Macrolactins from Marine-Derived Bacillus subtilis B5 Bacteria as Inhibitors of Inducible Nitric Oxide and Cytokines Expression
Abstract
:1. Introduction
2. Results
2.1. Structural Identification of 7,13-Epoxyl-macrolactin A (1)
2.2. Anti-Inflamatory Activity of Macrolactins
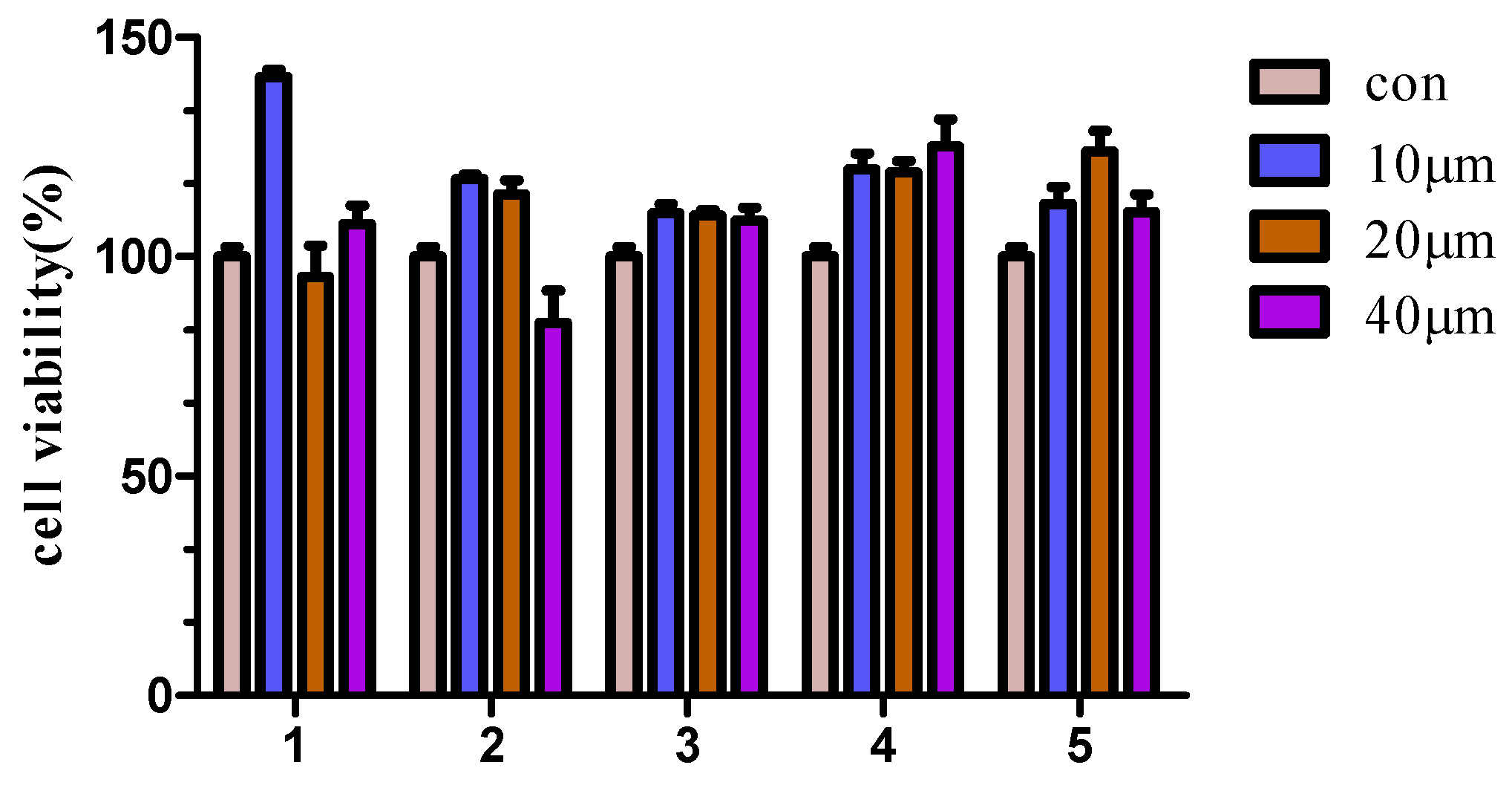
2.2.1. Cytotoxicity
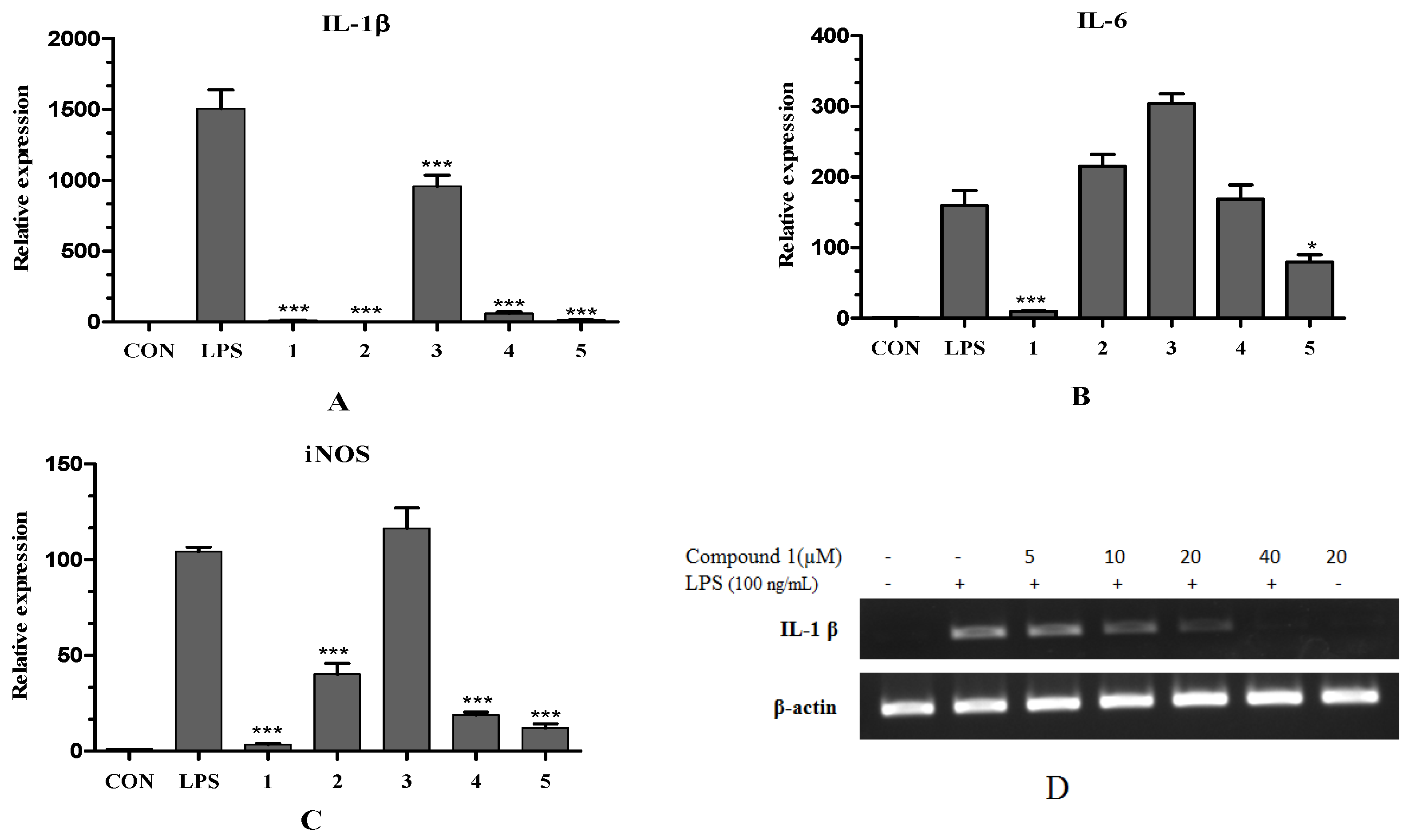
2.2.2. Inhibitory Effect of Compounds on LPS-Induced iNOS, IL-1β and IL-6 mRNA Expression
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Bacteria and Fermentation
3.3. Extraction and Isolation
3.4. Cell Cultivation
3.5. Cytotoxicity Assay
3.6. Total RNA Isolation
3.7. Real-Time PCR Analysis of iNOS, IL-1β and IL-6 mRNA
3.8. Reverse Transcription-PCR Analysis of IL-1β mRNA
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Libby, P.; Ridker, P.M.; Hansson, G.K. Inflammation in atherosclerosisfrom pathophysiology to practice. J. Am. Coll. Cardiol. 2009, 54, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, R.; Watanabe, M. Role of epithelial cells in the pathogenesis and treatment of inflammatory bowel disease. J. Gastroenterol. 2016, 51, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Crusz, S.M.; Balkwill, F.R. Inflammation and cancer: Advances and new agents. Nat. Rev. Clin. Oncol. 2015, 12, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Pickup, J.C. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care 2004, 27, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Kurakula, K.; Vos, M.; Logiantara, A.; Roelofs, J.J.; Nieuwenhuis, M.A.; Koppelman, G.H.; Postma, D.S.; van Rijt, L.S.; de Vries, C.J.M. Nuclear receptor Nur77 attenuates airway inflammation in mice by suppressing NF-κB activity in lung epithelial cells. J. Immunol. 