New Metabolites and Bioactive Actinomycins from Marine-Derived Streptomyces sp. ZZ338
Abstract
:1. Introduction
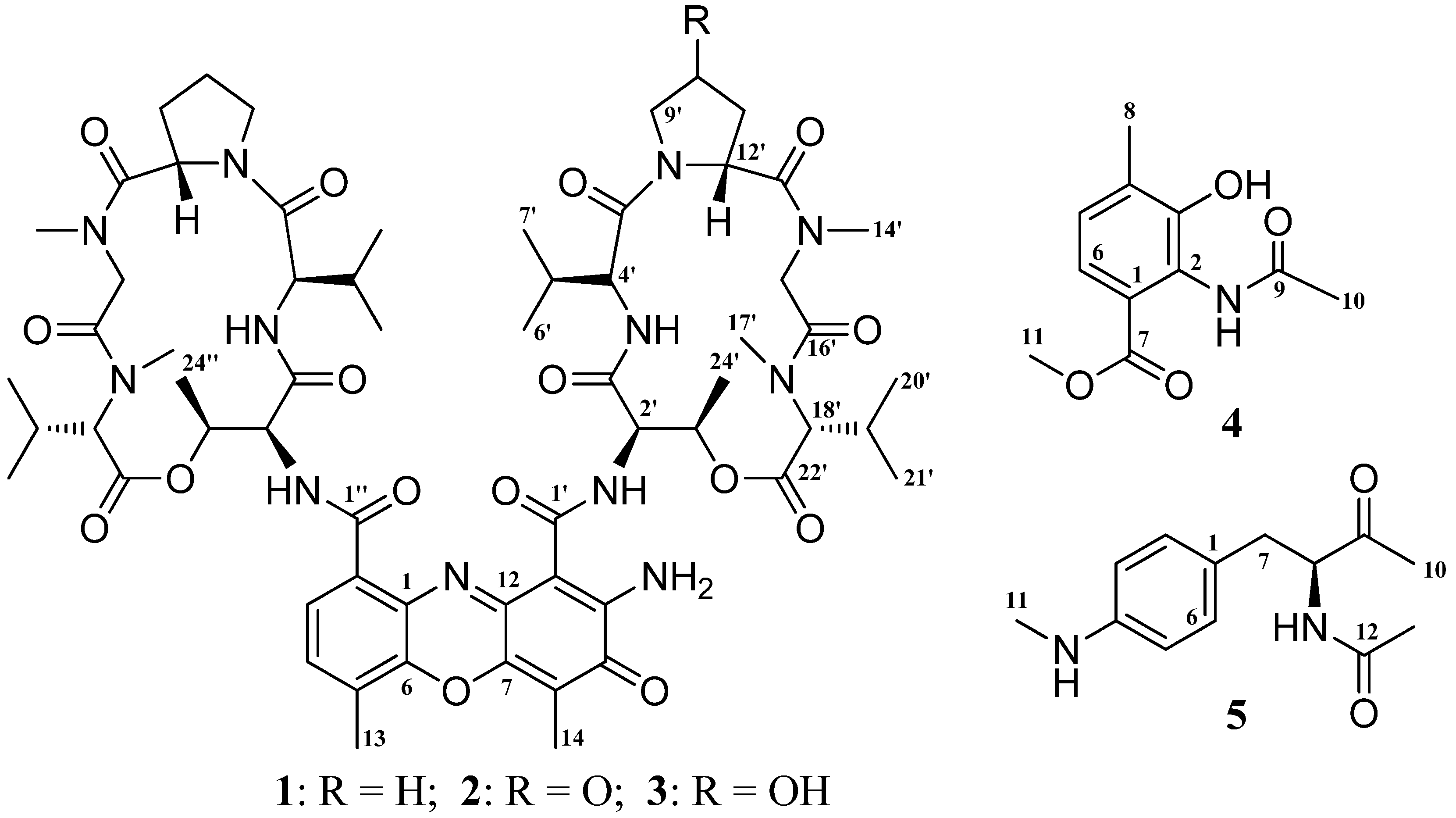
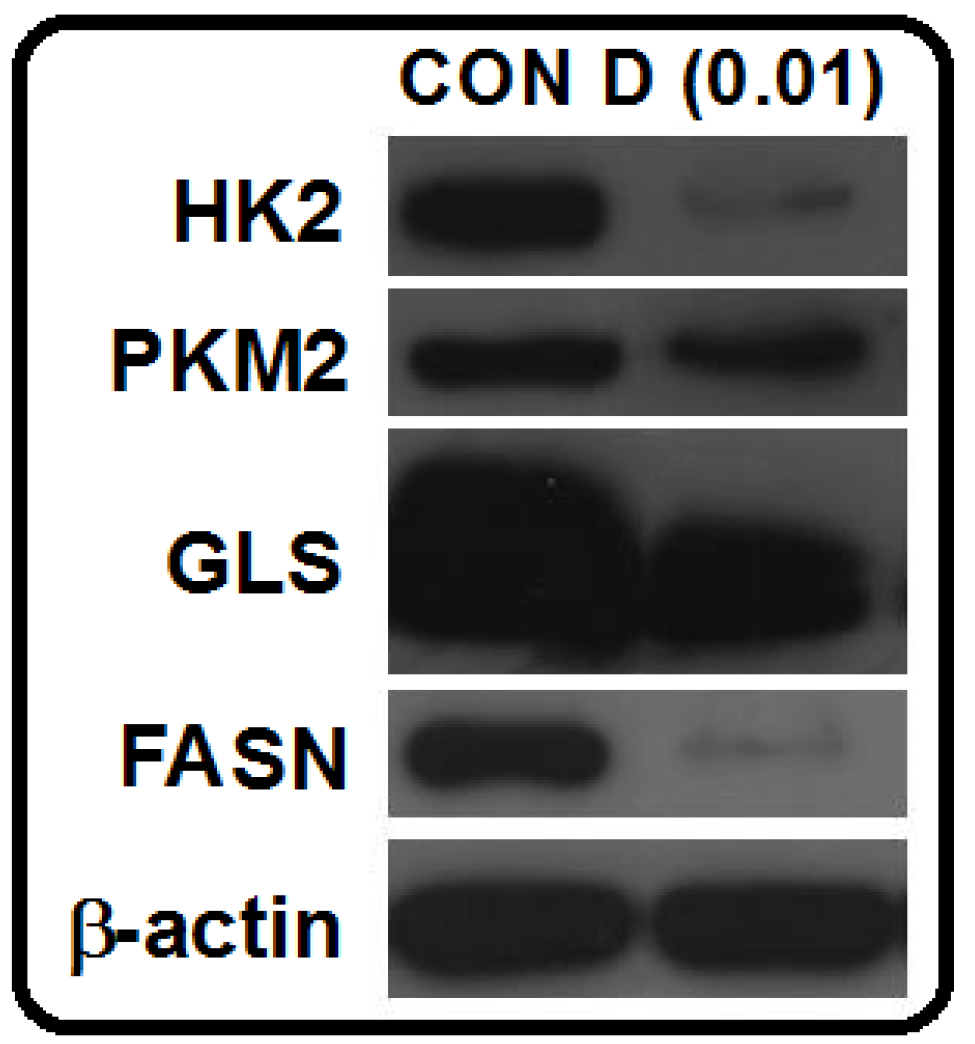
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Isolation and Identification of Strain ZZ338
3.3. Fermentation of Strain ZZ338
3.4. Isolation of Compounds 1–5
3.5. Antimicrobial Assay
3.6. Tumor Cell Culture
3.7. Sulforhodamine B (SRB) Assay
3.8. Western Blot Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kamran, N.; Calinescu, A.; Candolfi, M.; Chandran, M.; Mineharu, Y.; Asad, A.S.; Koschmann, C.; Nunez, F.J.; Lowenstein, P.R.; Castro, M.G. Recent advances and future of immunotherapy for glioblastoma. Expert Opin. Biol. Ther. 2016, 16, 1245–1264. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, M.C. Temozolomide: Therapeutic limitations in the treatment of adult high-grade gliomas. Expert Rev. Neurother. 2010, 10, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Piel, J. Metabolites from symbiotic bacteria. Nat. Prod. Rep. 2004, 21, 519–538. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.W.; Radax, R.; Steger, D.; Wagner, M. Sponge-associated microorganisms: Evolution, ecology, and biotechnological potential. Microbiol. Mol. Biol. Rev. 2007, 71, 295–347. [Google Scholar] [CrossRef] [PubMed]
- Searle, P.A.; Molinski, T.F. Phorboxazoles A and B: Potent cytostatic macrolides from marine sponge Phorbas species. J. Am. Chem. Soc. 1995, 117, 8126–8131. [Google Scholar] [CrossRef]
- Dunlap, W.C.; Battershill, C.N.; Liptrot, C.H.; Cobb, R.E.; Bourne, D.G.; Jaspars, M.; Long, P.F.; Newman, D.J. Biomedicinals from the phytosymbionts of marine invertebrates: A molecular approach. Methods 2007, 42, 358–376. [Google Scholar] [CrossRef] [PubMed]
