Larvicidal Potential of the Halogenated Sesquiterpene (+)-Obtusol, Isolated from the Alga Laurencia dendroidea J. Agardh (Ceramiales: Rhodomelaceae), against the Dengue Vector Mosquito Aedes aegypti (Linnaeus) (Diptera: Culicidae)
Abstract
:1. Introduction
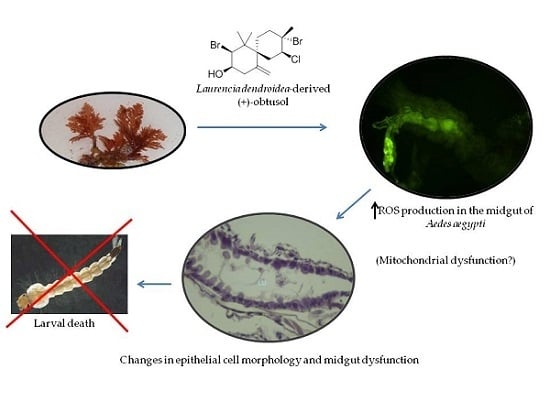
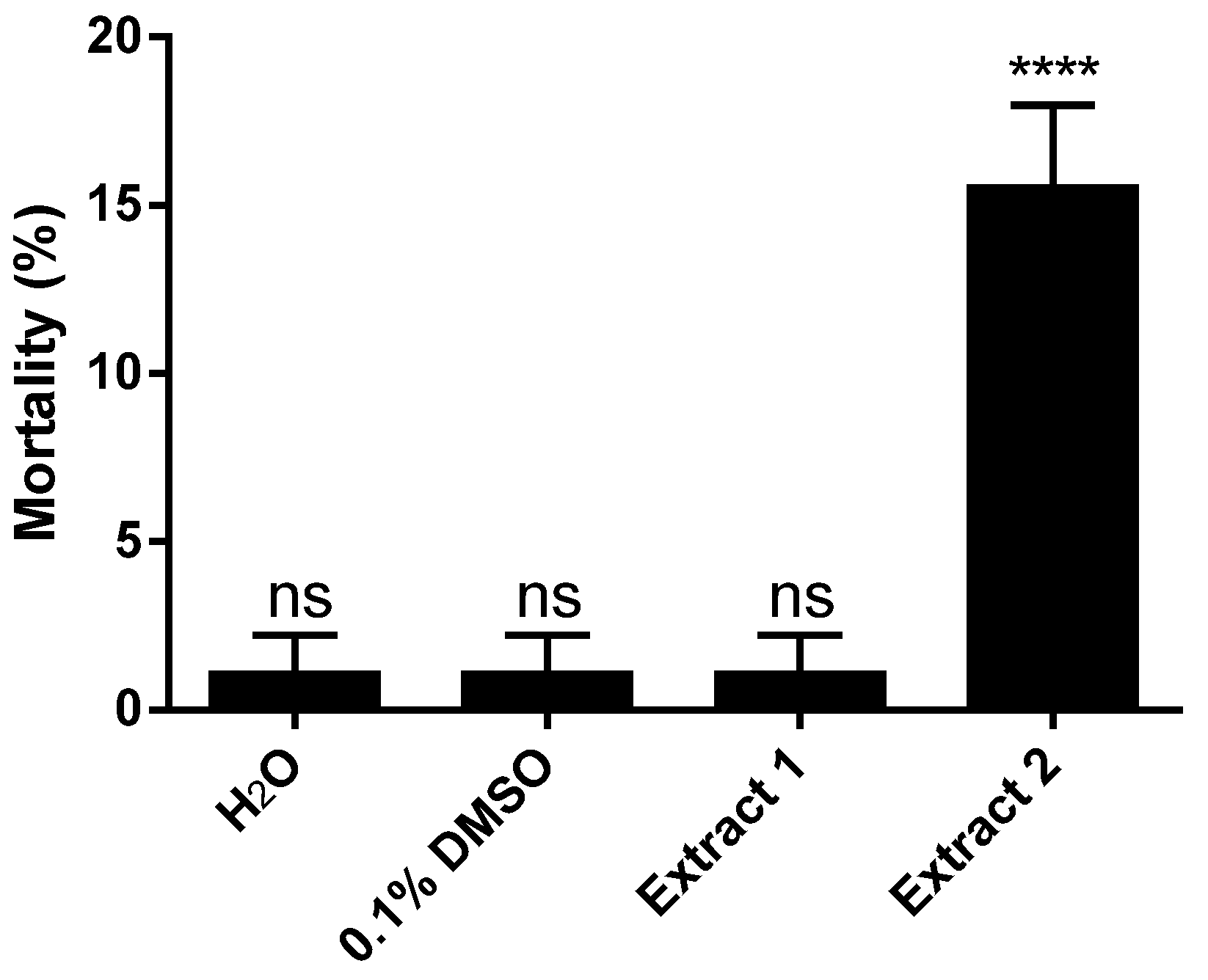
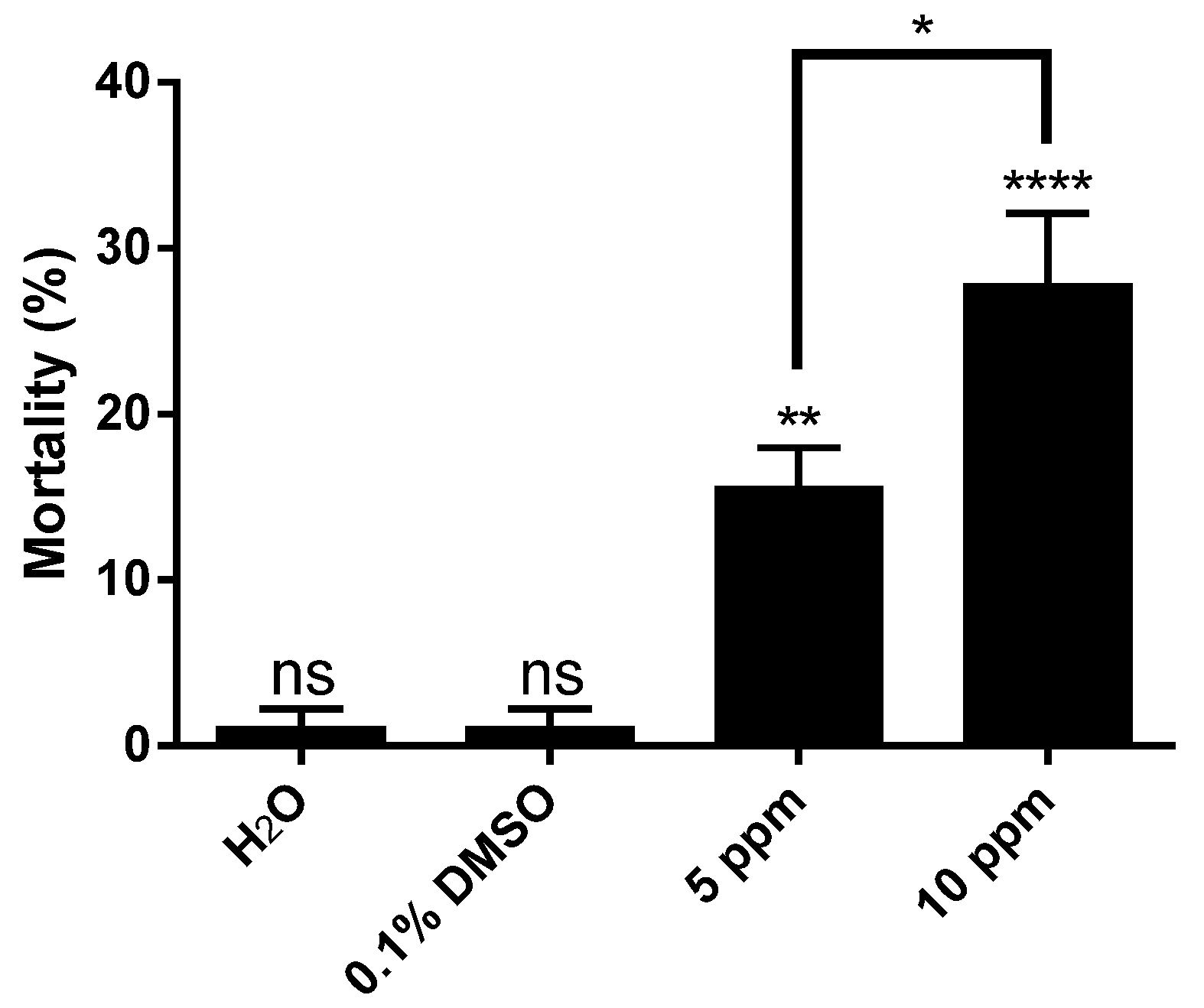
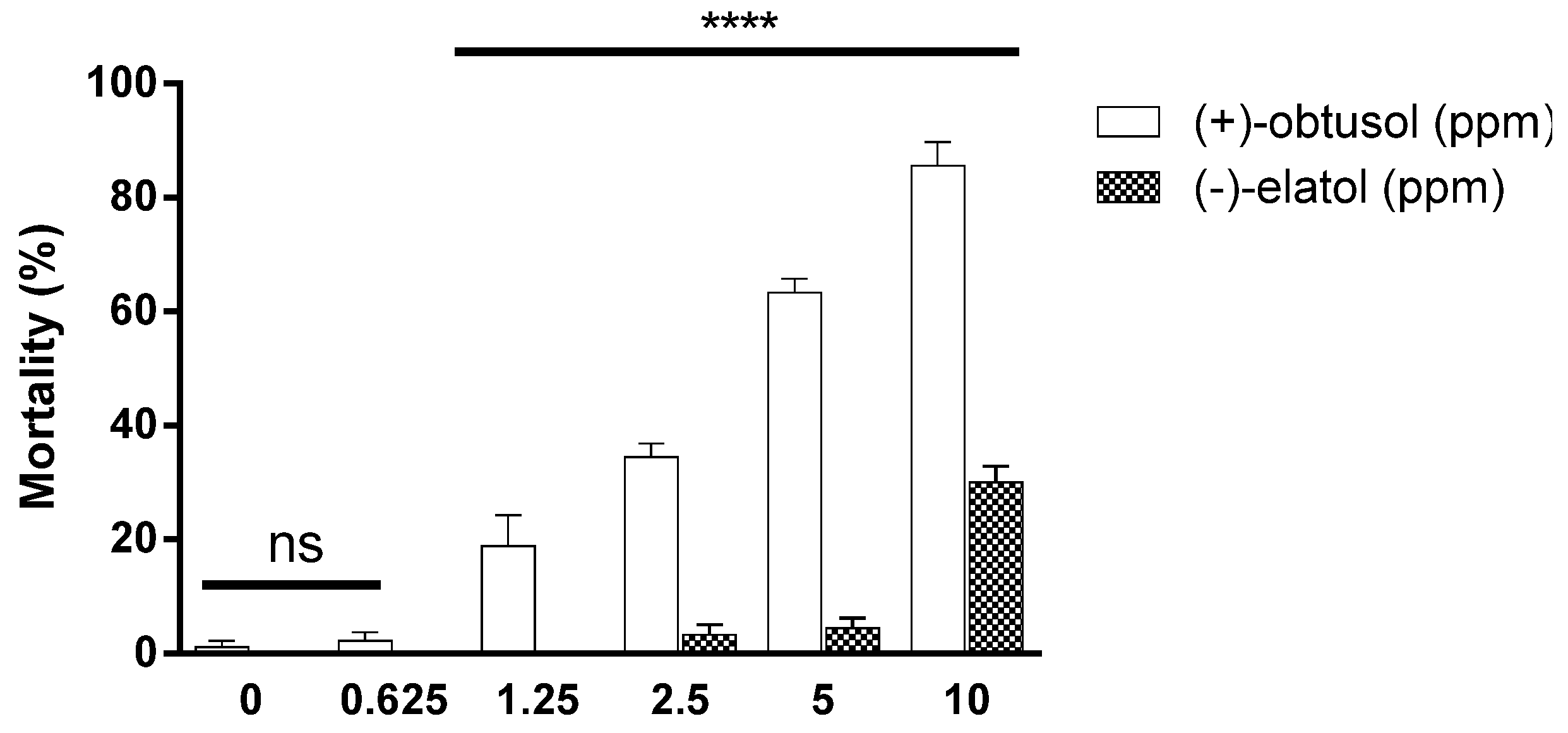
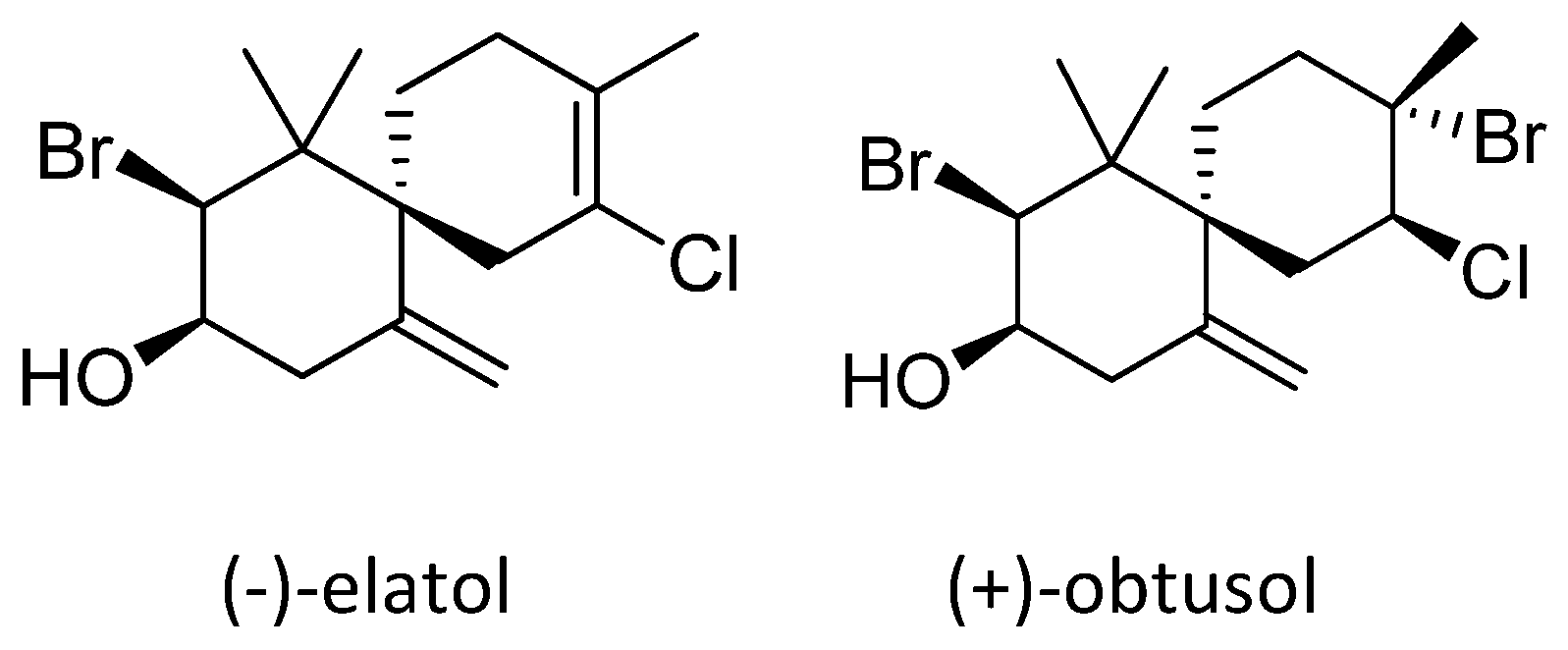
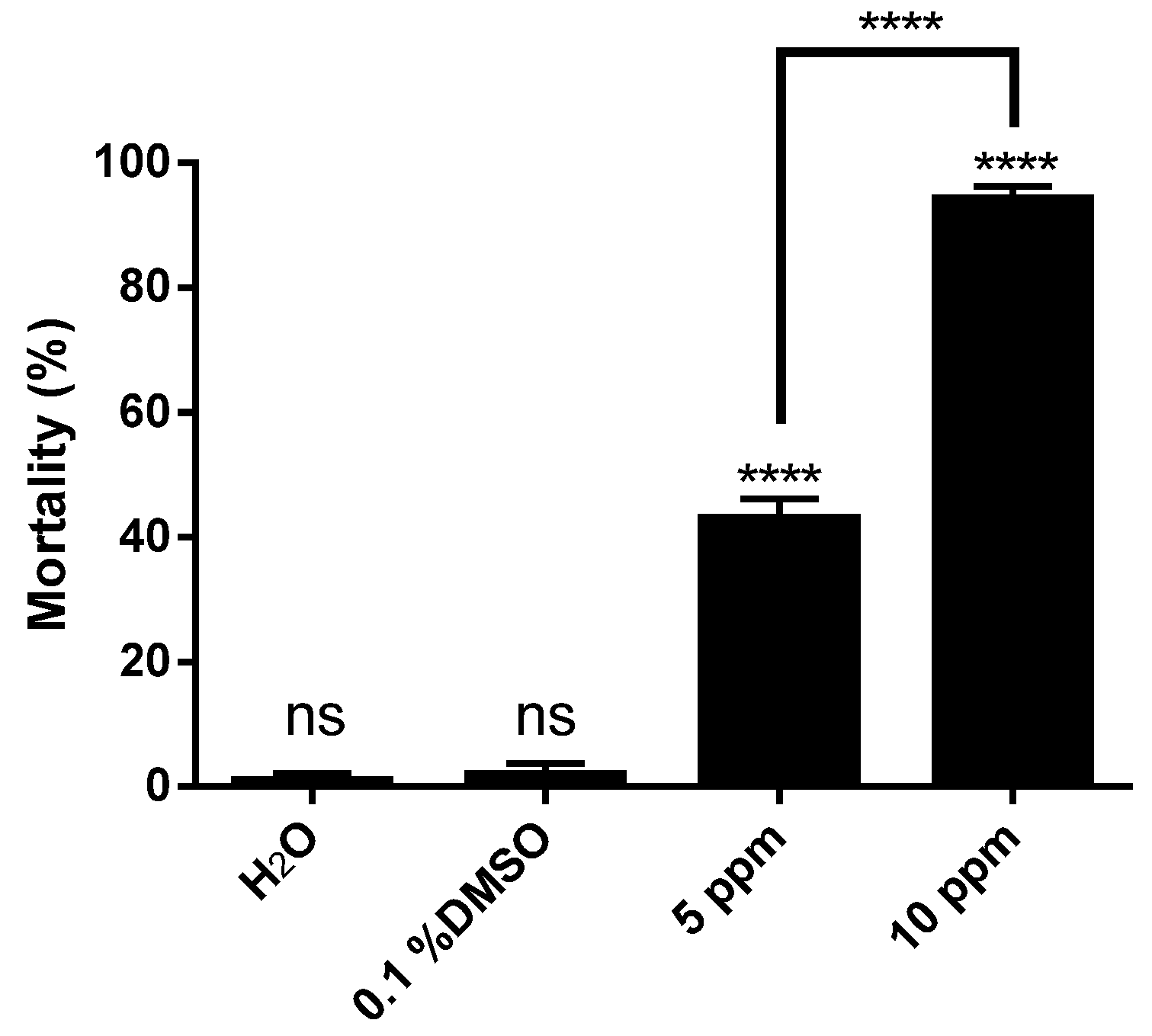
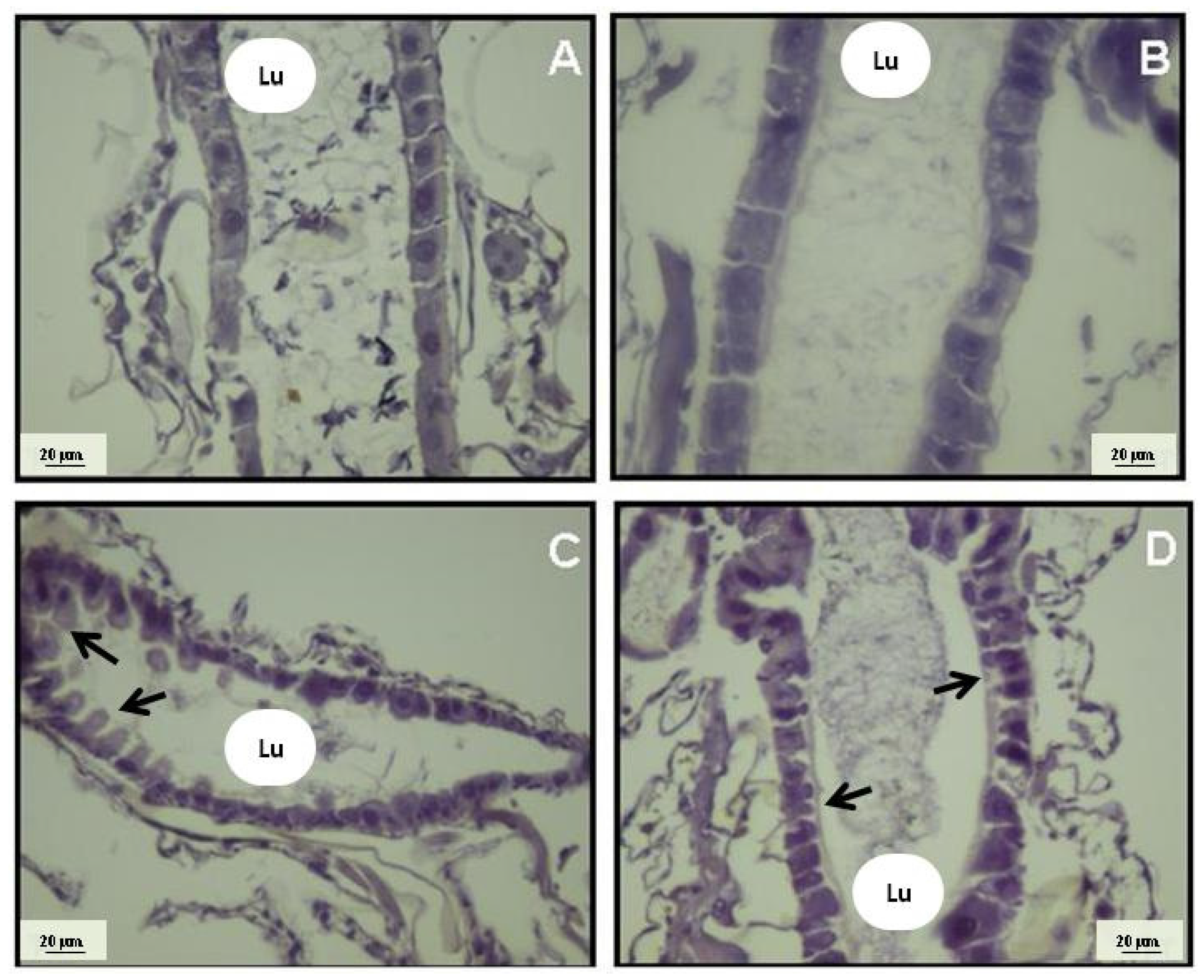
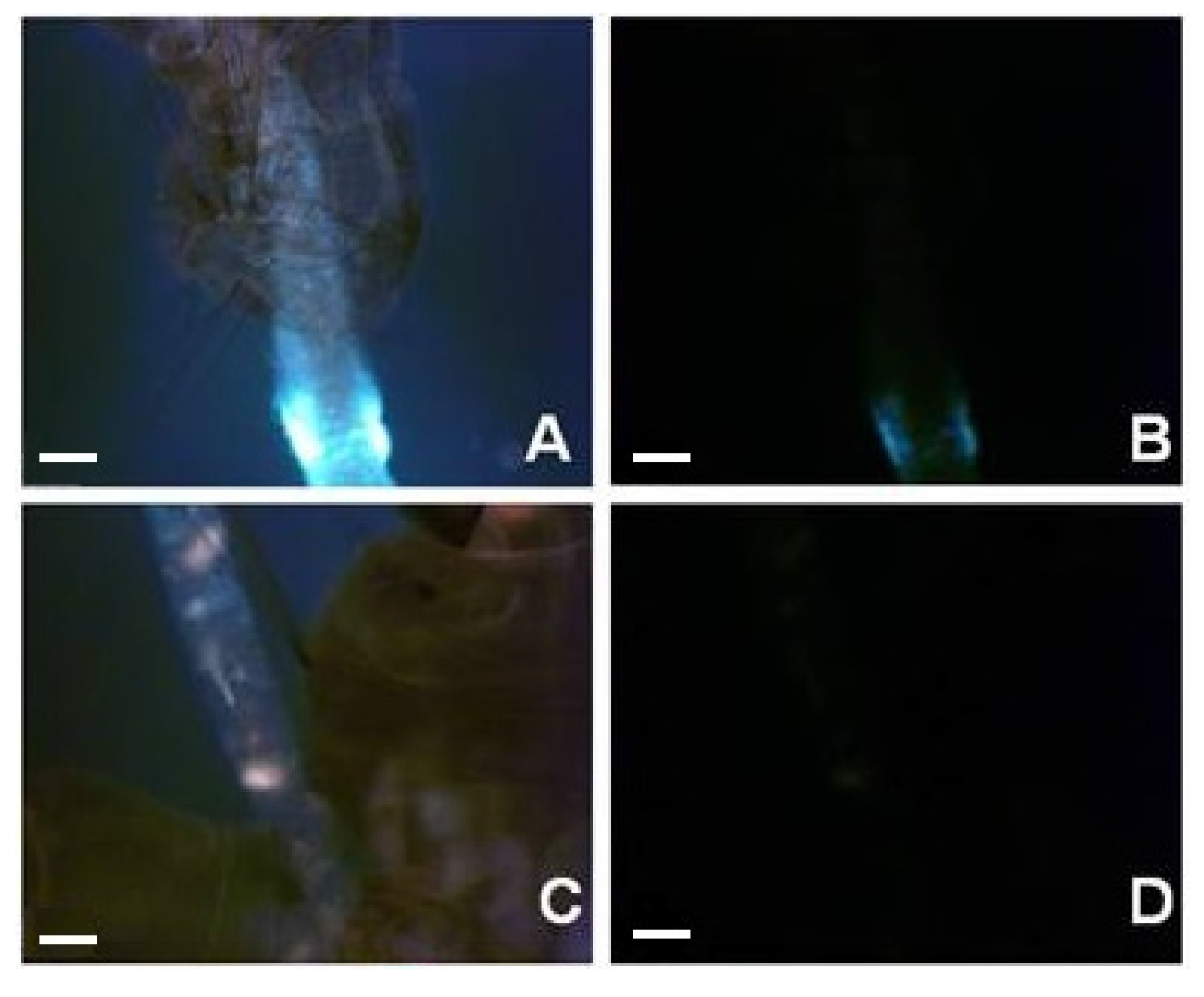
2. Results and Discussion



3. Experimental Section
3.1. Maintenance of the A. aegypti Colony
3.2. Seaweed Sampling
3.3. Extract Preparation and Sesquiterpene Purification
3.4. Analysis of Larvicidal Activity of Extracts and Pure Compounds
3.5. Histological Analysis
3.6. Detection of Reactive Oxidative Species (ROS) in Vivo