2015, 195, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Banerjee, S.; Sil, P.C. The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: A recent update. Food Chem. Toxicol. 2015, 83, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Jean, Y.-H.; Chen, W.-F.; Duh, C.-Y.; Huang, S.-Y.; Hsu, C.-H.; Lin, C.-S.; Sung, C.-S.; Chen, I.M.; Wen, Z.-H. Inducible nitric oxide synthase and cyclooxygenase-2 participate in anti-inflammatory and analgesic effects of the natural marine compound lemnalol from formosan soft coral lemnalia cervicorni. Eur. J. Pharmacol. 2008, 578, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.-X.; Zhang, J.-H.; Zhang, Y.; Meng, D.-L.; Yan, D. Anti-inflammatory activity studies on the stems and roots of Jasminum lanceolarium Roxb. J. Ethnopharmacol. 2015, 171, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.-S.; Huang, S.-S.; Lin, T.-H.; Lee, M.-M.; Kuo, C.-C.; Sung, P.-J.; Hou, W.-C.; Huang, G.-J.; Kuo, Y.-H. Analgesic and anti-inflammatory bioactivities of eburicoic acid and dehydroeburicoic acid isolated from antrodia camphorata on the inflammatory mediator expression in mice. J. Agric. Food Chem. 2013, 61, 5064–5071. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.Y.; Zhu, J.X.; Bian, T.; Gao, F.; Qian, X.F.; Du, Q.; Yuan, M.Y.; Sun, H.; Shi, L.Z.; Yu, M.H. Protective effects of astragaloside IV against ovalbumin-induced lung inflammation are regulated/mediated by T-bet/GATA-3. Pharmacology 2014, 94, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.