Identification of the Scopularide Biosynthetic Gene Cluster in Scopulariopsis brevicaulis
Abstract
:1. Introduction
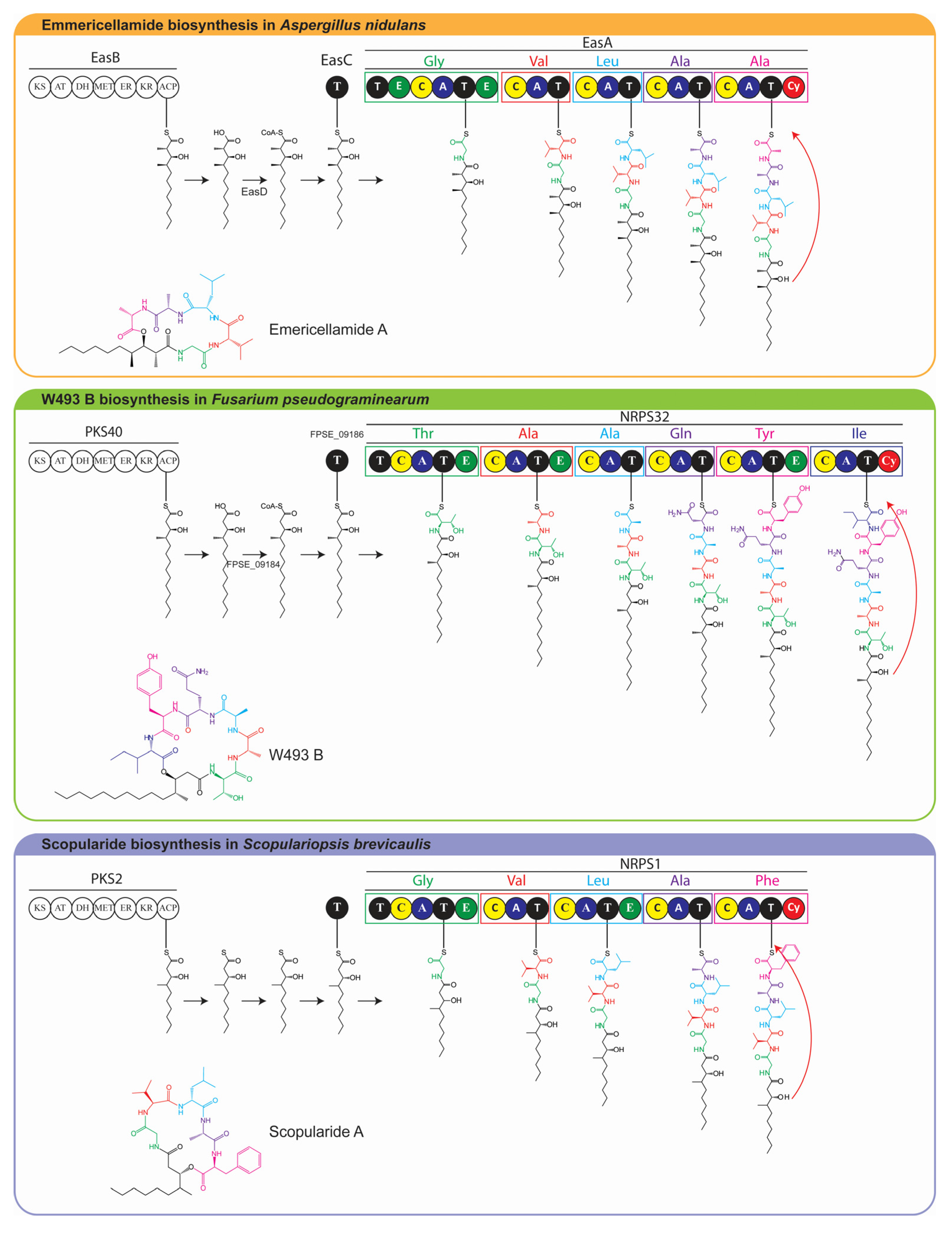
2. Results and Discussion
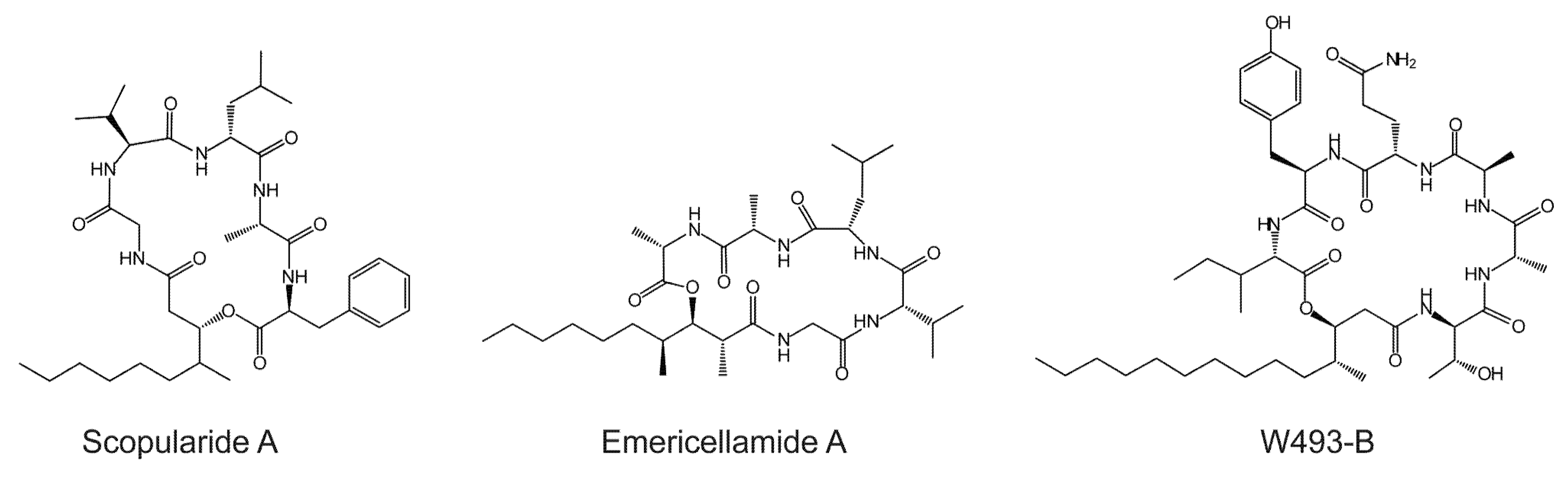
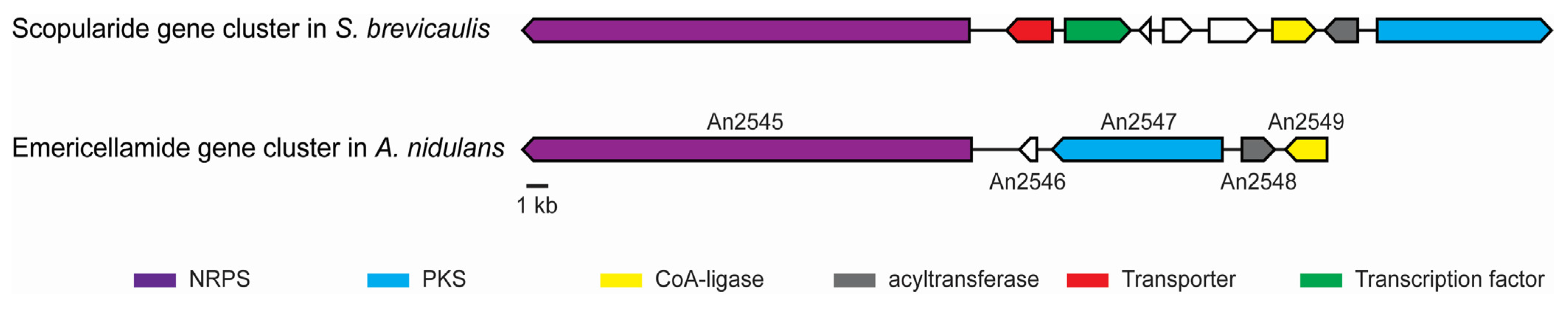
2.1. The Scopularide Gene Cluster Contains a Five Module NRPS and a Reducing PKS
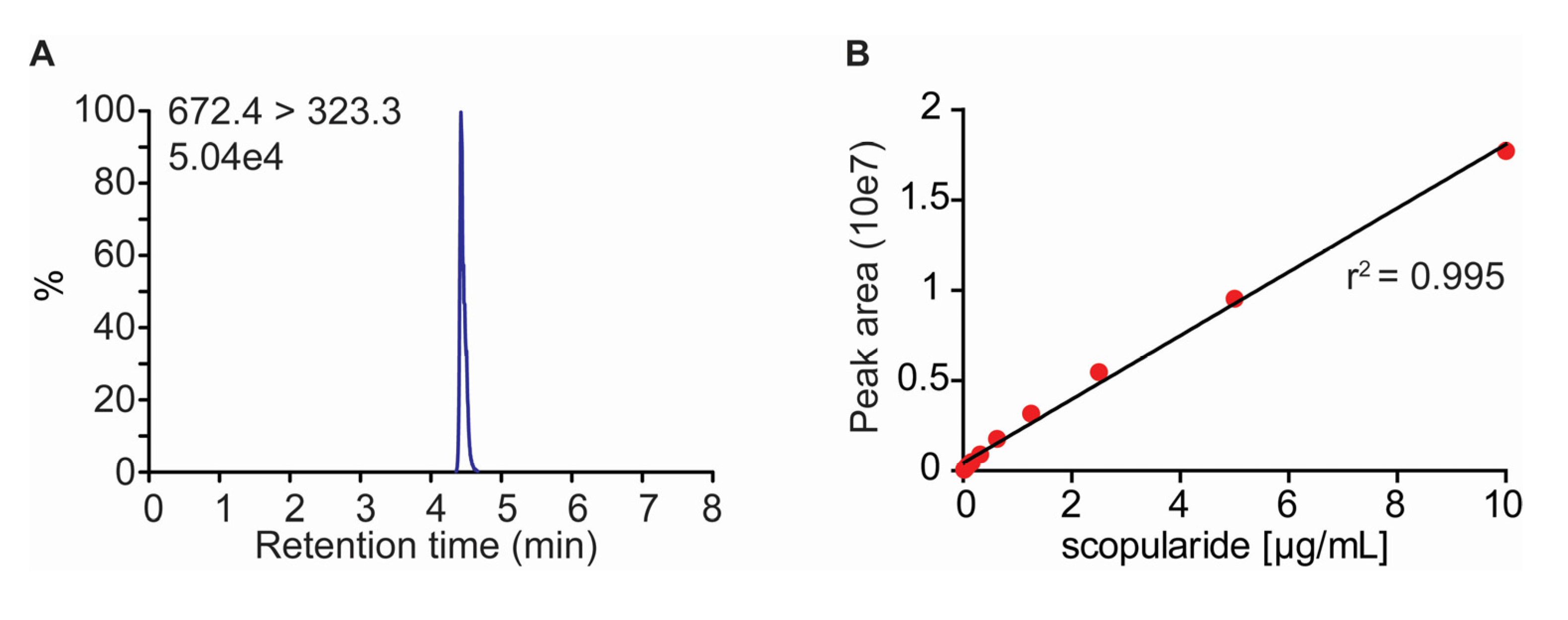
2.2. Development of a LC-MS/MS Method for Quantification of Scopularide A
2.3. Genetic Manipulation of S. brevicaulis
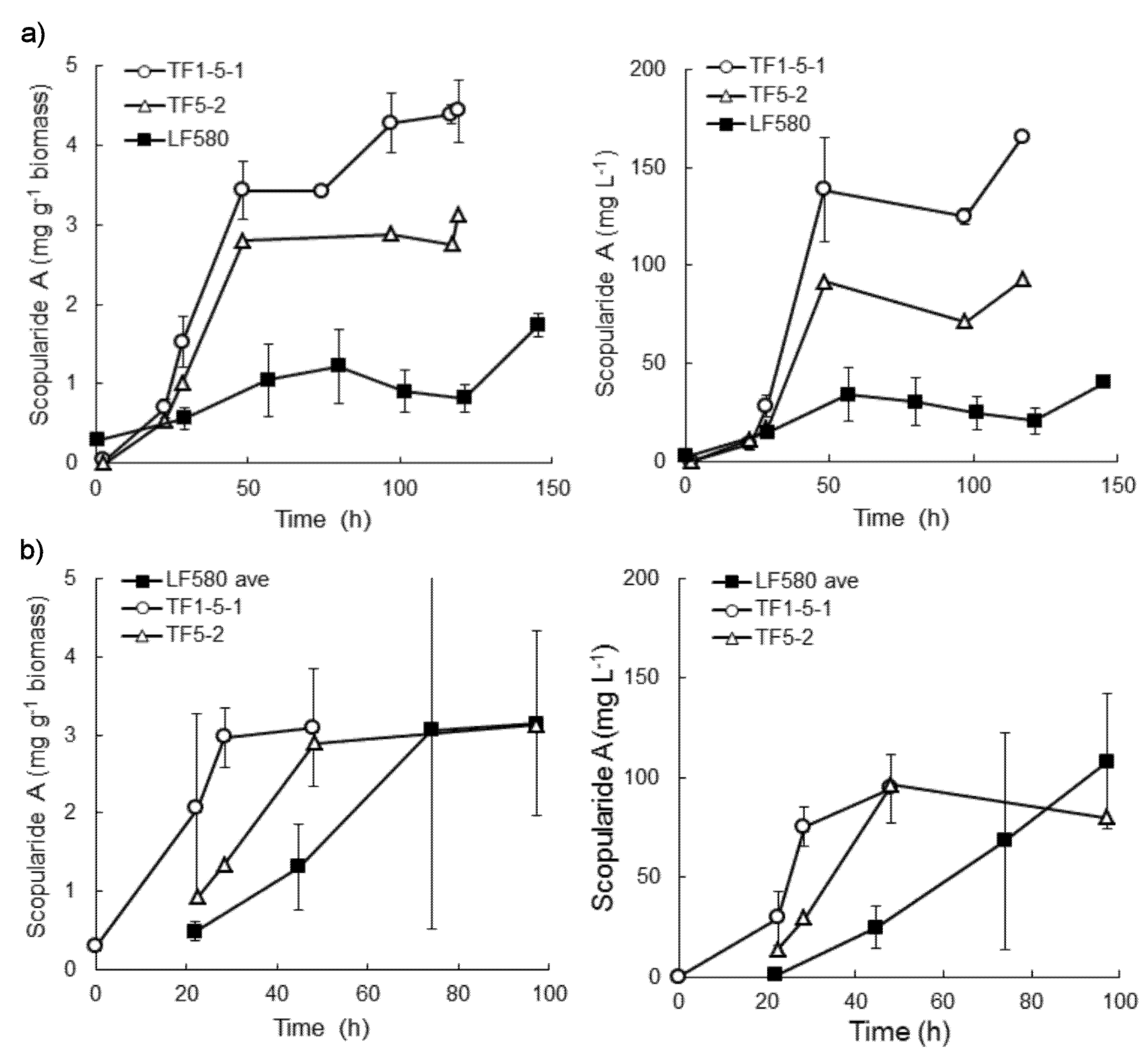
2.4. Production of Scopularide A in Batch Liquid Cultures
2.5. Model for Biosynthesis of Scopularide A
3. Experimental Section
3.1. In Silico Identification of the Scopularide Biosynthetic Gene Cluster in S. brevicaulis
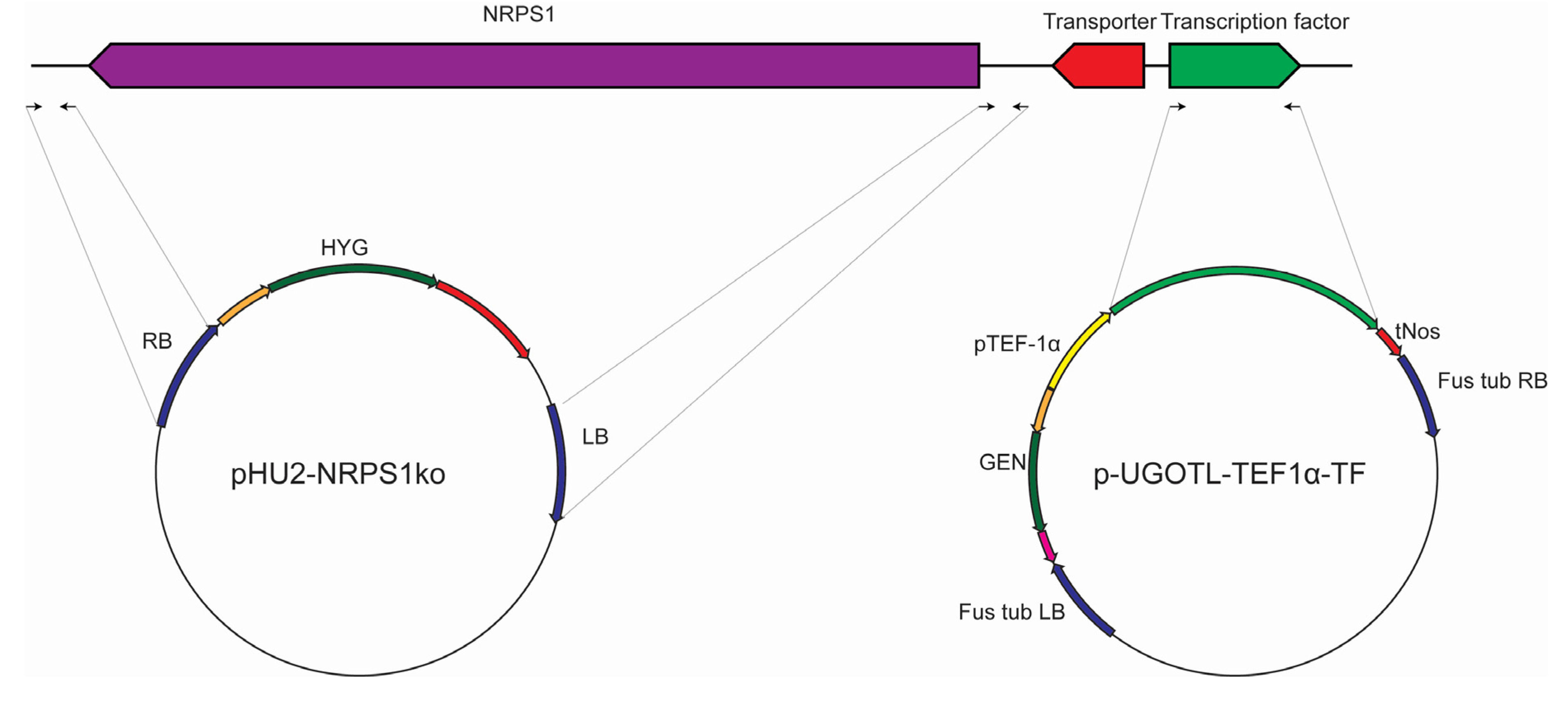
3.2. Strains and Genetic Manipulation of the Scopularide Biosynthetic Gene Cluster
| Primers | Sequence a | Amplicon |
|---|---|---|
| NRPS1-KO1 | 5′- TAAACGGCGCGCCGGCGATAACCCGGTCCGACCATAC | 707 bp |
| NRPS1-KO2 | 5′- AGTGCGCGATCGCGGGTAGGTGCCCGTTTGTCGTTTCAAATG | |
| NRPS1-KO3 | 5′- ATTAAACCCACAGCGGGTTGCTCTGGTGGGGCCGAGGGT | 717 bp |
| NRPS1-KO4 | 5′- GTGAATTCGAGCTCGCGTGGTGTTCCACACCAAGATTGGG | |
| TF1 | 5′-GC GGATCCATGACAAGCTGGCAATTG | 2288 bp |
| TF2 | 5′-GC GTCGACCTACCCAAAAAGAGATCG |
3.3. Solid State Growth of S. brevicaulis Transformants and Extraction of Scopularides
