Lipid Composition of Oil Extracted from Wasted Norway Lobster (Nephrops norvegicus) Heads and Comparison with Oil Extracted from Antarctic Krill (Euphasia superba)
Abstract
:1. Introduction
2. Results
2.1. Geographical Variation in Catch Composition and Lipid Content in Nephrops Head Waste
2.2. Seasonal Variation in Nephrops Head Waste in the Clyde Sea Area
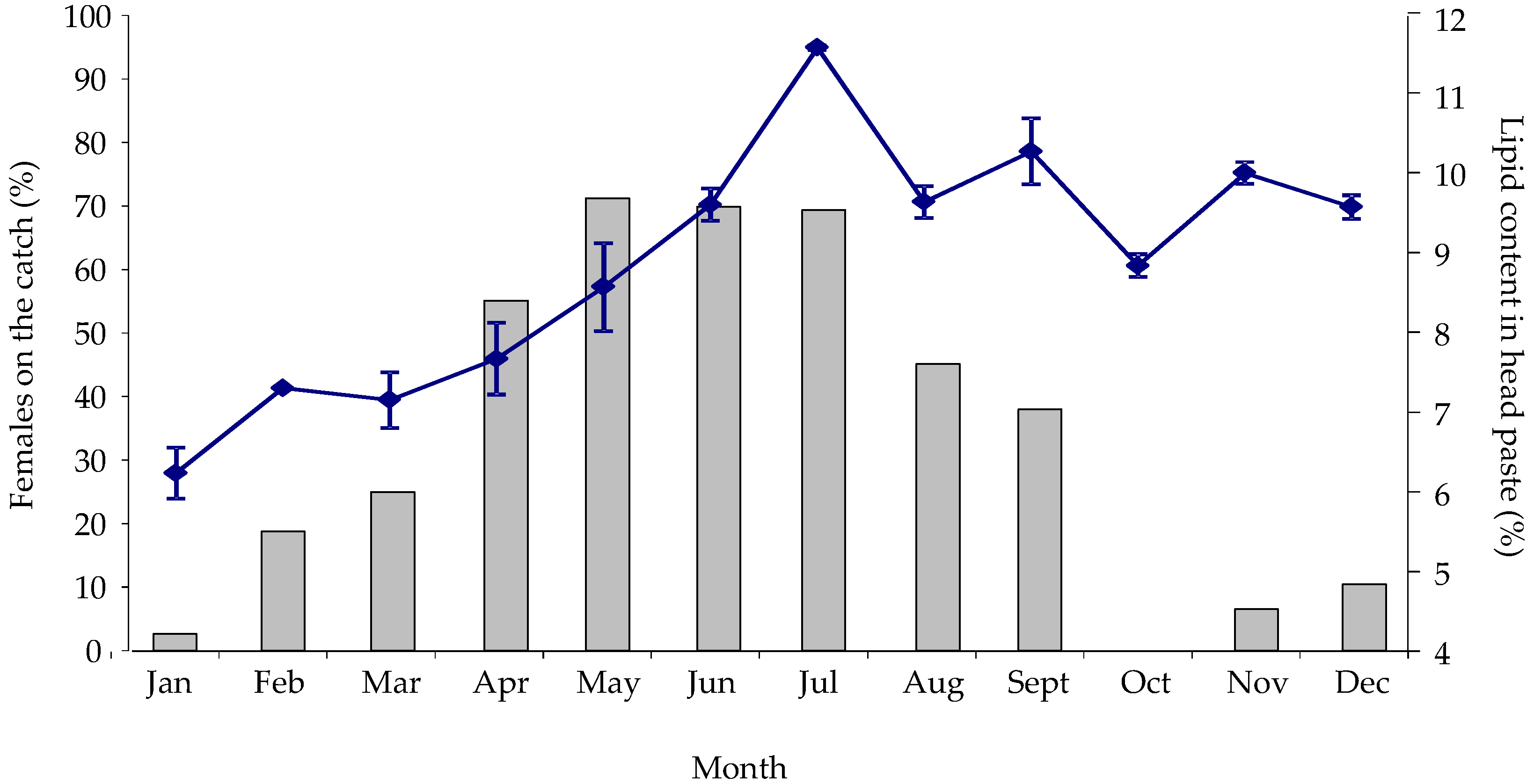
2.2.1. Seasonal Variability in Nephrops Catch Composition and Lipid Content
2.2.2. Body Indices and Lipid Content in Nephrops Tissue Samples
2.2.3. Fatty Acid Composition in Nephrops Head Waste
2.3. Comparison of Lipid Class and Fatty Acid Composition between Nephrops Head Waste and Krill Oil
3. Discussion
4. Materials and Methods
4.1. Geographic Variation in Catch Composition and Lipid Content in Nephrops Head Waste
4.2. Seasonality Variation in Nephrops Head Waste in the Clyde Sea Area
4.3. Initial Sample Processing and Transport of Samples
4.4. Comparison of Nephrops Head Waste Oil versus Oil Extracted from Krill
4.5. Head Waste and Tissue Sample Preparation
4.6. Total Lipid Extraction and Lipid Class Analysis
4.7. Fatty Acid Analysis: FAME Preparation and Gas Chromatography
4.8. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Barreto, E.; Bailey, N. Fish and shellfish stock. In Marine Scotland Communications, the Scottish Government; Marine Scotland, the Scottish Government: Edinburgh, UK, 2015. Available online: http://www.scotland.gov.uk/Publications/2015 (accessed on 9 September 2016). [Google Scholar]
- Bell, M.C.; Redant, F.; Tuck, I. Nephrops species. In Lobsters: Biology, Management, Aquaculture, and Fisheries; Phillips, B.F., Ed.; Blackwell Publishing Ltd.: Oxford, UK, 2006; pp. 412–461. [Google Scholar]
- Department for Environment, Food and Rural Affairs. New Common Fisheries Policy Deal Ends Discards. Available online: https://www.gov.uk/government/news/new-common-fisheries-policy-deal-ends-discards (accessed on 9 September 2016).
- Feekings, J.; Bartolino, V.; Madsen, N.; Catchpole, T. Fishery discards: Factors affecting their variability within a demersal trawl fishery. PLoS ONE 2012, 7, e36409. [Google Scholar] [CrossRef] [PubMed]