-J.; Heo, S.-J.; Han, S.-C.; Lee, H.-J.; Kang, G.-J.; Kang, H.-K.; Hyun, J.-W.; Koh, Y.-S.; Yoo, E.-S. Anti-inflammatory effect of sargachromanol G isolated from Sargassum siliquastrum in RAW 264.7 cells. Arch. Pharm. Res. 2012, 35, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.; Jachak, S.M. Recent developments in anti-inflammatory natural products. Med. Res. Rev. 2009, 29, 767–820. [Google Scholar] [CrossRef] [PubMed]
- Cuong, N.X.; Thao, N.P.; Luyen, B.T.T.; Ngan, N.T.T.; Thuy, D.T.T.; Song, S.B.; Nam, N.H.; Kiem, P.V.; Kim, Y.H.; Minh, C.V. Cembranoid diterpenes from the soft coral Lobophytum crassum and their anti-inflammatory activities. Chem. Pharm. Bull. 2014, 62, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-S.; Cui, X.; Lee, D.-S.; Sohn, J.; Yim, J.; Kim, Y.-C.; Oh, H. Anti-inflammatory effect of neoechinulin a from the marine fungus Eurotium sp. SF-5989 through the suppression of NF-κB and p38 MAPK pathways in lipopolysaccharide-stimulated RAW 264.7 macrophages. Molecules 2013, 18, 13245–13259. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-P.; Huang, S.-Y.; Lin, Y.-Y.; Wang, H.-M.; Jean, Y.-H.; Wu, S.-F.; Duh, C.-Y.; Wen, Z.-H. Soft coral-derived lemnalol alleviates monosodium urate-induced gouty arthritis in rats by inhibiting leukocyte infiltration and iNOS, COX-2 and c-Fos protein expression. Mar. Drugs 2013, 11, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.G.; Mesquita, J.X.; Aragão, K.S.; Franco, Á.X.; Souza, M.H.L.P.; Brito, T.V.; Dias, J.M.; Silva, R.O.; Medeiros, J.