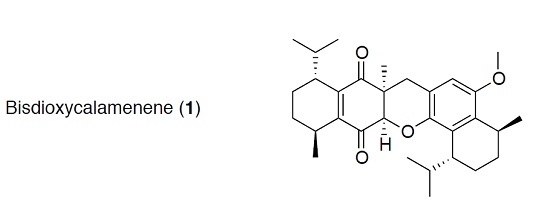
Bisdioxycalamenene: A Bis-Sesquiterpene from the Soft Coral Rhytisma fulvum fulvum
Abstract
:1. Introduction
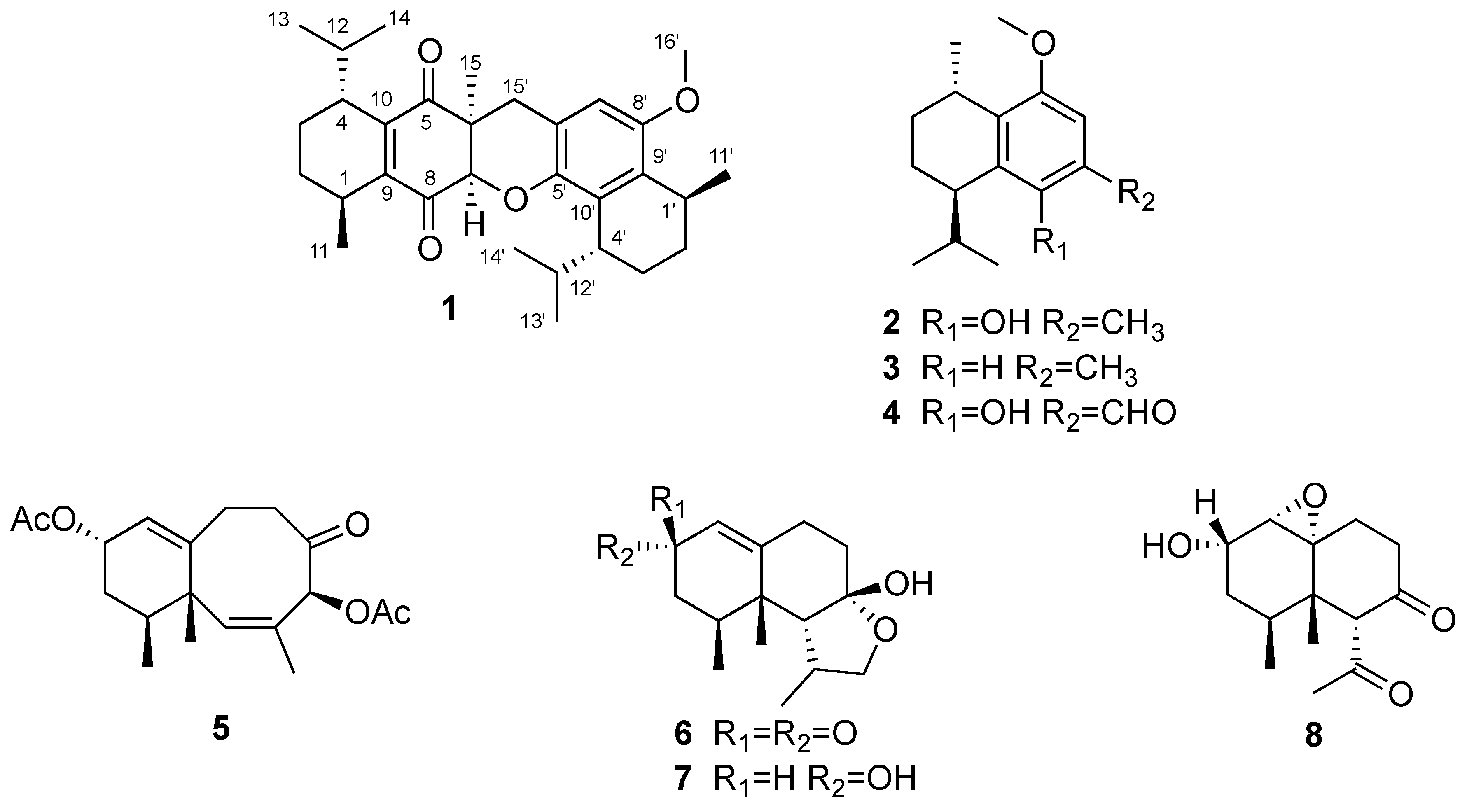
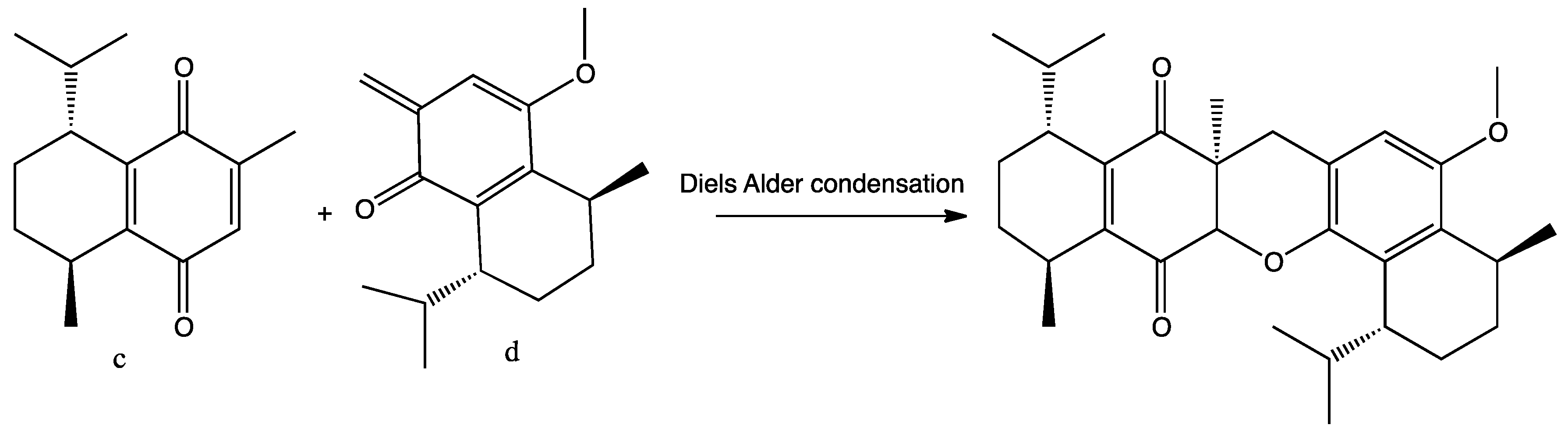
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Biological Material
3.3. Isolation Procedure
3.4. X-Ray Crystallography
3.5. Brine Shrimp Toxicity Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Benayahu, Y.; Loya, Y. Surface brooding in the Red Sea soft coral Parerythropodium fulvum fulvum (forskål, 1775). Biol. Bull. 1983, 165, 353–369. [Google Scholar] [CrossRef]
- Barneah, O.; Weis, V.M.; Perez, S.; Benayahu, Y. Diversity of dinoflagelate symbionts soft corals: Mode of symbiont acquisition matters. Mar. Ecol. Prog. Ser. 2004, 275, 89–95. [Google Scholar] [CrossRef]
- Kelman, D.; Benayahu, Y.; Kashman, Y. Chemical defence of the soft coral Parerythropodium fulvum fulvum (Forskål) in the Red Sea against generalist reef fish. J. Exp. Mar. Biol. Ecol. 1999, 238, 127–137. [Google Scholar] [CrossRef]