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2009, 8, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Gulder, T.A.; Moore, B.S. Chasing the treasures of the sea bacterial marine natural products. Curr. Opin. Microbiol. 2009, 12, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Rateb, M.E.; Ebel, R. Secondary metabolites of fungi from marine habitats. Nat. Prod. Rep. 2011, 28, 290–344. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.; Ye, X.; Yu, S.; Lian, X.Y.; Zhang, Z. New capoamycin-type antibiotics and polyene acids from marine Streptomyces fradiae PTZ0025. Mar. Drugs 2012, 10, 2388–2402. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Ye, X.; Chen, L.; Lian, X.Y.; Zhang, Z. Polyoxygenated 24,28-epoxyergosterols inhibiting the proliferation of glioma cells from sea anemone Anthopleura midori. Steroids 2014, 88, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Ye, X.; Huang, H.; Peng, R.; Su, Z.; Lian, X.Y.; Zhang, Z. Bioactive sulfated saponins from sea cucumber Holothuria moebii. Planta Med. 2015, 81, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liang, Y.; Song, T.; Anjum, K.; Wang, W.; Yu, S.; Huang, H.; Lian, X.Y.; Zhang, Z. Synthesis and bioactivity of tripolinolate A from Tripolium vulgare and its analogs. Bioorg. Med. Chem. Lett. 2015, 25, 2629–2633. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Anjum, K.; Song, T.; Wang, W.; Yu, S.; Huang, H.; Lian, X.Y.; Zhang, Z. A new curvularin glycoside and its cytotoxic and antibacterial analogues from marine actinomycete Pseudonocardia sp. HS7. Nat. Prod. Res. 2016, 30, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xie, X.; Chen, L.; Yan, S.; Ye, X.; Anjum, K.; Huang, H.; Lian, X.Y.; Zhang, Z. Bioactive polycyclic quinones from marine Streptomyces sp. 182SMLY. Mar. Drugs 2016, 14. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, H.; Eberhard, B.; Gunther, J. 13C nuclear magnetic resonance study of actinomycin D. J. Am. Chem. Soc. 1974, 96, 8036–8040. [Google Scholar]
- Mauger, A.B.; Thomas, W.A. NMR studies of actinomycins varying at the proline sites. Org. Magn. Reson. 1981, 17, 186–190. [Google Scholar] [CrossRef]