3.4. Quantification of Scopularide A
| RT a | Precursor Ion | Product Ions b | S-Lens | CE c | |
|---|---|---|---|---|---|
| Scopularide A | 4.45 | 672.4 [M + H]+ | 323.3/436.4 | 121 | 28/21 |
3.5. Cultivation of S. brevicaulis LF580, TF5-2 and TF1-5-1 in Bioreactors
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rateb, M.E.; Ebel, R. Secondary metabolites of fungi from marine habitats. Nat. Prod. Rep. 2011, 28, 290–344. [Google Scholar] [CrossRef] [PubMed]
- Debbab, A.; Aly, A.H.; Lin, W.H.; Proksch, P. Bioactive compounds from marine bacteria and fungi. Microb. Biotechnol. 2010, 3, 544–563. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.G.; Lang, G.; Kajahn, I.; Schmaljohann, R.; Imhoff, J.F. Scopularides A and B, cyclodepsipeptides from a marine sponge-derived fungus, Scopulariopsis brevicaulis. J. Nat. Prod. 2008, 71, 1052–1054. [Google Scholar] [CrossRef] [PubMed]
- Samson, R.A.; Houbraken, J.; Thrane, U.; Frisvad, J.C.; Andersen, B. Food and Indoor Fungi; CBS“KNAW”Fungal Biodiversity Centre: Utrecht, The Netherlands, 2010. [Google Scholar]
- Sandoval-Denis, M.; Sutton, D.A.; Fothergill, A.W.; Cano-Lira, J.; Gene, J.; Decock, C.A.; de Hoog, G.S.; Guarro, J. Scopulariopsis, a poorly known opportunistic fungus: Spectrum of species in clinical samples and in vitro responses to antifungal drugs. J. Clin. Microbiol. 2013, 51, 3937–3943. [Google Scholar] [CrossRef] [PubMed]
