakeya, H.; Osada, H. Asymmetric Total Synthesis of Pseurotin A. Org. Lett. 2003, 5, 2287–2290. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Shoji, M.; Mukaiyama, T.; Gotoh, H.; Yamaguchi, S.; Nakata, M.; Kakeya, H.; Osada, H. First Asymmetric Total Synthesis of Synerazol, an Antifungal Antibiotic, and Determination of Its Absolute Stereochemistry. J. Org. Chem. 2005, 70, 5643–5654. [Google Scholar] [CrossRef] [PubMed]
- Orellana, A.; Rovis, T. Towards the total synthesis of FD-838: Modular enantioselective assembly of the core. Chem. Commun. 2008, 730–732. [Google Scholar] [CrossRef]
- Hayashi, Y.; Sankar, K.; Ishikawa, H.; Nozawa, Y.; Mizoue, K.; Kakeya, H. Total synthesis and determination of the absolute configuration of FD-838, a naturally occurring azaspirobicyclic product. Bioorg. Med. Chem. Lett. 2009, 19, 3863–3865. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, Y.; Yabuta, Y.; Furumai, T. Determination of the absolute configuration of synerazol. J. Antibiotics 2004, 57, 537–540. [Google Scholar] [CrossRef] [Green Version]
- Yamada, T.; Imai, E.; Nakatsuji, K.; Numata, A.; Tanaka, R. Cephalimysin A, a potent cytotoxic metabolite from an Aspergillus species separated from a marine fish. Tetrahedron Lett. 2007, 48, 6294–6296. [Google Scholar] [CrossRef]
- Yamada, T.; Kitada, H.; Kajimoto, T.; Numata, A.; Tanaka, R. The Relationship between the CD Cotton Effect and the Absolute Configuration of FD-838 and Its Seven Stereoisomers. J. Org. Chem. 2010, 75, 4146–4153. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.Z.; Li, D.H.; Zhu, T.J.; Zhang, M.; Gu, Q.Q. Pseurotin A1 and A2, two new 1-oxa-7-azaspiro[4.4]non-2-ene-4,6-diones from the holothurian-derived fungus Aspergillus fumigatus WFZ-25. Can. J. Chem. 2011, 89, 72–76. [Google Scholar] [CrossRef]
| Position | Pseurotin A1 (1) | Pseurotin A2 | 3 | 5 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| δH a | m, J/Hz | δC | δH a | m, J/Hz | δC | δH a | m, J/Hz | δC | δH a | m, J/Hz | δC | |||||
| 1 | ||||||||||||||||
| 2 | 183.4 | qC | 183.5 | qC | 186.0 | qC | 186.4, | qC | ||||||||
| 3 | 113.2 | qC | 114.3 | qC | 113.4 | qC | 114.8, | qC | ||||||||
| 4 | 196.2 | qC | 199.7 | qC | 196.5 | qC | 201.1, | qC | ||||||||
| 5 | 89.5 | qC | 87.3 | qC | 92.7 | qC | 86.5, | qC | ||||||||
| 6 | 169.4 | qC | 166.9 | qC | 166.8 | qC | 167.5, | qC | ||||||||
| 7 | 8.53 | s | 7.70 | s | 8.38 | s | 7.34 | s | ||||||||
| 8 | 96.5 | qC | 93.2 | qC | 90.5 | qC | 96.4, | qC | ||||||||
| 9 | 4.88 | s | 76.6 | CH | 4.42 | br d, 12.0 (9-OH) | 74.2 | CH | 4.69 | br s | 73.2 | CH | 4.86 | d, 5.4 (9-OH) | 78.6, | CH |
| 10 | 4.60 | br s | 70.5 | CH | 4.73 | d, 3.0 (11) | 70.1 | CH | 4.59 | d, 5.4 (11) | 70.7 | CH | 4.69 | br d, 5.4 (11) | 70.9, | CH |
| 11 | 4.76 | d, 7.8 (12) | 71.0 | CH | 4.94 | dd, 8.4 (12) 3.0 (10) | 70.6 | CH | 4.75 | dd, 10.8 (12) 5.4 (10) | 70.7 | CH | 4.81 | dd, 10.8 (12) 5.4 (10) | 69.6, | CH |