3.7. Statistical Analysis
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tomori, O. Yellow fever: The recurring plague. Crit. Rev. Clin. Lab. Sci. 2004, 41, 391–427. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Dengue and Severe Dengue. Updated May 2015. Available online: http://www.who.int/mediacentre/factsheets/fs117/en/ (accessed on 5 November 2015).
- Thiboutot, M.M.; Kannan, S.; Kawalekar, O.U.; Shedlock, D.J.; Khan, A.S.; Sarangan, G.; Srikanth, P.; Weiner, D.B.; Muthumani, K. Chikungunya: A Potentially Emerging Epidemic? PLoS Negl. Trop. Dis. 2010. [Google Scholar] [CrossRef] [PubMed]
- Marchette, N.J.; Garcia, R.; Rudnick, A. Isolation of Zika Virus from Aedes aegypti Mosquitoes in Malaysia. Am. J. Trop. Med. Hyg. 1969, 18, 411–415. [Google Scholar] [PubMed]
- Ranson, H.; Burhani, J.; Lumjuan, N.; Black, I.V.W.C. Insecticide Resistance in Dengue Vectors. TropIKA.net.(online) 2010, 1, 0–0, ISSN 2078-8606. Available online: http://journal.tropika.net/scielo.php?script=sci_arttext (accessed on 24 November 2014). [Google Scholar]
- Sun, C.N.; Georghiou, G.P.; Weiss, K. Toxicity of Bacillus thuringiensis subsp. israelensis to mosquito larvae variously resistant to conventional insecticides. Mosq. News 1980, 40, 614–618. [Google Scholar]
- Scholte, E.-J.; Takken, W.; Knols, B.G.J. Infection of adult Aedes aegypti and Ae. albopictus mosquitoes with the entomopathogenic fungus Metarhizium anisopliae. Acta Trop. 2007, 102, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Paula, A.R.; Brito, E.S.; Pereira, C.R.; Carrera, M.P.; Samuels, R.I. Susceptibility of adult Aedes aegypti (Diptera: Culicidae) to infection by Metarhizium anisopliae and Beauveria bassiana: Prospects for Dengue vector control. Biocontrol. Sci. Technol. 2008, 18, 1017–1025. [Google Scholar] [CrossRef]