- Rosa, R.; Nunes, M.L. Changes in organ indices and lipid dynamics during the reproductive cycle of Aristeus antennatus, Parapenaeus longirostris, and Nephrops norvegicus (Decapoda) from the Portuguese south coast. Crustaceana 2002, 75, 1095–1105. [Google Scholar] [CrossRef]
- Tou, J.C.; Jaczynski, J.; Chen, Y. Krill for human consumption: Nutritional value and potential health benefits. Nutr. Rev. 2007, 65, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Ulven, S.M.; Kirkhus, B.; Lamglait, A.; Basu, S.; Elind, E.; Haider, T.; Berge, K.; Vik, H.; Pedersen, J.I. Metabolic effects of krill oil are essentially similar to those of fish oil but at a lower dose of EPA and DHA, in healthy volunteers. Lipids 2011, 46, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Salem, N.J.; Kuratko, C.N. A reexamination of krill oil bioavailability studies. Lipids Health Dis. 2014, 13. [Google Scholar] [CrossRef] [PubMed]
- Ghasenmifard, S.; Turchini, G.M.; Sinclair, A.J. Omega-3 long chain fatty acid “bioavailability”: A review of evidence and methodological considerations. Prog. Lipid Res. 2014, 56, 92–108. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre-Delaunay, D.; Pachiaudi, C.; Laville, M.; Pousin, J.; Amstrong, M.; Lagarde, M. Blood compartmental metabolism of docosahexaenoic acid (DHA) in humans after ingestión of a single dose of [13C]DHA in phosphatidylcholine. J. Lipid Res. 1999, 40, 1867–1874. [Google Scholar] [PubMed]
- Queiros, A.M.; Weetman, A.; McLay, H.A.; Dobby, H. Geographical variation in size at the onset of maturity of male and female Norway lobster Nephrops norvegicus (L., Homarida: Decapoda) in Scottish waters. Fish. Res. 2013, 139, 132–144. [Google Scholar] [CrossRef]
- Chapman, C.J.; Rice, A.L. Some direct observations on the ecology and behavior of the Norway lobster Nephrops norvegicus. Mar. Biol. 1971, 10, 321–329. [Google Scholar] [CrossRef]
- Mente, E.; Karapanagiotidis, I.T.; Logothetis, P.; Vafidis, D.; Malandrakis, E.; Neofitou, N.; Exadactylos, A.; Stratakos, A. The reproductive cycle of Norway lobster. J. Zool. 2009, 278, 324–332. [Google Scholar] [CrossRef]
- Milligan, R.J.; Albalat, A.; Atkinson, R.J.A.; Neil, D.M. The effects of trawling on the physical condition of the Norway lobster Nephrops norvegicus in relation to seasonal cycles in the Clyde Sea area. ICES J. Mar. Sci. 2009, 66, 488–494. [Google Scholar] [CrossRef]