| 12 | 5.23 | dd, 10.8 (13) 7.8 (11) | 126.4 | CH | 5.28 | dd, 10.8 (13) 8.4 (11) | 125.3 | CH | 5.28 | dd, 11.2 (13) 10.8 (11) | 126.4 | CH | 5.43 | dd, 11.2 (13) 10.8 (11) | 125.6, | CH |
| 13 | 5.64 | dt, 10.8 (12) 7.2 (14) | 136.9 | CH | 5.64 | dt, 10.8 (12) 7.2 (14) | 137.4 | CH | 5.59 | dt, 11.2 (12) 7.8 (14) | 136.8 | CH | 5.74 | dt, 11.2 (12) 7.8 (14) | 138.2, | CH |
| 14A | 2.09 | m | 21.4 | CH2 | 2.14 | m | 21.4 | CH2 | 2.09 | m | 21.4 | CH2 | 2.15 | m | 21.5, | CH2 |
| 14B | 2.15 | m | 2.19 | m | 2.15 | m | 2.21 | m | ||||||||
| 15 | 0.99 | t, 7.8 (14) | 14.1 | CH3 | 1.03 | t, 7.2 (14) | 14.1 | CH3 | 0.98 | t, 9.0 (14) | 14.1 | CH3 | 1.05 | t, 7.2 (14) | 14.2, | CH3 |
| 16 | 1.68 | s | 6.2 | CH3 | 1.67 | s | 5.9 | CH3 | 1.68 | s | 6.0 | CH3 | 1.78 | s | 5.6, | CH3 |
| 17 | 194.3 | qC | 194.0 | qC | 195.2 | qC | 192.4, | qC | ||||||||
| 18 | 133.5 | qC | 132.8 | qC | 132.4 | qC | 133.8, | qC | ||||||||
| 19 | 8.27 | d, 8.4 (20) | 130.0 | CH | 8.34 | d, 8.4 (20) | 130.7 | CH | 8.31 | d, 8.4 (20) | 130.7 | CH | 8.20 | d, 8.4 (20) | 129.4, | CH |
| 20 | 7.49 | t, 8.4 (19, 21) | 128.8 | CH | 7.48 | t, 8.4 (19, 21) | 128.6 | CH | 7.49 | t, 8.4 (19, 21) | 128.7 | CH | 7.50 | t, 8.4 (19, 21) | 128.8, | CH |
| 21 | 7.64 | t, 8.4 (20, 22) | 134.4 | CH | 7.63 | t, 8.4 (20, 22) | 134.6 | CH | 7.64 | t, 8.4 (20, 22) | 134.7 | CH | 7.63 | t, 8.4 (20, 22) | 134.2, | CH |
| 22 | 7.49 | t, 8.4 (21, 23) | 128.8 | CH | 7.48 | t, 8.4 (21, 23) | 128.6 | CH | 7.49 | t, 8.4 (21, 23) | 128.7 | CH | 7.50 | t, 8.4 (21, 23) | 128.8, | CH |
| 23 | 8.27 | d, 8.4 (22) | 130.0 | CH | 8.34 | d, 8.4 (22) | 130.7 | CH | 8.31 | d, 8.4 (22) | 130.7 | CH | 8.20 | d, 8.4 (22) | 129.4, | CH |
| 8-OCH3 | 3.37 | s | 51.7 | CH3 | 3.30 | s | 51.9 | CH3 | 3.44 | s | 51.8 | CH3 | 3.27 | s | 51.5, | CH3 |
| 9-OH | 3.94 | br s | 4.22 | br d, 12.0 (9) | 4.25 | br s | 4.97 | d, 5.4 (9) | ||||||||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, T.; Ohshima, M.; Yuasa, K.; Kikuchi, T.; Tanaka, R. Assignment of the CD Cotton Effect to the Chiral Center in Pseurotins, and the Stereochemical Revision of Pseurotin A2. Mar. Drugs 2016, 14, 74. https://doi.org/10.3390/md14040074
Yamada T, Ohshima M, Yuasa K, Kikuchi T, Tanaka R. Assignment of the CD Cotton Effect to the Chiral Center in Pseurotins, and the Stereochemical Revision of Pseurotin A2. Marine Drugs. 2016; 14(4):74. https://doi.org/10.3390/md14040074
Chicago/Turabian StyleYamada, Takeshi, Mina Ohshima, Kaori Yuasa, Takashi Kikuchi, and Reiko Tanaka. 2016. "Assignment of the CD Cotton Effect to the Chiral Center in Pseurotins, and the Stereochemical Revision of Pseurotin A2" Marine Drugs 14, no. 4: 74. https://doi.org/10.3390/md14040074
APA StyleYamada, T., Ohshima, M., Yuasa, K., Kikuchi, T., & Tanaka, R. (2016). Assignment of the CD Cotton Effect to the Chiral Center in Pseurotins, and the Stereochemical Revision of Pseurotin A2. Marine Drugs, 14(4), 74. https://doi.org/10.3390/md14040074