- Reyes-Villanueva, F.; Garza-Hernandez, J.A.; Garcia-Munguia, A.M.; Tamez-Guerra, P.; Howard, A.F.V.; Rodriguez-Perez, M.A. Dissemination of Metarhizium anisopliae of low and high virulence by mating behavior in Aedes aegypti. Parasit. Vectors 2011, 4, 171. [Google Scholar] [CrossRef] [PubMed]
- Rubio, Y.; Ayesta, C. Laboratory observations on the biology of Toxorhynchites theobaldi. Mosq. News 1984, 44, 86–90. [Google Scholar]
- Marti, G.A.; Micieli, M.V.; Scorsetti, A.C.; Liljesthrom, G. Evaluation of Mesocyclops annulatus (Copepoda: Cyclopoidea) as a control agent of Aedes aegypti (Diptera: Culicidae) in Argentina. Mem. Inst. Oswaldo Cruz 2004, 99, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Kay, B.H.; Hanh, T.T.T.; Le, N.H.; Quy, T.M.; Nam, V.S.; Hang, P.V.D.; Yen, N.T.; Hill, P.S.; Vos, T.V.; Ryan, P.A. Sustainability and cost of a community-based strategy against Aedes aegypti in northern and central Vietnam. Am. J. Trop. Med. Hyg. 2010, 82, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ibarra, J.A.; Guillen, Y.G.; Arredondo-Jimenez, J.I.; Rodriguez-Lopez, M.H. Indigenous fish species for the control of Aedes aegypti in water storage tanks in Southern Mexico. BioControl 2002, 47, 481–486. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, M.A.; Howard, A.F.V.; Reyes-Villanueva, F. Biological Control of Dengue Vectors, Integrated Pest Management and Pest Control—Current and future Tactics; Soloneski, S., Ed.; ISBN 978-953-51-0050-8. InTech: Rijeka, Croatia, 2012; Available online: http://www.intechopen.com/books/integrated-pest-management-and-pest-control-current-and-future-tactics/biological-control-of-dengue-vectors (accessed on 14 January 2016).