- Imhoff, J.F.; Kajahn, I.; Lang, G.; Wiese, J.; Peters, A. Production and Use of Antimumoral, Antibiotic and Insecticidal Cyclodepsipeptides. WO 2010/142258, December 2010. [Google Scholar]
- Chooi, Y.-H.; Tang, Y. Adding the lipo to lipopeptides: Do more with less. Chem. Biol. 2010, 17, 791–793. [Google Scholar] [CrossRef] [PubMed]
- Condurso, H.L.; Bruner, S.D. Structure and noncanonical chemistry of nonribosomal peptide biosynthetic machinery. Nat. Prod. Rep. 2012, 29, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Hertweck, C. The Biosynthetic Logic of Polyketide Diversity. Angew. Chem. Int. Ed. 2009, 48, 4688–4716. [Google Scholar] [CrossRef] [PubMed]
- Kraas, F.I.; Helmetag, V.; Wittmann, M.; Strieker, M.; Marahiel, M.A. Functional dissection of surfactin synthetase initiation module reveals insights into the mechanism of lipoinitiation. Chem. Biol. 2010, 17, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.C.; Kauffman, C.A.; Jensen, P.R.; Fenical, W. Induced production of emericellamides A and B from the marine-derived fungus Emericella sp. in competing co-culture. J. Nat. Prod. 2007, 70, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Nihei, K.; Itoh, H.; Hashimoto, K.; Miyairi, K.; Okuno, T. Antifungal cyclodepsipeptides, W493 A and B, from Fusarium sp.: Isolation and structural determimation. Biosci. Biotechnol. Biochem. 1998, 62, 858–863. [Google Scholar] [CrossRef]