- Kelman, D.; Benayahu, Y.; Kashman, Y. Variation in secondary metabolite concentrations in yellow and grey morphs of the Red Sea soft coral Parerythropodium fulvum fulvum: Possible ecological implications. J. Chem. Ecol. 2000, 26, 1123–1133. [Google Scholar] [CrossRef]
- Bowden, B.F.; Coll, J.C.; Mitchell, S.J.; Nemorin, J.L.E.; Sternhell, S. Studies of Australian soft corals. XXIII the co-occurrence of bicyclogermacrene and lemnacarnol derivatives in Parerythropodium fulvum. Tetrahedron Lett. 1980, 21, 3105–3108. [Google Scholar] [CrossRef]
- Green, D.; Kashman, Y. Secondary Metabolites of the Yellow and Gray Morphs of the Soft Coral Parerythropodium fulvum fulvum: Comparative Aspects. J. Nat. Prod. 1992, 55, 1186–1196. [Google Scholar] [CrossRef]
- Jurek, J.; Scheuer, P.J. Sesquiterpenoids and norsesquiterpenoids from the soft coral Lemnalia africana. J. Nat. Prod. 1993, 56, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Wessels, M.; Konig, G.M.; Wright, A.D. 3-Acetoxyspathulenol, a New Aromadendrane-Type Natural Product from the Soft Coral Parerythropodium fulvum fulvum. J. Nat. Prod. 2001, 64, 370–372. [Google Scholar] [CrossRef] [PubMed]