- Krishnapillai, A.M.; Taylor, A.; Morris, A.E.J.; Quantick, P.C. Extraction and purification of hyaluronoglucosidase (EC 3.2.1.35) from Norway lobster (Nephrops norvegicus). Food Chem. 1999, 65, 359–365. [Google Scholar] [CrossRef]
- Rødde, R.H.; Einbu, A.; Varum, K.M. A seasonal study of the chemical composition and chitin quality of shrimp shells obtained from northern shrimp (Pandalus borealis). Carbohydr. Polym. 2008, 71, 388–393. [Google Scholar] [CrossRef]
- Kolakowska, A. The influence of sex and maturity stage of krill (Euphausia superba Dana) upon the content and composition of its lipids. Pol. Polar Res. 1991, 12, 73–78. [Google Scholar]
- Eiríksson, H. On the biennial breeding cycle of Nephrops at Iceland and how it relates to the fishery. ICES CM 1993, K:5, 1–18. [Google Scholar]
- Eiríksson, H. Reproductive biology of female Norway lobster, Nephrops norvegicus (Linnaeus, 1978) Leach, in Icelandic waters during the period 1960–2010: Comparative overview of distribution areas in the Northeast Atlantic and the Mediterranean. Adv. Mar. Biol. 2014, 68, 65–210. [Google Scholar] [PubMed]
- Saether, O.; Ellingsen, T.E.; Mohr, V. Lipids of North Atlantic krill. J. Lipid Res. 1986, 27, 274–285. [Google Scholar] [PubMed]
- Chapman, C.J. Ecology of juvenile and adult Nephrops. In The Biology and Management of Lobsters; Cobb, J.S., Phillips, B.F., Eds.; Academic Press: London, UK, 1980; pp. 143–178. [Google Scholar]
- Bailey, N. Some aspects of reproduction in Nephrops. ICES CM 1984, K:33, 1–26. [Google Scholar]
- Watts, A.J.R.; Albalat, A.; Smith, I.P.; Atkinson, R.J.A.; Neil, D.M. Seasonal nutritional status in Norway lobsters, Nephrops norvegicus (L.): Are females nutritionally compromised over the winter? Mar. Biol. Res. 2016, 12, 563–572. [Google Scholar] [CrossRef]
- Tuck, I.D.; Taylor, A.C.; Atkinson, R.J.A.; Gramitto, M.E.; Smith, C. Biochemical composition of Nephrops norvegicus: Changes associated with ovary maturation. Mar. Biol. 1997, 129, 505–511. [Google Scholar] [CrossRef]
- Rotllant, G.; Company, J.B.; Alvarez-Fernández, I.; García, J.A.; Aguzzi, J.; Durfort, M. The effects of seasonal variation on the nutritional condition of Nephrops norvegicus (Astacidae: Nephropidae) from wild populations in the western Mediterranean. J. Mar. Biol. Assoc. UK 2014, 94, 763–773. [Google Scholar] [CrossRef]