- Keller, N.P.; Hohn, T.M. Metabolic pathway gene clusters in filamentous fungi. Fungal Genet. Biol. 1997, 21, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, J.L.; Sondergaard, T.E.; Covarelli, L.; Fuertes, P.R.; Hansen, F.T.; Frandsen, R.J.N.; Saei, W.; Lukassen, M.B.; Wimmer, R.; Nielsen, K.F; et al. Identification of the biosynthetic gene clusters for the lipopeptides fusaristatin A and W493 B in Fusarium graminearum and F. pseudograminearum. J. Nat. Prod. 2014, 77, 2619–2615. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.M.; Szewczyk, E.; Nayak, T.; Davidson, A.D.; Sanchez, J.F.; Lo, H.C.; Ho, W.Y.; Simityan, H.; Kuo, E.; Praseuth, A.; et al. Molecular genetic mining of the Aspergillus secondary metabolome: Discovery of the emericellamide biosynthetic pathway. Chem. Biol. 2008, 15, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Tamminen, A.; Kramer, A.; Labes, A.; Wiebe, M.G. Production of scopularide A in submerged culture with Scopulariopsis brevicaulis. Microb. Cell Fact. 2014, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.; Paun, L.; Imhoff, J.F.; Kempken, F.; Labes, A. Development and validation of a fast and optimized screening method for enhanced production of secondary metabolites using the marine Scopulariopsis brevicaulis strain LF580 producing anti-cancer active scopularide A and B. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.; Beck, H.C.; Kumar, A.; Kristensen, L.P.; Imhoff, J.F.; Labes, A. Proteomic analysis of anti-cancerous scopularide production by a marine Microascus brevicaulis strain and its UV-mutant. PLoS ONE 2015. Submitted for publication. [Google Scholar]
- Kumar, A.; Bernard, H.; Arvas, M.; Syed, F.; Sørensen, J.L.; Eric, R.; Pöggeler, S.; Kempken, F. de novo assembly and genome analyses of the marine sponge derived Scopulariopsis brevicaulis strain LF580 unravels life-style traits and anticancerous scopularide producing hybrid NRPS-PKS cluster. PLoS Genet. 2015. Submitted for publication. [Google Scholar]
- Medema, M.H.; Blin, K.; Cimermancic, P.; de Jager, V.; Zakrzewski, P.; Fischbach, M.A.; Weber, T.; Takano, E.; Breitling, R. antiSMASH: Rapid identification, annotation and analysis of secondary metabolite biosynthesis gene clusters in bacterial and fungal genome sequences. Nucleic Acids Res. 2011, 39, W339–W346. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, J.L.; Knudsen, M.; Hansen, F.T.; Olesen, C.; Fuertes, P.R.; Lee, T.V.; Sondergaard, T.E.; Pedersen, C.N.S.; Brodersen, D.E.; Giese, H. Fungal NRPS-dependent siderophores: From function to prediction. In Biosynthesis and Molecular Genetics of Fungal Secondary Metabolites; Martín, J.-F., Garcia-Estrada, C., Zeilinger, S., Eds.; Springer: New York, NY, USA, 2014. [Google Scholar]