-V.R.; Oliveira, J.S.; et al. Polysaccharides isolated from Digenea simplex inhibit inflammatory and nociceptive responses. Carbohydr. Polym. 2014, 108, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.; Guihéneuf, F.; Bahar, B.; Schmid, M.; Stengel, D.; Fitzgerald, G.; Ross, R.; Stanton, C. The anti-inflammatory effect of algae-derived lipid extracts on lipopolysaccharide (LPS)-stimulated human THP-1 macrophages. Mar. Drugs 2015, 13, 5402–5424. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Pil, G.; Heo, S.-J.; Lee, H.-S.; Lee, J.; Lee, Y.-J.; Lee, J.; Won, H. Anti-inflammatory activity of tanzawaic acid derivatives from a marine-derived fungus Penicillium steckii 108YD142. Mar. Drugs 2016, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tang, X.-X.; Yan, X.; Wu, Z.; Yi, Z.-W.; Fang, M.-J.; Su, X.; Qiu, Y.-K. A new macrolactin antibiotic from deep sea-derived bacteria Bacillus subtilis B5. Nat. Prod. Res. 2016, 1–6. [Google Scholar] [CrossRef]
- Lu, X.-L.; Xu, Q.-Z.; Liu, X.-Y.; Cao, X.; Ni, K.-Y.; Jiao, B.-H. Marine drugs—Macrolactins. Chem. Biodivers. 2008, 5, 1669–1674. [Google Scholar] [CrossRef] [PubMed]
- Mondol, M.A.M.; Tareq, F.S.; Kim, J.H.; Lee, M.A.; Lee, H.-S.; Lee, Y.-J.; Lee, J.S.; Shin, H.J. Cyclic ether-containing macrolactins, antimicrobial 24-membered isomeric macrolactones from a marine Bacillus sp. J. Nat. Prod. 2011, 74, 2582–2587. [Google Scholar] [CrossRef] [PubMed]