- Parslow-Williams, P.J. Nutritional Limitation in Populations of the Norway Lobster, Nephrops norvegicus (L.) in the Firth of Clyde, Scotland. Ph.D. Thesis, University of Glasgow, Glasgow, UK, 1998; p. 238. [Google Scholar]
- Glencross, B.D. Exploring the nutritional demand for essential fatty acids by aquaculture species. Rev. Aquac. 2009, 1, 71–124. [Google Scholar] [CrossRef]
- Maki, K.C.; Reeves, M.S.; Farmer, M.; Griinari, M.; Berge, K.; Vik, H.; Hubacher, R.; Rains, T.M. Krill oil supplementation increases plasma concentrations of eicospentaenoic and docosahexaenoic acids in overweight and obese men and women. Nutr. Res. 2009, 29, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Kwantes, J.M.; Grundmann, O. A brief review of krill oil history, research and the commercial market. J. Diet. Suppl. 2015, 12, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Wijendran, V.; Huang, M.C.; Diau, G.Y.; Boehm, F.; Nathanielsz, P.W.; Brenna, J.T. Efficacy of dietary arachidonic acid provided as triglyceride or phospholipid as substrates for brain arachidonic acid accretion in baboon neonates. Pediatr. Res. 2002, 51, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Farmer, A.S.D. Reproduction in Nephrops norvegicus (Decapoda: Nephropidae). J. Zool. 1974, 174, 161–183. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Tocher, D.R.; Harvie, D.G. Fatty acid compositions of the major phosphoglycerides from fish neural tissues; (n-3) and (n-6) polyunsaturated fatty acids in rainbow trout (Salmo gairdneri) and cod (Gadus morhua) brains and retinas. Fish Physiol. Biochem. 1988, 5, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Christie, W.W. Lipid Analyses, 2nd ed.; Pergamon Press: Oxford, UK, 1982; pp. 52–56. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014; Available online: http://www.R-project.org/ (accessed on 6 April 2016).
| Winter | Spring | Summer | Autumn | |
|---|---|---|---|---|
| Carapace Length (mm) | ||||
| Male | 33.0 ± 0.8 | 32.6 ± 1.4 | 32.6 ± 1.3 | 32.3 ± 0.8 |
| Female | 27.2 ± 0.5 a | 31.6 ± 1.1 a | 35.5 ± 1.9 b | 30.8 ± 1.8 a |
| Body weight (g) | ||||