- Bishara, A.; Yeffet, D.; Sisso, M.; Shmul, G.; Schleyer, M.; Benayahu, Y.; Rudi, A.; Kashman, Y. Nardosinanols A–I and Lemnafricanol, Sesquiterpenes from Several Soft Corals, Lemnalia sp., Paralemnalia clavata, Lemnalia africana, and Rhytisma fulvum fulvum. J. Nat. Prod. 2008, 71, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Kashman, Y. 7-Hydroxy (and acetoxy)-α-muurolene from the soft-coral Heteroxenia fuscescens. Tetrahedron 1979, 35, 263–266. [Google Scholar] [CrossRef]
- Izac, R.R.; Tagle, B.; Clardy, J.; Fenical, W. Neolemnane and eremophilane sesquiterpenoids from the pacific soft coral Lemnalia africana. Tetrahedron 1981, 37, 2569–2573. [Google Scholar] [CrossRef]
- Izac, R.R.; Schmeider, P.; Swain, M.; Fenical, W. New nor-sesquiterpenoids of apparent nardosinane origin from the pacific soft-coral Paralemnalia thyrsoides. Tetrahedron Lett. 1982, 23, 817–820. [Google Scholar] [CrossRef]
- Parsons, S.; Flack, H.D.; Wagner, T. Use of intensity quotients and differences in absolute structure refinement. Acta Crystallogr. 2013, 69, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.-J.; David, J.G.; Feng, Z.-G.; Weaver, M.G.; Wu, K.-L.; Pettus, T.R.R. The domestication of ortho-Quinone Methides. Acc. Chem. Res. 2014, 47, 3655–3664. [Google Scholar] [CrossRef] [PubMed]