- Carolino, A.T.; Paula, A.R.; Silva, C.P.; Butt, T.M.; Samuels, R.I. Monitoring persistence of the entomopathogenic fungus Metarhizium anisopliae under simulated field conditions with the aim of controlling adult Aedes aegypti (Diptera: Culicidae). Parasit. Vectors 2014, 7, 198. [Google Scholar] [CrossRef] [PubMed]
- Shaalan, E.A.-S.D.; Canyon, M.W.F.; Younes, H.; Abdel-Waheb, A.-H. Mansour. A review of botanical phytochemicals with mosquitocidal potential. Environ. Int. 2005, 31, 1149–1166. [Google Scholar] [CrossRef] [PubMed]
- Amer, A.; Mehlhorn, H. Larvicidal effects of various essential oils against Aedes, Anopheles, and Culex larvae (Diptera, Culicidae). Parasitol. Res. 2006, 99, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Mohan, L.; Srivastava, C.N. Phytoextract-induced developmental deformities in malaria vector. Bioresour. Technol. 2006, 97, 1599–1604. [Google Scholar] [CrossRef] [PubMed]
- Fallatah, S.A.B.; Khater, E.I.M. Potential of medicinal plants in mosquito control. J. Egypt. Soc. Parasitol. 2010, 40, 1–26. [Google Scholar] [PubMed]
- Gomes, S.A.; Paula, A.R.; Ribeiro, A.; Moraes, C.O.P.; Santos, J.W.A.B.; Silva, C.P.; Samuels, R.I. Neem oil increases the efficiency of the entomopathogenic fungus Metarhizium anisopliae for the control of Aedes aegypti (Diptera: Culicidae) larvae. Parasit. Vectors 2015, 8, 669. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Hu, W.P.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2007, 24, 31–86. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.M.; Mendis, E.; Kim, S.K. Laurencia okamurai extract containing laurinterol induces apoptosis in melanoma cells. J. Med. Food 2008, 11, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.; Souza, C.B.; Lhullier, C.; Falkenberg, M.; Schenkel, E.P.; Ribeiro-do-Valle, R.M.; Siqueira, J.M. Anti-tumour effects of elatol, a marine derivative compound obtained from red algae Laurencia microcladia. J. Pharm. Pharmacol. 2012, 64, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Topcu, G.; Aydogmus, Z.; Imre, S.; Gören, A.C.; Pezzuto, J.M.; Clement, J.A.; Kingston, D.G. Brominated sesquiterpenes from the red alga Laurencia obtuse. J. Nat. Prod. 2003, 66, 1505–1508. [Google Scholar] [CrossRef] [PubMed]
- Veiga-Santos, P.; Rocha, K.J.P.; Santos, A.O.; Ueda-Nakamura, T.; Dias-Filho, B.P.; Lautenschlager, S.O.S.; Sudatti, D.B.; Bianco, E.M.; Pereira, R.C.; Nakamura, C.V. In vitro anti-trypanosomal activity of elatol isolated from red seaweed Laurencia dendroidea. Parasitology 2010, 137, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.O.; Veiga-Santos, P.; Ueda-Nakamura, T.; Dias-Filho, B.P.; Sudatti, D.B.; Bianco, E.M.; Pereira, R.C.; Nakamura, C.V. Effect of elatol, isolated from red seaweed Laurencia dendroidea, on Leishmania amazonensis. Mar. Drugs 2010, 8, 2733–2743. [Google Scholar] [CrossRef] [PubMed]
- Machado, F.L.S.; Pacienza-Lima, W.; Rossi-Bergman, B.; Gestinari, L.M.S.; Fujii, M.T.; Paula, J.C.; Costa, S.S.; Lopes, N.P.; Kaiser, C.R.; Soares, A.R. Antileishmanial sesquiterpenes from the Brazilian red alga Laurencia dendroidea. Planta Med. 2011, 77, 733–735. [Google Scholar] [CrossRef] [PubMed]
- Vairappan, C.S.; Daitoh, M.; Suzuki, M.; Abe, T.; Masuda, M. Antibacterial halogenated metabolites from the Malaysian Laurencia species. Phytochemistry 2001, 58, 291–297. [Google Scholar] [CrossRef]
- Vairappan, C.S.; Kawamoto, T.; Miwa, H.; Suzuki, M. Potent antibacterial activity of halogenated compounds against antibiotic-resistent bacteria. Planta Med. 2004, 70, 1087–1090. [Google Scholar] [CrossRef] [PubMed]
- Paradas, W.C.; Salgado, L.T.; Sudatti, D.B.; Crapez, M.A.; Fujii, M.T.; Coutinho, R.; Pereira, R.C.; Amado-Filho, G.M. Induction of halogenated vesicle transport in cells of the red seaweed Laurencia obtusa. Biofouling 2010, 26, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Thangam, T.S.; Kathiresan, K. Marine plants for mosquito control. In Proceedings of the Second International Conference on Urban Pests, Edinburgh, Scotland, UK, 7–10 July 1996; Wildey, K.B., Ed.; pp. 431–435.