- Inglis, D.; Binkley, J.; Skrzypek, M.; Arnaud, M.; Cerqueira, G.; Shah, P.; Wymore, F.; Wortman, J.; Sherlock, G. Comprehensive annotation of secondary metabolite biosynthetic genes and gene clusters of Aspergillus nidulans, A. fumigatus, A. niger and A. oryzae. BMC Microbiol. 2013, 13, 91. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, B.O.; Ravel, J. Methods for in silico prediction of microbial polyketide and nonribosomal peptide biosynthetic pathways from DNA sequence data. In Complex Enzymes in Microbial Natural Product Biosynthesis Methods in Enzymology; Hopwood, D.A., Ed.; Elsevier Academic Press Inc: San Diego, CA, USA, 2009; Volume 458. [Google Scholar]
- Prieto, C.; Garcia-Estrada, C.; Lorenzana, D.; Martin, J.F. NRPSsp: Non-ribosomal peptide synthase substrate predictor. Bioinformatics 2012, 28, 426–427. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, S.; Schumann, J.; Scherlach, K.; Lange, C.; Brakhage, A.A.; Hertweck, C. Genomics-driven discovery of PKS-NRPS hybrid metabolites from Aspergillus nidulans. Nat. Chem. Biol. 2007, 3, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, J.L.; Hansen, F.T.; Sondergaard, T.E.; Staerk, D.; Lee, T.V.; Wimmer, R.; Klitgaard, L.G.; Purup, S.; Giese, H.; Frandsen, R.J. Production