- Willis, N.J.; Bray, C.D. Ortho-Quinone Methides in Natural Product Synthesis. Chem. Eur. J. 2012, 18, 9160–9173. [Google Scholar] [CrossRef] [PubMed]
- Solis, P.N.; Wright, C.W.; Anderson, M.M.; Gupta, M.P.; Phillipson, J.D. A microwell cytotoxicity assay using Artemia salina (brine shrimp). Planta Med. 1993, 59, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Kupchan, S.M.; Tsou, G. Bruceantin, a new potent antileukemic simaroubolide from Brucea antidysenterica. J. Org. Chem. 1973, 38, 178–179. [Google Scholar] [CrossRef] [PubMed]
- Coll, J.C.; Bowden, B.F.; Clayton, M.N. Chemistry and coral reproduction. Chem. Br. 1990, 26, 761–763. [Google Scholar]
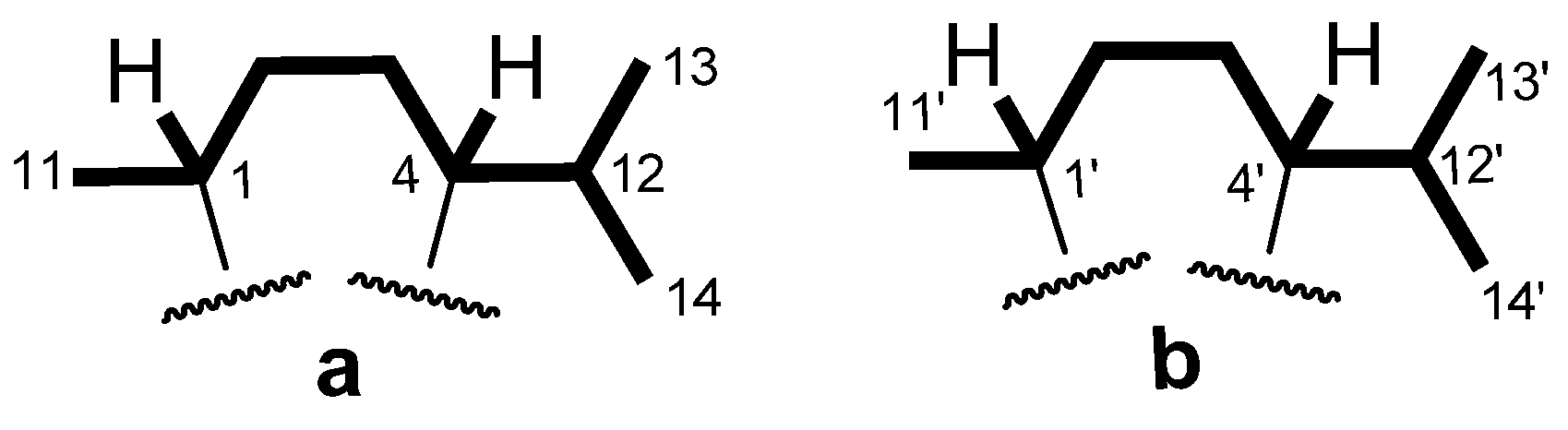
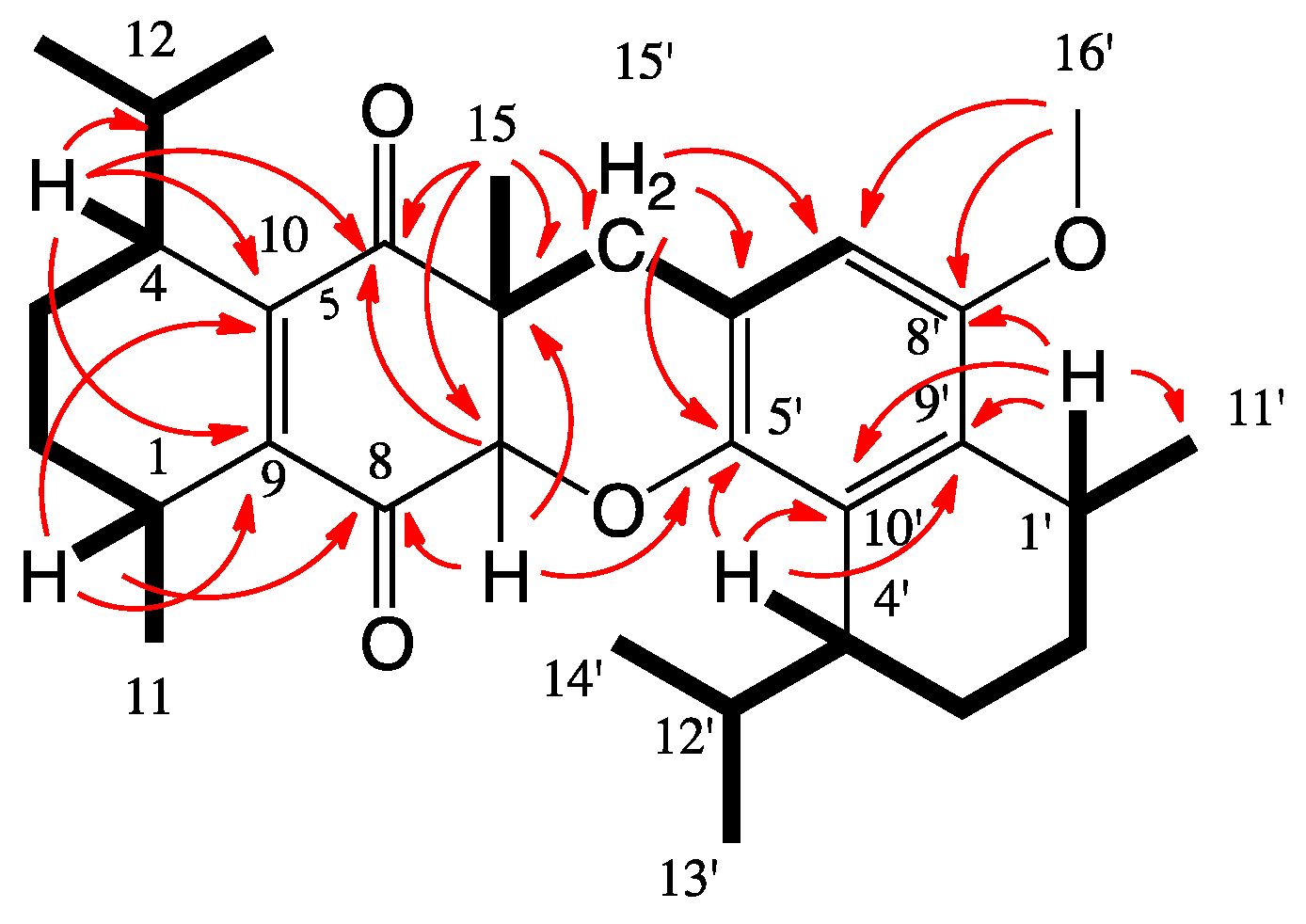
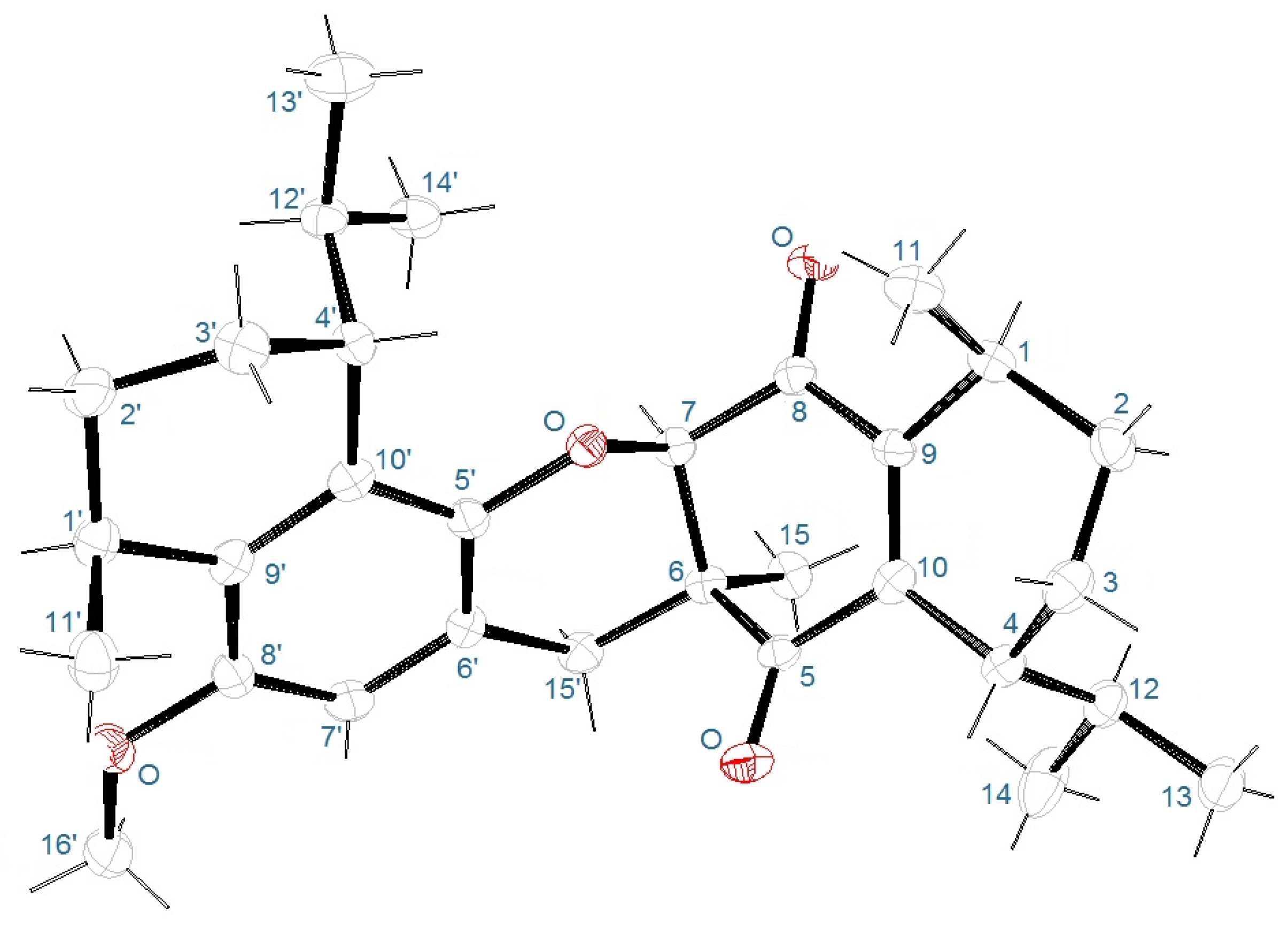
| Position | δC, Type b | δH, Mult (J in Hz) | HMBC Correlations c | COSY Correlations |
|---|---|---|---|---|
| 1 | 27.2, CH | 2.99, ddq (1.3, 6.8, 7.0) | 2, 3, 5, 8, 9, 10, 11 | 2a, 11 |
| 2 | 25.5, CH2 | 1.84, m | 10 | 1 |
| 1.46, m | 3a, 3b, 12 | |||
| 3 | 18.5, CH2 | 1.74, m | 10 | 2b, 3b, 4, 12 |
| 1.61, m | 4, 10 | 2b, 3a, 4 | ||
| 4 | 36.9, CH | 2.66, dt (2.3, 6.0) | 2, 3, 5, 9, 10, 12, 14 | 3a, 3b, 12 |