- Manilal, A.; Thajuddin, N.; Selvin, J.; Idhayadhulla, A.; Kumar, R.S.; Sujith, S. In vitro mosquito larvicidal activity of marine algae against the human vectors, Culex quinquefasciatus (Say) and Aedes aegypti (Linnaeus) (Diptera: Culicidae). Int. J. Zool. Res. 2011, 7, 272–278. [Google Scholar] [CrossRef]
- Nisha, L.L.J.L.; Vincent, L.; Poonguzhali, T.V. Larvicidal activity of two seaweeds, Cheatomorpha antenina (Bory de Saint-Vincent) Kutzing and Sargassum wightii Greville against mosquito vector, Aedes aegypti. Asian J. Biochem. Pharm. Res. 2013, 2, 705–711. [Google Scholar]
- Ali, M.Y.S.; Ravikumar, S.; Beula, J.M. Mosquito larvicidal activity of seaweeds extracts against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus. Asian Pac. J. Trop. Dis. 2013, 3, 196–201. [Google Scholar] [CrossRef]
- Watanabe, K.; Umeda, K.; Miyakado, M. Isolation and identification of three insecticidal principles from the red alga Laurencia nipponica. Yamada. Agric. Biol. Chem. Takyo 1989, 53, 2513–2515. [Google Scholar] [CrossRef]
- Watanabe, K.; Miyakado, M.; Ohono, N.; Okada, A.; Yanagi, K.; Moriguchi, K. A polyhalogenated insecticidal monoterpene from the red alga, Plocamitrm telfairiae. Phytochemistry 1989, 28, 77–78. [Google Scholar] [CrossRef]
- Watanabe, K.; Umeda, K.; Kurita, Y.; Takayama, C.; Miyakado, M. Two insecticidal monoterpenes, telfairine and aplysiaterpenoid A, from the red alga Plocamiurn telfairiae: Structure elucidation, biological activity, and molecular topographical consideration by a semiempirical molecular orbital study. Pestic. Biochem. Phys. 1990, 37, 275–286. [Google Scholar] [CrossRef]
- Rovirosa, J.; Sepulveda, M.; Quezada, E.; San-Martin, A. Isoepitaondiol, a diterpenoid of Stypopodium flabelliforme and the insecticidal activity of stypotriol, epitaondiol and derivatives. Phytochemistry 1992, 31, 2679–2681. [Google Scholar] [CrossRef]
- Yu, K.X.; Jantan, I.; Ahmad, R.; Wong, C.L. The major bioactive components of seaweeds and their mosquitocidal potential. Parasitol. Res. 2014, 113, 3121–3141. [Google Scholar] [CrossRef] [PubMed]
- Bianco, E.M.; Pires, L.; Santos, G.K.N.; Dutra, K.; Reis, T.N.V.; Vasconcelos, E.R.T.P.P.; Cocentino, A.L.M.; Navaro, D.M.A.F. Larvicidal activity of seaweeds from northeastern Brazil and of a hologenated sesquiterpene against the dengue mosquito (Aedes aegypti). Ind. Crop. Prod. 2013, 43, 270–275. [Google Scholar] [CrossRef]
- Souto, M.L.; Manriquez, C.P.; Norte, M.; Fernandez, J.J. Novel marine polyethers. Tetrahedron 2002, 58, 8119–8125. [Google Scholar] [CrossRef]
- Wang, B.G.; Gloer, J.B.; Ji, N.Y.; Zhao, J.C. Halogenated organic molecules of Rhodomelaceae origin: Chemistry and biology. Chem. Rev. 2013, 113, 3632–3685. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.C.; Gama, B.A.P.; Teixeira, V.L.; Yoneshigue-Valentin, Y. Ecological roles of natural products of the Brazilian red seaweed Laurencia obtusa. Braz. J. Biol. 2003, 63, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Sudatti, D.B.; Rodrigues, S.V.; Coutinho, R.; Gama, B.A.P.; Salgado, L.T.; Amado-Filho, G.M.; Pereira, R.C. Transport and defensive role of elatol at the surface of the red seaweed Laurencia obtusa (Ceramiales, Rhodophyta). J. Phycol. 2008, 44, 584–591. [Google Scholar] [CrossRef]
- Yu, K.X.; Wong, C.L.; Ahmad, R.; Jantan, I. Mosquitocidal and oviposition repellent activities of the extracts of seaweed Bryopsis pennata on Aedes aegypti and Aedes albopictus. Molecules 2015, 20, 14082–14102. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.D.; Paul, V.J. Intraplant variation in secondary metabolite concentration in three species of Caulerpa (Chlorophyta: Caulerpales) and its effect on herbivorous fishes. Mar. Ecol. Prog. Ser. 1992, 82, 249–257. [Google Scholar] [CrossRef]
- Wright, J.T.; de Nys, R.; Steinberg, P.D. Geografic variation in halogenated furacones from the red alga Delisea pulchra and associated herbivores and epiphytes. Mar. Ecol. Prog. Ser. 2000, 207, 227–241. [Google Scholar] [CrossRef]
- Machado, F.L.S.; Lima, W.P.; Duarte, H.M.; Rossi-Bergmann, B.; Gestinari, L.M.; Fujii, M.T.; Kaiser, C.R.; Soares, A.R. Chemical diversity and antileishmanial activity of crude extracts of Laurencia complex (Ceramiales, Rhodophyta) from Brazil. Rev. Bras. Farmacogn. 2014, 24, 635–643. [Google Scholar] [CrossRef]
- Bansemir, A.; Just, N.; Michalik, M.; Lindequist, U.; Lalk, M. Extracts and sesquiterpene derivatives from the red alga Laurencia chondrioides with antibacterial activity against fish and human pathogenic bacteria. Chem. Biodivers. 2004, 1, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Konig, G.M.; Wright, A.D. Laurencia rigida: Chemical investigations of its antifouling dichloromethane extract. J. Nat. Prod. 1997, 60, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Lhullier, C.; Donnangelo, A.; Caro, M.; Palermo, J.; Horta, P.A.; Falkenberg, M.; Schenkel, E.P. Isolation of elatol from Laurencia microcladia and its palatability to the sea urchin Echinometra lucunter. Biochem. Syst. Ecol. 2009, 37, 254–259. [Google Scholar] [CrossRef]