- Tareq, F.S.; Kim, J.H.; Lee, M.A.; Lee, H.-S.; Lee, J.-S.; Lee, Y.-J.; Shin, H.J. Antimicrobial gageomacrolactins characterized from the fermentation of the marine-derived bacterium Bacillus subtilis under optimum growth conditions. J. Agric. Food Chem. 2013, 61, 3428–3434. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, K.; Roman, M.; Fenical, W. The macrolactins, a novel class of antiviral and cytotoxic macrolides from a deep-sea marine bacterium. J. Am. Chem. Soc. 1989, 111, 7519–7524. [Google Scholar] [CrossRef]
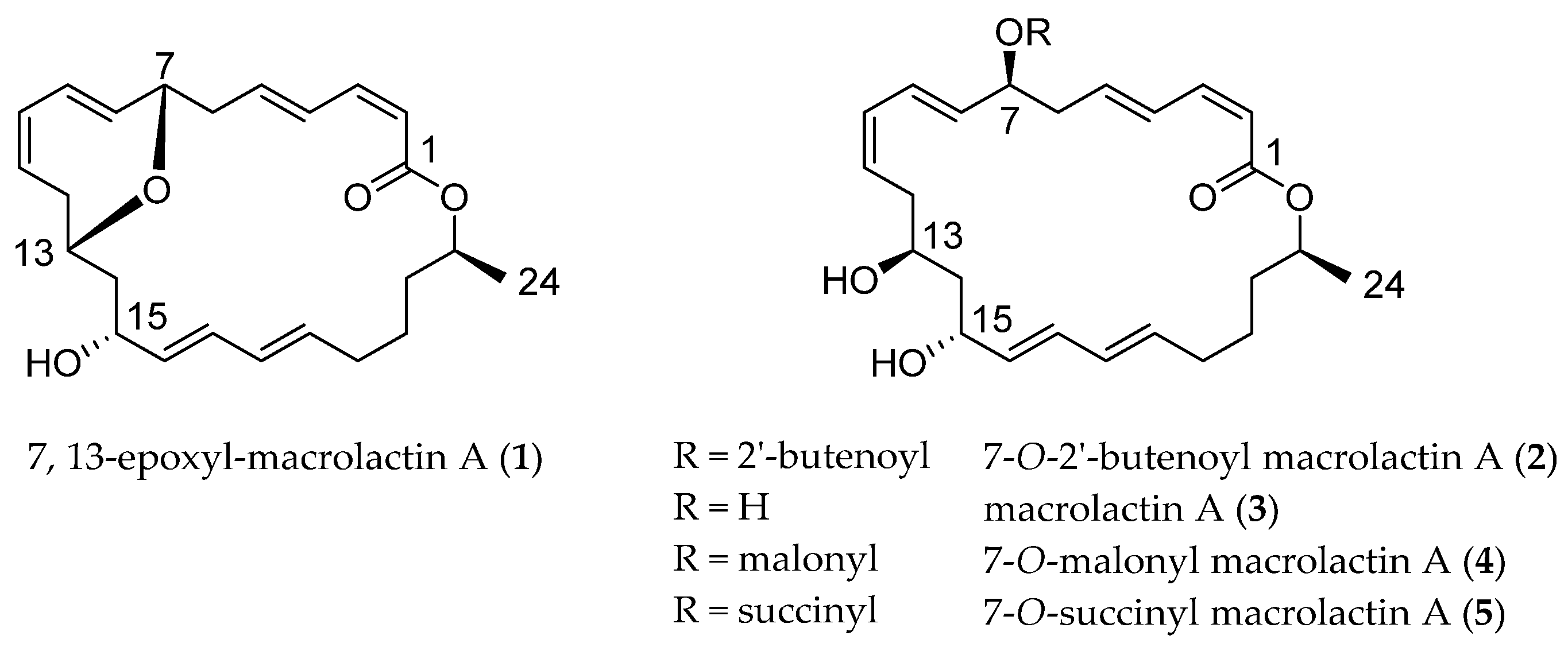
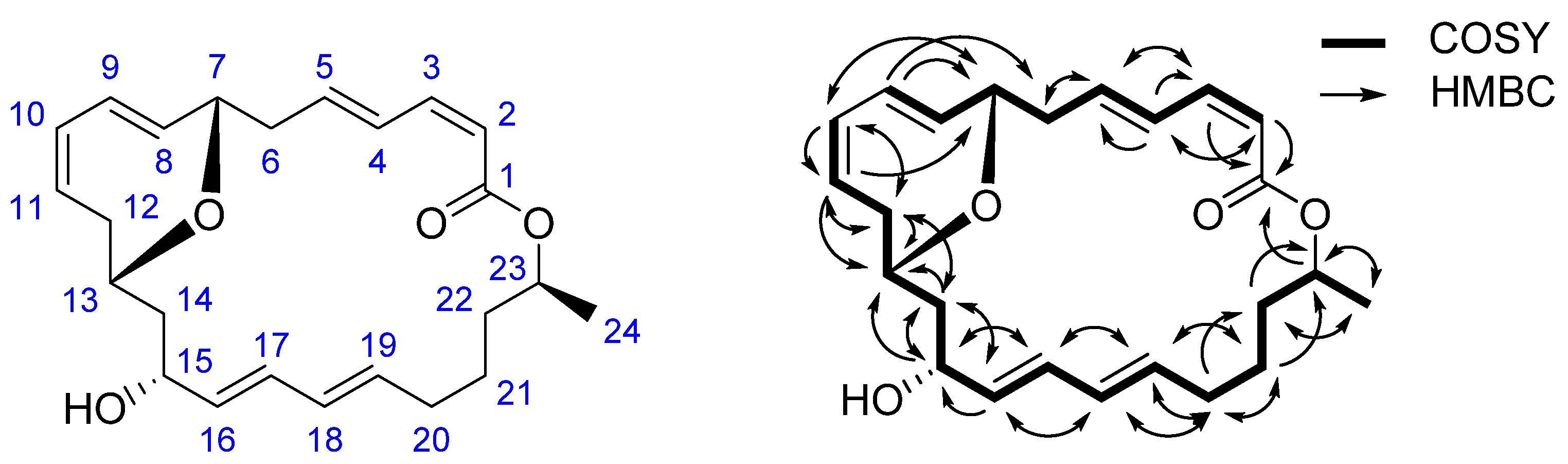
| Position | δH (J in Hz) | δC, Multiple | 1H–1H COSY | HMBC | |
|---|---|---|---|---|---|
| 1 | 166.0 | C | |||
| 2 | 5.59 br.d (11.4) | 117.8 | CH | H-3 | C-1,4 |
| 3 | 6.69 br.t (11.4) | 143.4 | CH | H-2,4 | C-1,5 |
| 4 | 7.11 br.dd (14.7, 11.7) | 128.1 | CH | H-3,5 | C-2,3,5 |
| 5 | 6.17 dt (14.9, 7.4) | 142.1 | CH | H-4,6 | C-3,6 |
| 6 | 2.91 m | 36.0 | CH2 | H-5 | C-5 |
| 7 | 4.56 br.s | 72.2 | CH | H-8 | n. o. a |
| 8 | 5.48 overlapped | 130.6 | CH | H-7,9 | C-6 |
| 9 | 5.54 m | 129.7 | CH | H-8 | C-5,6,7 |
| 10 | 5.71 br.d (10.5) | 128.4 | CH | H-11 | C-7 |
| 11 | 5.81 m | 125.2 | CH | H-10,12 | C-12 |
| 12 | 1.98 m and 1.87 m | 31.3 | CH2 | H-11,13 | C-8,10,13,14 |
| 13 | 3.50 overlapped | 65.0 | CH | H-12,14 | n. o. |
| 14 | 1.68 m and 1.61 m | 44.1 | CH2 | H-13,15 | C-15,16 |
| 15 | 3.93 br.s | 69.4 | CH | H-14,16 | C-14 |
| 16 | 5.48 overlapped | 135.3 | CH | H-15,17 | C-14,15 |
| 17 | 6.06 br.dd (14.9, 10.5) | 130.1 | CH | H-16,18 | C-15 |
| 18 | 5.97 br.dd (14.9, 10.6) | 130.3 | CH | H-17,19 | C-16,20 |
| 19 | 5.67 m | 133.9 | CH | H-18,20 | C-20,21 |