| 5 | 199.4, qC | |||
| 6 | 48.0, qC | |||
| 7 | 83.9, CH | 4.39, s | 5, 6, 8, 9, 15, 5′, 15′ | |
| 8 | 195.3, qC | |||
| 9 | 150.3, qC | |||
| 10 | 147.3, qC | |||
| 11 | 20.1, CH3 | 1.10, d (7.0) | 1, 2, 9, 10 | 1 |
| 12 | 31.1, CH | 1.82, m | 3, 4 | 2b, 3a, 4, 13, 14 |
| 13 | 21.3, CH3 | 0.87, d (7.0) | 4, 12, 14 | 12 |
| 14 | 20.2, CH3 | 0.85, d (7.0) | 4, 12 | 12 |
| 15 | 21.8, CH3 | 1.33, s | 5, 6, 7, 6′, 15′ | |
| 1′ | 26.5, CH | 3.09, dq (6.5,7.0) | 2′, 3′, 8′, 9′, 10′, 11′ | 2′a, 11′ |
| 2′ | 25.6, CH2 | 1.93, m | 10′ | 1′, 2′b, 3′ |
| 1.41, m | 4′, 9′ | 3′ | ||
| 3′ | 19.5, CH2 | 1.70, m | 1′, 4′, 10′, 12′ | 2′a, 2′b, 4′ |
| 4′ | 37.0, CH | 2.72, dt (2.3, 5.9) | 2′, 5′, 9′, 10′, 12′, 14′ | 1′, 3′, 12′ |
| 5′ | 144.0, qC | |||
| 6′ | 115.4, qC | |||
| 7′ | 107.5, CH | 6.36, s | 5′, 8′, 9′, 15′ | |
| 8′ | 151.7, qC | |||
| 9′ | 130.6, qC | |||
| 10′ | 129.1, qC | |||
| 11′ | 21.9, CH3 | 1.07, d (7.0) | 1′, 2′, 9′ | 1′ |
| 12′ | 32.3, CH | 1.86, m | 3′, 10′, 14′ | 4′, 13′, 14′ |
| 13′ | 21.7, CH3 | 0.86, d (7.0) | 4′, 12′ | 12′ |
| 14′ | 21.4, CH3 | 0.77, d (7.0) | 4′, 12′ | 12′ |
| 15′ | 33.1, CH2 | 3.35, d (16.5) | 5, 6, 7, 8, 15, 5′, 6′, 7′, 8′, 9′ | |