| Male | 18.7 ± 1.6 | 20.4 ± 2.6 | 18.7 ± 2.1 | 17.1 ± 1.3 |
| Female | 10.6 ± 0.6 a | 18.7 ± 2.3 b | 25.8 ± 4.4 c | 19.6 ± 3.7 b |
| HSI (%) | ||||
| Male | 5.1 ± 0.2 a | 5.5 ± 0.2 a | 5.0 ± 0.3 a | 7.1 ± 0.7 b |
| Female | 5.2 ± 0.3 a | 6.1 ± 0.4 b | 5.5 ± 0.4 ab | 5.1 ± 0.4 ab |
| GSI (%) | ||||
| Female | 0.7 ± 0.1 a | 2.9 ± 0.3 b | 6.4 ± 0.5 c | 2.5 ± 1.0 ab |
| Stage 3/4 gonads (%) | ||||
| Female | 0 | 45.4 | 86.4 | 23.1 |
| Lipid content (%) | ||||
| Hepato Male | 46.4 ± 0.2 a | 28.5 ± 1.2 b | 46.2 ± 0.6 a | 47.6 ± 0.5 a |
| Hepato Female | 56.1 ± 0.7 a | 41.9 ± 1.3 b | 46.4 ± 1.6 b | 58.4 ± 1.6 a |
| Gonad (Female) | 17.4 ± 1.1 a | 23.4 ± 0.5 b | 26.4 ± 0.8 b | 24.3 ± 0.50 b |
| Sampling Location | |||
|---|---|---|---|
| Clyde | Minch | Iceland | |
| Females in the catch (%) | 70 | 66 | 42 |
| Total lipid head paste (%) | 9.6 ± 0.2 c | 7.7 ± 0.2 b | 5.3 ± 0.1 a |
| HSI (%) | |||
| Males | 5.5 ± 0.3 b | 3.3 ± 0.9 a | 3.2 ± 0.2 a |
| Females | 5.8 ± 0.3 c | 3.7 ± 0.2 b | 2.11 ± 0.2 a |
| GSI (%) | |||
| Females | 4.1 ± 0.4 b | 3.9 ± 1.4 b | 2.6 ± 0.3 a |
| Females at gonad stage 2 | 17 | 14 | 58 |
| Females at gonad stage 3/4 | 74 | 81 | 0 |
| Lipid content (%) | |||
| Hepato Males | 37.7 ± 0.4 a | 46.3 ± 0.1 b | 43.3 ± 1.6 b |
| Hepato Females | 45.0 ± 0.5 c | 38.4 ± 1.7 b | 31.3 ± 0.1 a |
| Gonad (Females) | 25.9 ± 0.6 b | 23.9 ± 0.5 b | 13.7 ± 0.5 a |
| Fatty Acid (%) | Winter | Spring | Summer | Autumn |
|---|---|---|---|---|
| 14:0 | 1.1 | 1.4 | 1.6 | 1.7 |
| 15:0 | 0.7 | 0.6 | 0.6 | 0.6 |
| 16:0 | 12.5 | 12.1 | 12.6 | 13.6 |
| 18:0 | 4.2 | 3.8 | 3.9 | 4.1 |
| 19:0 | 0.2 | 0.2 | 0.2 | 0.2 |
| 20:0 | 0.4 | 0.4 | 0.2 | 0.4 |
| 22:0 | 0.1 | 0.4 | n.d. | 0.4 |
| Total saturated | 22.9 | 22.2 | 22.0 | 24.4 |
| 16:1n-9 | 0.2 | 0.2 | 0.3 | 0.3 |
| 16:1n-7 | 5.6 | 6.6 | 7.3 | 6.2 |
| 17:1 | 0.8 | 0.6 | 0.6 | 0.6 |
| 18:1n-9 | 14.8 | 13.3 | 13.0 | 13.0 |
| 18:1n-7 | 8.3 | 7.8 | 7.8 | 8.3 |
| 19:1 | 0.3 | 0.4 | 0.4 | 0.4 |
| 20:1n-11 | 4.1 | 3.1 | 3.4 | 3.1 |
| 20:1n-9 | 3.8 | 3.1 | 2.6 | 3.1 |
| 20:1n-7 | 5.7 | 4.5 | 4.5 | 4.2 |
| 22:1n-11 | 1.0 | 1.2 | 0.5 | 0.9 |
| 22:1n-9 | 0.6 | 0.7 | 0.5 | 0.9 |
| 22:1n-7 | 1.3 | 1.0 | 0.8 | 1.0 |
| Total monounsaturated | 46.5 | 42.4 | 41.8 | 42.1 |
| 18:2n-6 | 0.7 | 0.7 | 0.8 | 0.9 |
| 18:3n-6 | 0.2 | 0.2 | 0.1 | n.d. |