of novel fusarielins by ectopic activation of the polyketide synthase 9 cluster in Fusarium graminearum. Environ. Microbiol. 2012, 14, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.M.; Lee, S.; Lee, J.; Baek, S.R.; Kim, J.C.; Yun, S.H.; Park, S.Y.; Kang, S.C.; Lee, Y.W. Functional characterization and manipulation of the apicidin biosynthetic pathway in Fusarium semitectum. Mol. Microbiol. 2010, 76, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, R.J.N.; Andersson, J.A.; Kristensen, M.B.; Giese, H. Efficient four fragment cloning for the construction of vectors for targeted gene replacement in filamentous fungi. BMC Mol. Biol. 2008, 9. [Google Scholar] [CrossRef] [PubMed]
- Josefsen, L.; Droce, A.; Sondergaard, T.E.; Sørensen, J.L.; Bormann, J.; Schaefer, W.; Giese, H.; Olsson, S. Autophagy provides nutrients for nonassimilating fungal structures and is necessary for plant colonization but not for infection in the necrotrophic plant pathogen Fusarium graminearum. Autophagy 2012, 8, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Hansen, F.T.; Droce, A.; Sørensen, J.L.; Fojan, P.; Giese, H.; Sondergaard, T.E. Overexpression of NRPS4 leads to increased surface hydrophobicity in Fusarium graminearum. Fungal Biol. 2012, 116, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Vogel, H. A convenient growth medium for Neurospora (medium N). Microb. Genet. Bull. 1956, 13, 2–43. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukassen, M.B.; Saei, W.; Sondergaard, T.E.; Tamminen, A.; Kumar, A.; Kempken, F.; Wiebe, M.G.; Sørensen, J.L. Identification of the Scopularide Biosynthetic Gene Cluster in Scopulariopsis brevicaulis. Mar. Drugs 2015, 13, 4331-4343. https://doi.org/10.3390/md13074331
Lukassen MB, Saei W, Sondergaard TE, Tamminen A, Kumar A, Kempken F, Wiebe MG, Sørensen JL. Identification of the Scopularide Biosynthetic Gene Cluster in Scopulariopsis brevicaulis. Marine Drugs. 2015; 13(7):4331-4343. https://doi.org/10.3390/md13074331
Chicago/Turabian StyleLukassen, Mie Bech, Wagma Saei, Teis Esben Sondergaard, Anu Tamminen, Abhishek Kumar, Frank Kempken, Marilyn G. Wiebe, and Jens Laurids Sørensen. 2015. "Identification of the Scopularide Biosynthetic Gene Cluster in Scopulariopsis brevicaulis" Marine Drugs 13, no. 7: 4331-4343. https://doi.org/10.3390/md13074331
APA StyleLukassen, M. B., Saei, W., Sondergaard, T. E., Tamminen, A., Kumar, A., Kempken, F., Wiebe, M. G., & Sørensen, J. L. (2015). Identification of the Scopularide Biosynthetic Gene Cluster in Scopulariopsis brevicaulis. Marine Drugs, 13(7), 4331-4343. https://doi.org/10.3390/md13074331