| 2.53, d (16.5) | 5, 6, 7, 15, 5′, 6′, 7′, 8′, 9′, 10′ | |||
| 16′ | 55.2, CH3 | 3.74, s | 7′, 8′ |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trifman, Y.J.; Aknin, M.; Gauvin-Bialecki, A.; Benayahu, Y.; Carmeli, S.; Kashman, Y. Bisdioxycalamenene: A Bis-Sesquiterpene from the Soft Coral Rhytisma fulvum fulvum. Mar. Drugs 2016, 14, 41. https://doi.org/10.3390/md14020041
Trifman YJ, Aknin M, Gauvin-Bialecki A, Benayahu Y, Carmeli S, Kashman Y. Bisdioxycalamenene: A Bis-Sesquiterpene from the Soft Coral Rhytisma fulvum fulvum. Marine Drugs. 2016; 14(2):41. https://doi.org/10.3390/md14020041
Chicago/Turabian StyleTrifman, Yuval J., Maurice Aknin, Anne Gauvin-Bialecki, Yehuda Benayahu, Shmuel Carmeli, and Yoel Kashman. 2016. "Bisdioxycalamenene: A Bis-Sesquiterpene from the Soft Coral Rhytisma fulvum fulvum" Marine Drugs 14, no. 2: 41. https://doi.org/10.3390/md14020041
APA StyleTrifman, Y. J., Aknin, M., Gauvin-Bialecki, A., Benayahu, Y., Carmeli, S., & Kashman, Y. (2016). Bisdioxycalamenene: A Bis-Sesquiterpene from the Soft Coral Rhytisma fulvum fulvum. Marine Drugs, 14(2), 41. https://doi.org/10.3390/md14020041