- Pereira, R.C.; Teixeira, V.L. Sesquiterpenos das algas marinhas Laurencia lamouroux (Ceramiales, Rhodophyta) 1. Significado ecológico. Quim. Nova 1999, 22, 369–374. [Google Scholar] [CrossRef]
- Da Gama, B.A.P.; Pereira, R.C.; Soares, A.R.; Teixeira, V.L.; Yoneshige-Valentin, Y. Is the mussel test a good indicator of fouling activity? A comparison between laboratory and field assays. Biofouling 2003, 19, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Salgado, L.T.; Viana, N.B.; Andrade, L.R.; Leal, R.N.; Gama, B.A.P.; Attias, M.; Pereira, R.C.; Amado-Filho, G.M. Intra-cellular storage, transport and exocytosis of halogenated compounds in marine red alga Laurencia obtusa. J. Struct. Biol. 2008, 162, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Al-Mehmadi, R.M.; Al-Khalaf, A.A. Larvicidal and histological effects of Melia azedarach extract on Culex quinquefasciatus Say larvae (Diptera: Culicidae). J. King Saud Univ. Sci. 2010, 22, 77–85. [Google Scholar] [CrossRef]
- Desoti, V.C.; Lazarin-Bidóia, D.; Sudatti, D.B.; Pereira, R.C.; Alonso, A.; Ueda-Nakamura, T.; Filho, B.P.D.; Nakamura, C.V.; Oliveira, S. Trypanosomal action of (−)-elatol involves an oxidative stress triggered by mitochondrial dysfunction. Mar. Drugs 2012, 10, 1631–1646. [Google Scholar] [CrossRef] [PubMed]
- Casida, J.E. Pyrethrum flowers and pyrethroid insecticides. Environ. Health Perspect. 1980, 34, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Kukel, C.F.; Jennings, K.R. Delphinium alkaloids as inhibitors of alpha-bungarotoxin binding to rat and insect neural membranes. Can. J. Physiol. Pharm. 1994, 72, 104–107. [Google Scholar] [CrossRef]
- Senthil-Nathan, S.; Choi, M.Y.; Paik, C.H.; Seo, H.Y.; Kalaivani, K.; Kim, J.D. Effect of azadirachtin on acetylcholinesterase (AChE) activity and histology of the brown planthopper Nilaparvata lugens (Stål). Ecotoxicol. Environ. Saf. 2008, 70, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, M.; Karuppannan, P. Mosquito larvicidal and ovicidal properties of Eclipta alba (L.) Hassk (Asteraceae) against chikungunya vector, Aedes aegypti (Linn.) (Diptera: Culicidae). Asian Pac. J. Trop. Med. 2011, 4, 24–28. [Google Scholar] [CrossRef]
- Selvaraj, M.; Mosses, M. Efficacy of Melia azedarach on the larvae of three mosquito species Anopheles stephensi, Culex quinquefasciatus and Aedes aegypti (Diptera: Culicidae). Eur. Mosq. Bull. 2011, 29, 116–121. [Google Scholar]
- Shivakumar, M.S.; Srinivasan, R.; Natarajan, D. Larvicidal potential of some India medicinal plant extracts against Aedes aegypti (L). Asian J. Pharm. Clin. Res. 2013, 6, 77–80. [Google Scholar]
- World Health Organization (WHO). Guidelines for Laboratory and Field Testing of Mosquito Larvicides. Communicable Disease Control, Prevention and Eradication, WHO Pesticide Evaluation Scheme. Available online: http://www.who.int/whopes/guidelines/en/ (accessed on 14 April 2013).
- Oliveira, J.H.M.; Gonçalves, R.L.S.; Lara, F.A.; Dias, F.A.; Gandara, A.C.P.; Menna-Barreto, R.F.S.; Edwards, M.C.; Laurindo, F.R.M.; Silva-Neto, M.A.C.; Sorgine, M.H.F.; et al. Blood meal-derived heme decreases ROS levels in the midgut of Aedes aegypti and allows proliferation of intestinal microbiota. PLoS Pathog. 2011, 7, e1001320. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvador-Neto, O.; Gomes, S.A.; Soares, A.R.; Machado, F.L.d.S.; Samuels, R.I.; Nunes da Fonseca, R.; Souza-Menezes, J.; Moraes, J.L.d.C.; Campos, E.; Mury, F.B.; et al. Larvicidal Potential of the Halogenated Sesquiterpene (+)-Obtusol, Isolated from the Alga Laurencia dendroidea J. Agardh (Ceramiales: Rhodomelaceae), against the Dengue Vector Mosquito Aedes aegypti (Linnaeus) (Diptera: Culicidae). Mar. Drugs 2016, 14, 20. https://doi.org/10.3390/md14020020
Salvador-Neto O, Gomes SA, Soares AR, Machado FLdS, Samuels RI, Nunes da Fonseca R, Souza-Menezes J, Moraes JLdC, Campos E, Mury FB, et al. Larvicidal Potential of the Halogenated Sesquiterpene (+)-Obtusol, Isolated from the Alga Laurencia dendroidea J. Agardh (Ceramiales: Rhodomelaceae), against the Dengue Vector Mosquito Aedes aegypti (Linnaeus) (Diptera: Culicidae). Marine Drugs. 2016; 14(2):20. https://doi.org/10.3390/md14020020
Chicago/Turabian StyleSalvador-Neto, Orlando, Simone Azevedo Gomes, Angélica Ribeiro Soares, Fernanda Lacerda da Silva Machado, Richard Ian Samuels, Rodrigo Nunes da Fonseca, Jackson Souza-Menezes, Jorge Luiz da Cunha Moraes, Eldo Campos, Flávia Borges Mury, and et al. 2016. "Larvicidal Potential of the Halogenated Sesquiterpene (+)-Obtusol, Isolated from the Alga Laurencia dendroidea J. Agardh (Ceramiales: Rhodomelaceae), against the Dengue Vector Mosquito Aedes aegypti (Linnaeus) (Diptera: Culicidae)" Marine Drugs 14, no. 2: 20. https://doi.org/10.3390/md14020020
APA StyleSalvador-Neto, O., Gomes, S. A., Soares, A. R., Machado, F. L. d. S., Samuels, R. I., Nunes da Fonseca, R., Souza-Menezes, J., Moraes, J. L. d. C., Campos, E., Mury, F. B., & Silva, J. R. (2016). Larvicidal Potential of the Halogenated Sesquiterpene (+)-Obtusol, Isolated from the Alga Laurencia dendroidea J. Agardh (Ceramiales: Rhodomelaceae), against the Dengue Vector Mosquito Aedes aegypti (Linnaeus) (Diptera: Culicidae). Marine Drugs, 14(2), 20. https://doi.org/10.3390/md14020020