- Cao, X.; Yang, R.L.; Yuan, X.W.; Xi, T.; Yang, Z.Z. Separation, purification, and structural identification of antitumor active components from marine actinomycete ACMA006. J. Oceanogr. Taiwan Haixia 2010, 30, 400–404. [Google Scholar]
- Zhang, Z.; Peng, G.; Yang, G.; Xiao, C.L.; Hao, X.Q. Isolation, purification, identification of structures and study of bioactivity of anti-TB active component 9005B. Chin. J. Antibiot. 2009, 34, 399–402. [Google Scholar]
- Chen, C.; Song, F.; Wang, Q.; Abdel-Mageed, W.M.; Guo, H.; Fu, C.; Hou, W.; Dai, H.; Liu, X.; Yang, N.; et al. A marine-derived Streptomyces sp. MS449 produces high yield of actinomycin X2 and actinomycin D with potent anti-tuberculosis activity. Appl. Microbiol. Biotechnol. 2012, 95, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Pakulski, Z.; Demchuk, O.M.; Frelek, J.; Luboradzki, R.; Pietrusiewicz, K.M. New monodentate p,c-stereogenic bicyclic phosphanes: 1-phenyl-1,3a,4,5,6,6a-hexahydrocyclopenta[b] phosphole and 1-phenyloctahydrocyclopenta[b]phosphole. Eur. J. Org. Chem. 2004, 18, 3913–3918. [Google Scholar] [CrossRef]
- Emese, P.; József, S.; Ferenc, F.; Elemér, F. The influence of molecular structure and crystallization time on the efficiency of diastereoisomeric salt forming resolutions. Tetrahedron. Asymmetry 2010, 21, 2429–2434. [Google Scholar]
- Tacara, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.; Agnihotri, S.; Micallef, J.; Mukherjee, J.; Sabha, N.; Cairns, R.; Hawkins, C.; Guha, A. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J. Exp. Med. 2011, 208, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G. Targeting cancer metabolism: A therapeutic window opens. Nat. Rev. Drug Discov. 2011, 10, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Kefas, B.; Comeau, L.; Erdle, N.; Montgomery, E.; Amos, S.; Purow, B. Pyruvate kinase M2 is a target of the tumor-suppressive microRNA-326 and regulates the survival of glioma cells. Neuro Oncol. 2010, 12, 1102–1112. [Google Scholar] [CrossRef] [PubMed]