| 20 | 2.13 m and 2.00 m | 32.1 | CH2 | H-19,21 | C-19,21,22 |
| 21 | 1.44 m | 25.4 | CH2 | H-20,22 | C-19,20,22 |
| 22 | 1.59 m | 35.3 | CH2 | H-21,23 | C-20,21,24 |
| 23 | 4.96 m | 70.8 | CH | H-22,24 | C-1,21,22,24 |
| 24 | 1.22 d (6.2) | 20.4 | CH3 | H-23 | C-22,23 |
| 15-OH | 4.65 br.s | ||||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Zhou, Y.-X.; Tang, X.-X.; Liu, X.-X.; Yi, Z.-W.; Fang, M.-J.; Wu, Z.; Jiang, F.-Q.; Qiu, Y.-K. Macrolactins from Marine-Derived Bacillus subtilis B5 Bacteria as Inhibitors of Inducible Nitric Oxide and Cytokines Expression. Mar. Drugs 2016, 14, 195. https://doi.org/10.3390/md14110195
Yan X, Zhou Y-X, Tang X-X, Liu X-X, Yi Z-W, Fang M-J, Wu Z, Jiang F-Q, Qiu Y-K. Macrolactins from Marine-Derived Bacillus subtilis B5 Bacteria as Inhibitors of Inducible Nitric Oxide and Cytokines Expression. Marine Drugs. 2016; 14(11):195. https://doi.org/10.3390/md14110195
Chicago/Turabian StyleYan, Xia, Yun-Xia Zhou, Xi-Xiang Tang, Xiu-Xiu Liu, Zhi-Wei Yi, Mei-Juan Fang, Zhen Wu, Fu-Quan Jiang, and Ying-Kun Qiu. 2016. "Macrolactins from Marine-Derived Bacillus subtilis B5 Bacteria as Inhibitors of Inducible Nitric Oxide and Cytokines Expression" Marine Drugs 14, no. 11: 195. https://doi.org/10.3390/md14110195
APA StyleYan, X., Zhou, Y.-X., Tang, X.-X., Liu, X.-X., Yi, Z.-W., Fang, M.-J., Wu, Z., Jiang, F.-Q., & Qiu, Y.-K. (2016). Macrolactins from Marine-Derived Bacillus subtilis B5 Bacteria as Inhibitors of Inducible Nitric Oxide and Cytokines Expression. Marine Drugs, 14(11), 195. https://doi.org/10.3390/md14110195