| 20:2n-6 | 1.2 | 1.1 | 1.0 | 1.0 |
| 20:3n-6 | 0.2 | 0.1 | 0.1 | 0.2 |
| 20:4n-6 | 4.0 | 3.8 | 3.8 | 3.4 |
| 22:4n-6 | 0.7 | 0.6 | 0.8 | 0.8 |
| 22:5n-6 | 0.6 | 0.5 | 0.4 | 0.5 |
| Total n-6 PUFA | 7.6 | 7.0 | 7.1 | 6.7 |
| 18:3n-3 | 0.2 | 0.2 | 0.2 | 0.3 |
| 18:4n-3 | 0.3 | 0.7 | 0.8 | 0.7 |
| 20:3n-3 | 0.2 | 0.2 | 0.2 | 0.2 |
| 20:4n-3 | 0.3 | 0.4 | 0.4 | 0.4 |
| 20:5n-3 | 10.5 | 13.9 | 15.5 | 13.1 |
| 22:5n-3 | 1.8 | 1.7 | 2.1 | 2.0 |
| 22:6n-3 | 8.5 | 9.6 | 8.4 | 8.5 |
| Total n-3 PUFA | 21.7 | 26.5 | 27.6 | 25.2 |
| Lipid Class (% Total Lipid) | Nephrops | Krill |
|---|---|---|
| Sterol esters | 3.85 ± 0.01 | 5.60 ± 0.55 |
| Triacylglycerols | 33.93 ± 2.52 | 37.99 ± 1.77 |
| Free fatty acids | 14.99 ± 0.43 | 9.90 ± 0.44 |
| Cholesterol/sterols | 14.89 ± 0.89 | 10.53 ± 0.58 |
| Unknown neutral lipid ¶ | 0.57 ± 0.17 | 3.48 ± 0.11 |
| Total neutral lipids | 68.23 ± 1.01 | 67.49 ± 0.30 |
| Monogalactosyldiacylglycerols | n.d. | n.d. |
| Unknown glycolipid | n.d. | n.d. |
| Digalactosyldiacylglycerols | n.d. | n.d. |
| Unknown polar lipid * | n.d. | 1.22 ± 0.05 |
| Phosphatidylethanolamine | 7.42 ± 0.05 | 6.70 ± 0.11 |
| Phosphatidic acid/Phosphatidylglycerol/cardiolipin | 1.50 ± 0.06 | 1.52 ± 0.18 |
| Phosphatidylinositol | 2.42 ± 0.03 | 1.29 ± 0.11 |
| Phosphatidylserine | 2.65 ± 0.20 | 0.90 ± 0.17 |
| Phosphatidylcholine | 14.04 ± 0.76 | 18.01 ± 0.72 |
| Sphingomyelin | 1.15 ± 0.18 | n.d. |
| Lysophosphatidylcholine | 1.11 ± 0.15 | 2.49 ± 0.26 |
| Pigmented material | 1.48 ± 0.06 | 0.39 ± 0.13 |
| Total polar lipids | 31.77 ± 1.01 | 32.51 ± 0.30 |
| Fatty Acid (%) | Neutral Lipids | Polar Lipids | ||
|---|---|---|---|---|
| Nephrops | Krill | Nephrops | Krill | |
| 14:0 | 3.3 | 18.7 | 1.0 | 2.2 |
| 15:0 | 0.7 | 0.6 | 0.6 | 0.4 |
| 16:0 | 12.7 | 23.7 | 12.6 | 21.8 |
| 18:0 | 3.4 | 1.8 | 6.2 | 1.6 |
| 19:0 | 0.5 | n.d. | 0.2 | 0.2 |
| 20:0 | 0.3 | 0.2 | 0.3 | n.d. |
| 22:0 | 0.2 | n.d. | n.d. | 0.2 |
| Total saturated | 21.3 | 45.1 | 21.3 | 26.5 |
| 16:1n-9 | 0.4 | 1.0 | 0.8 | 0.2 |
| 16:1n-7 | 9.6 | 12.5 | 5.3 | 1.9 |
| 17:1 | 0.5 | 0.4 | 0.5 | 0.2 |
| 18:1n-9 | 13.1 | 18.3 | 11.7 | 7.9 |
| 18:1n-7 | 7.2 | 8.1 | 5.7 | 6.8 |
| 19:1 | 0.4 | n.d. | 0.3 | n.d. |
| 20:1n-11 | 3.3 | n.d. | 1.3 | n.d. |