- Daye, D.; Wellen, K.E. Metabolic reprogramming in cancer: Unraveling the role of glutamine in tumorigenesis. Semin. Cell Dev. Biol. 2012, 23, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Ru, P.; Williams, T.M.; Chakravarti, A.; Guo, D. Tumor metabolism of malignant gliomas. Cancers 2013, 5, 1469–1484. [Google Scholar] [CrossRef] [PubMed]
- Menendez, J.A.; Lupu, R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat. Rev. Cancer 2007, 7, 763–777. [Google Scholar] [CrossRef] [PubMed]
- Imamichi, T.; Murphy, M.A.; Adelsberger, J.W.; Yang, J.; Watkins, C.M.; Berg, S.C.; Baseler, M.W.; Lempicki, R.A.; Guo, J.; Levin, J.G.; et al. Actinomycin D induces high-level resistance to thymidine analogs in replication of human immunodeficiency virus type 1 by interfering with host cell thymidine kinase expression. J. Virol. 2003, 77, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Imamichi, T.; Conrads, T.P.; Zhou, M.; Liu, Y.; Adelsberger, J.W.; Veenstra, T.D.; Lane, H.C. A transcription inhibitor, actinomycin D, enhances HIV-1 replication through an interleukin-6-dependent pathway. J. Acquir. Immune Defic. Syndr. 2005, 40, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Sobell, H.M. Actinomycin and DNA transcription. Proc. Natl. Acad. Sci. USA 1985, 82, 5328–5331. [Google Scholar] [CrossRef] [PubMed]

| No. | 4 | 5 | ||||
|---|---|---|---|---|---|---|
| 13C, Type | 1H (J = Hz) | HMBC (H → C) | 13C, Type | 1H (J = Hz) | HMBC (H → C) | |
| 1 | 124.3, C | – | – | 124.5, C | – | – |
| 2 | 124.9, C | – | – | 129.9, CH | 6.92, d (8.2) | C-4, 6, 7 |
| 3 | 149.9, C | – | – | 112.1, CH | 6.43, d (8.2) | C-1, 4, 5 |
| 4 | 131.1, C | – | – | 148.8, C | – | – |
| 5 | 127.4, CH | 7.07, d (7.9) | C-1, 3 | 112.1, CH | 6.43, d (8.2) | C-1, 3, 4 |
| 6 | 120.7, CH | 7.19, d (7.9) | C-2, 4, 7 | 129.9, CH | 6.92, d (8.2) | C-2, 4, 7 |
| 7 | 166.6, C | – | – | 35.0, CH2 | 2.56, dd (14.0, 9.0); 2.80, dd (14.0, 5.4) | C-1, 2, 6, 8, 9 |
| 8 | 16.6, CH3 | 2.21, s | C-3, 4, 5 | 61.0, CH | 4.27, m | C-1, 7, 9, 12 |
| 9 | 170.2, C | – | – | 208.5, C | – | – |
| 10 | 23.1, CH3 | 2.07, s | C-9 | 27.5, CH3 | 2.02, s | C-8, 9 |
| 11 | 51.8, CH3 | 3.71, s | C-7 | 30.2, CH3 | 2.61, s | C-4 |
| 12 | 170.4, C | – | – | |||
| 13 | 22.5, CH3 | 1.80, s | C-12 | |||
| NH | 9.68, s | 8.21, d (6.8) | C-7, 8, 12 | |||
| Microbes | Actinomycin D (1) | Actinomycin V (2) | Actinomycin X0β (3) | Gentamicin | Amphotericin |
|---|---|---|---|---|---|
| S. aureus | 0.08 | 0.08 | 0.61 | 0.26 | - |
| E. coli | 0.12 | 0.12 | 0.61 | 0.51 | - |
| C. albicans | 9.96 | 9.85 | 9.83 | - | 0.05 |
| Glioma Cells | Actinomycin D (1, nM) | Actinomycin A5 (2, nM) | Actinomycin X0β (3, nM) | DOX (μM) |
|---|---|---|---|---|
| U251 | 10.06 ± 0.68 | 1.80 ± 0.19 | 8.71 ± 0.66 | 9.61 ± 1.25 |
| SHG44 | 3.31 ± 0.25 | 1.37 ± 0.07 | 3.26 ± 0.32 | 2.54 ± 0.23 |
| C6 | 1.01 ± 0.05 | 0.42 ± 0.23 | 25.18 ± 0.47 | 0.70 ± 0.01 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Ye, X.; Chai, W.; Lian, X.-Y.; Zhang, Z. New Metabolites and Bioactive Actinomycins from Marine-Derived Streptomyces sp. ZZ338. Mar. Drugs 2016, 14, 181. https://doi.org/10.3390/md14100181
Zhang X, Ye X, Chai W, Lian X-Y, Zhang Z. New Metabolites and Bioactive Actinomycins from Marine-Derived Streptomyces sp. ZZ338. Marine Drugs. 2016; 14(10):181. https://doi.org/10.3390/md14100181
Chicago/Turabian StyleZhang, Xiufang, Xuewei Ye, Weiyun Chai, Xiao-Yuan Lian, and Zhizhen Zhang. 2016. "New Metabolites and Bioactive Actinomycins from Marine-Derived Streptomyces sp. ZZ338" Marine Drugs 14, no. 10: 181. https://doi.org/10.3390/md14100181
APA StyleZhang, X., Ye, X., Chai, W., Lian, X.-Y., & Zhang, Z. (2016). New Metabolites and Bioactive Actinomycins from Marine-Derived Streptomyces sp. ZZ338. Marine Drugs, 14(10), 181. https://doi.org/10.3390/md14100181