| 20:1n-9 | 3.2 | 1.4 | 2.1 | 0.6 |
| 20:1n-7 | 4.6 | 0.5 | 2.0 | 0.2 |
| 22:1n-11 | 2.0 | n.d. | 0.4 | n.d. |
| 22:1n-9 | 0.9 | 0.4 | n.d. | 1.0 |
| 24:1n-9 | 0.3 | n.d. | n.d. | 0.4 |
| Total monounsaturated | 45.5 | 42.5 | 30.0 | 19.3 |
| 18:2n-6 | 1.1 | 1.3 | 1.2 | 2.3 |
| 18:3n-6 | 0.2 | 0.2 | 0.3 | n.d. |
| 20:2n-6 | 1.0 | n.d. | 0.8 | n.d. |
| 20:3n-6 | 0.2 | n.d. | n.d. | n.d. |
| 20:4n-6 | 2.5 | 0.1 | 4.0 | 1.0 |
| 22:4n-6 | 0.5 | n.d. | 0.5 | 0.2 |
| 22:5n-6 | 0.4 | n.d. | 0.3 | 0.7 |
| Total n-6 PUFA | 5.9 | 1.7 | 7.1 | 4.1 |
| 18:3n-3 | 0.3 | 0.5 | 0.4 | 1.0 |
| 18:4n-3 | 0.9 | 2.1 | 0.8 | 1.5 |
| 20:3n-3 | 0.2 | n.d. | n.d. | n.d. |
| 20:4n-3 | 0.4 | 0.1 | 0.4 | 0.4 |
| 20:5n-3 | 15.0 | 4.3 | 22.0 | 26.1 |
| 22:5n-3 | 1.4 | 0.1 | 2.1 | n.d. |
| 22:6n-3 | 8.3 | 2.3 | 15.4 | 20.8 |
| Total n-3 PUFA | 26.6 | 9.4 | 41.3 | 49.8 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albalat, A.; Nadler, L.E.; Foo, N.; Dick, J.R.; Watts, A.J.R.; Philp, H.; Neil, D.M.; Monroig, O. Lipid Composition of Oil Extracted from Wasted Norway Lobster (Nephrops norvegicus) Heads and Comparison with Oil Extracted from Antarctic Krill (Euphasia superba). Mar. Drugs 2016, 14, 219. https://doi.org/10.3390/md14120219
Albalat A, Nadler LE, Foo N, Dick JR, Watts AJR, Philp H, Neil DM, Monroig O. Lipid Composition of Oil Extracted from Wasted Norway Lobster (Nephrops norvegicus) Heads and Comparison with Oil Extracted from Antarctic Krill (Euphasia superba). Marine Drugs. 2016; 14(12):219. https://doi.org/10.3390/md14120219
Chicago/Turabian StyleAlbalat, Amaya, Lauren E. Nadler, Nicholas Foo, James R. Dick, Andrew J. R. Watts, Heather Philp, Douglas M. Neil, and Oscar Monroig. 2016. "Lipid Composition of Oil Extracted from Wasted Norway Lobster (Nephrops norvegicus) Heads and Comparison with Oil Extracted from Antarctic Krill (Euphasia superba)" Marine Drugs 14, no. 12: 219. https://doi.org/10.3390/md14120219
APA StyleAlbalat, A., Nadler, L. E., Foo, N., Dick, J. R., Watts, A. J. R., Philp, H., Neil, D. M., & Monroig, O. (2016). Lipid Composition of Oil Extracted from Wasted Norway Lobster (Nephrops norvegicus) Heads and Comparison with Oil Extracted from Antarctic Krill (Euphasia superba). Marine Drugs, 14(12), 219. https://doi.org/10.3390/md14120219






