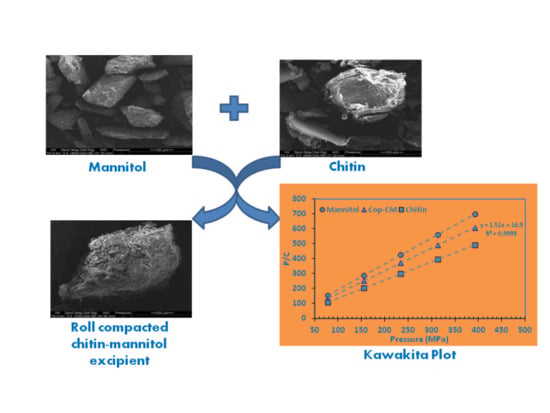
Co-Processed Chitin-Mannitol as a New Excipient for Oro-Dispersible Tablets
Abstract
:1. Introduction
2. Results and Discussion
2.1. Selection of Processing Methods and Ratios for Co-Processed Chitin–Mannitol Excipient

2.2. Characterization of Cop–CM Powder
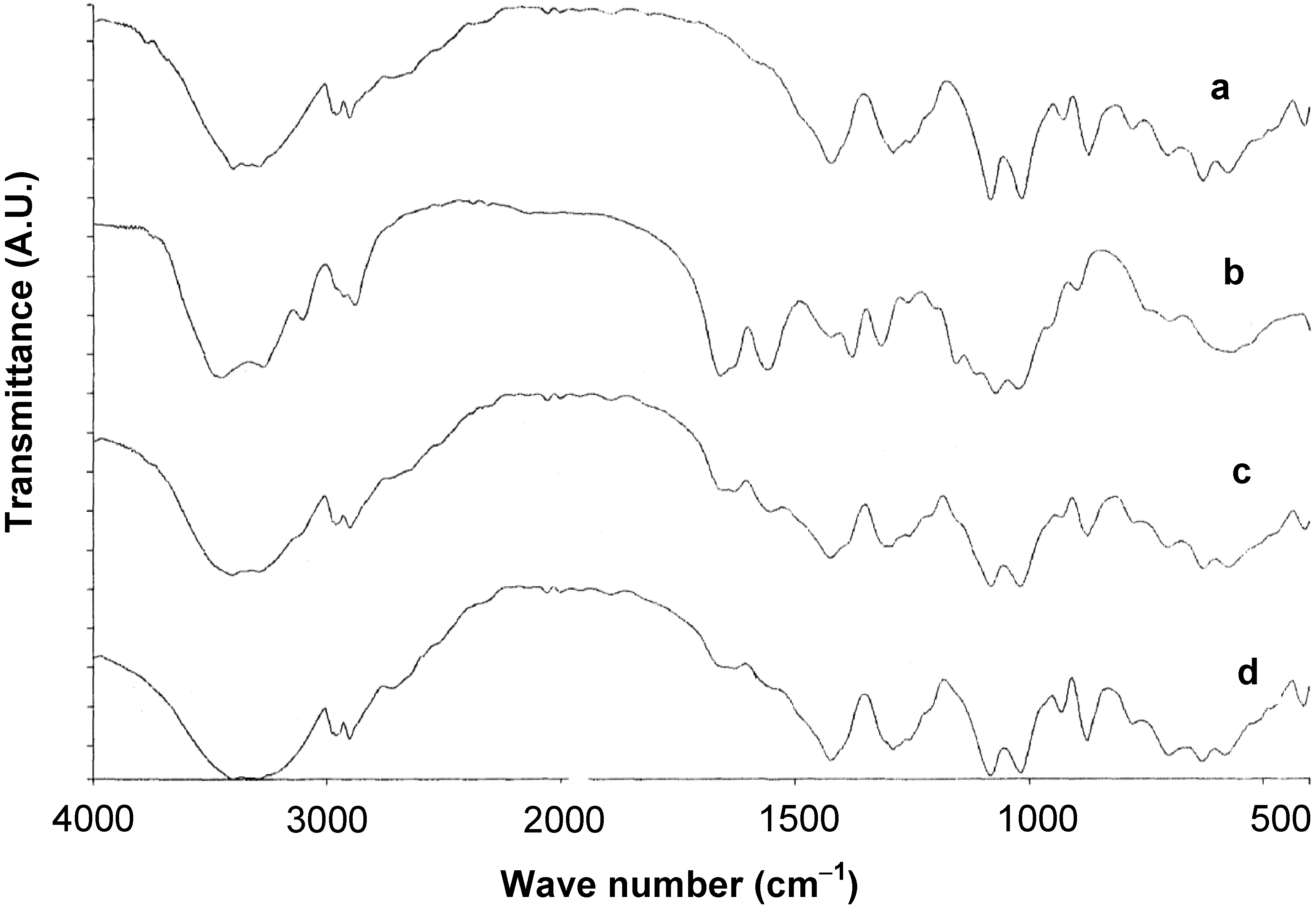
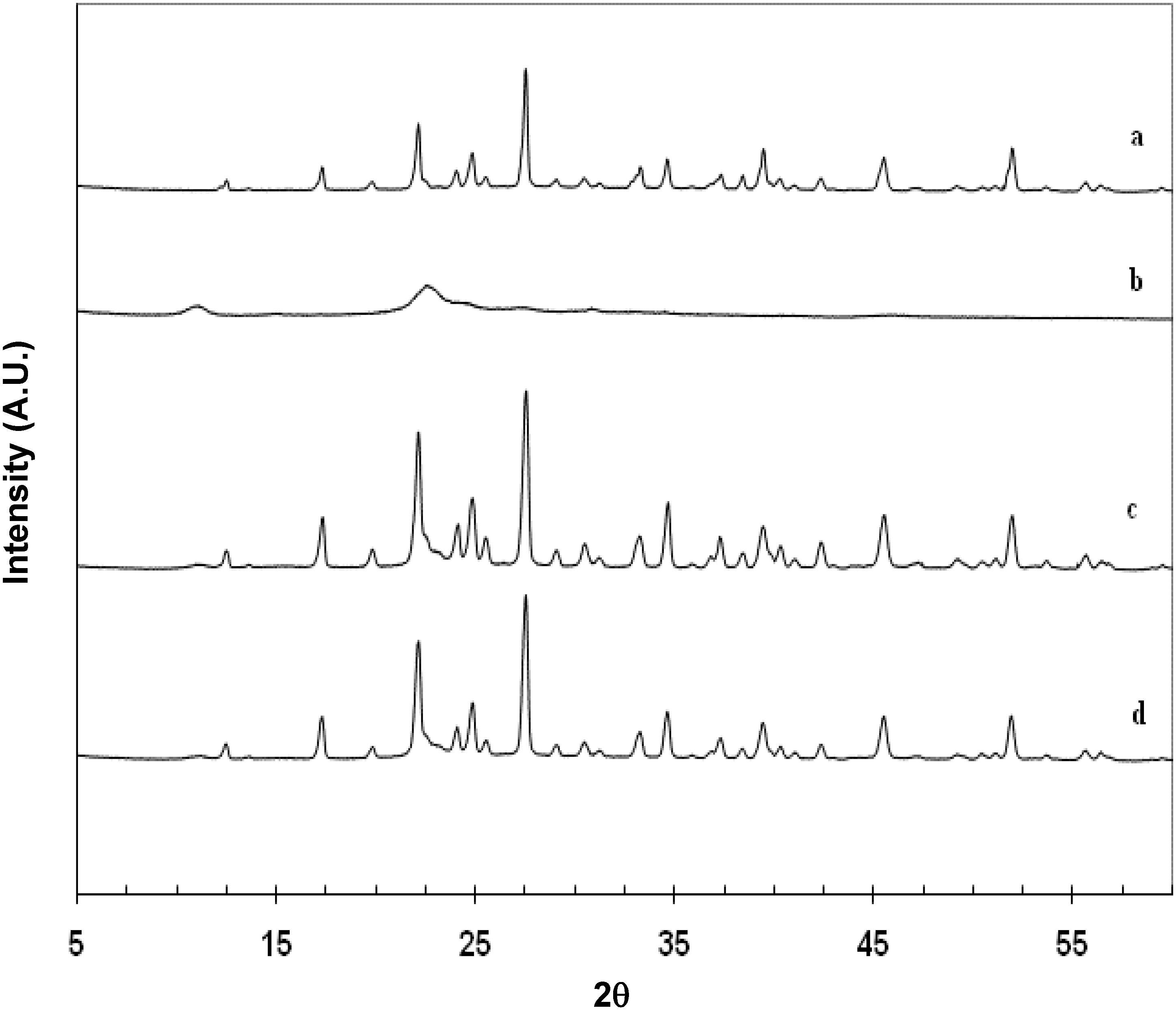
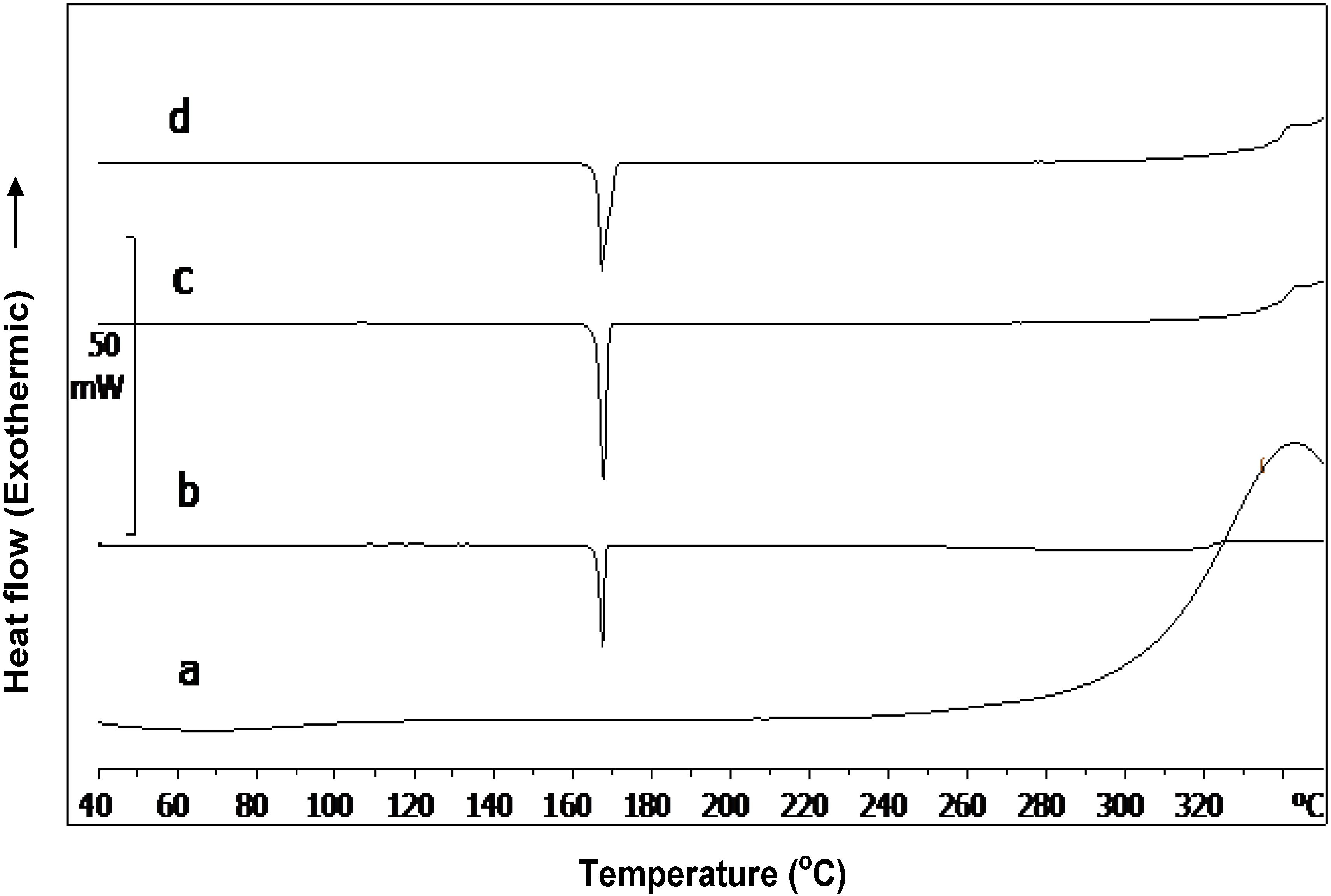
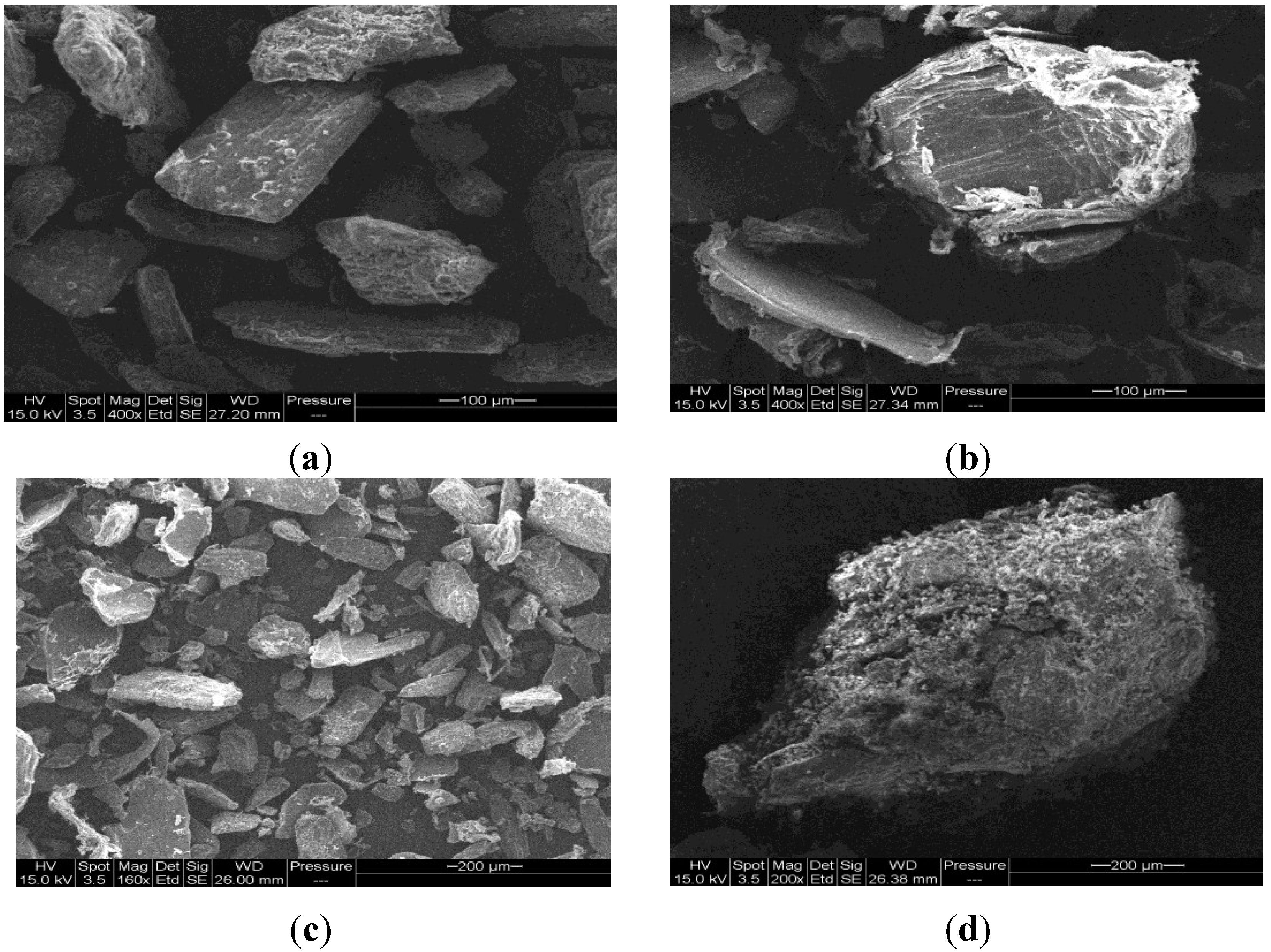
2.3. Physical Properties of Cop–CM Powder
| Parameter | Value |
|---|---|
| Water content (w/w%) | 1.5 |
| pH | 6.0–8.0 |
| Bulk density (gm/mL) | 0.50–0.55 |
| Tapped density (gm/mL) | 0.55–0.65 |
| Particle size distribution: | |
| - Milling through 710 μm | D10: 5 μm; D50: 145 μm; D90: 496 μm |
| - Milling through 1000 μm | D10: 7 μm; D50: 170 μm; D90: 584 μm |
| Hausner Ratio | 1.13 |
| Carr Index | 11.86 |
| Angle of Repose | 32° |
2.4. Moisture Uptake by Cop–CM
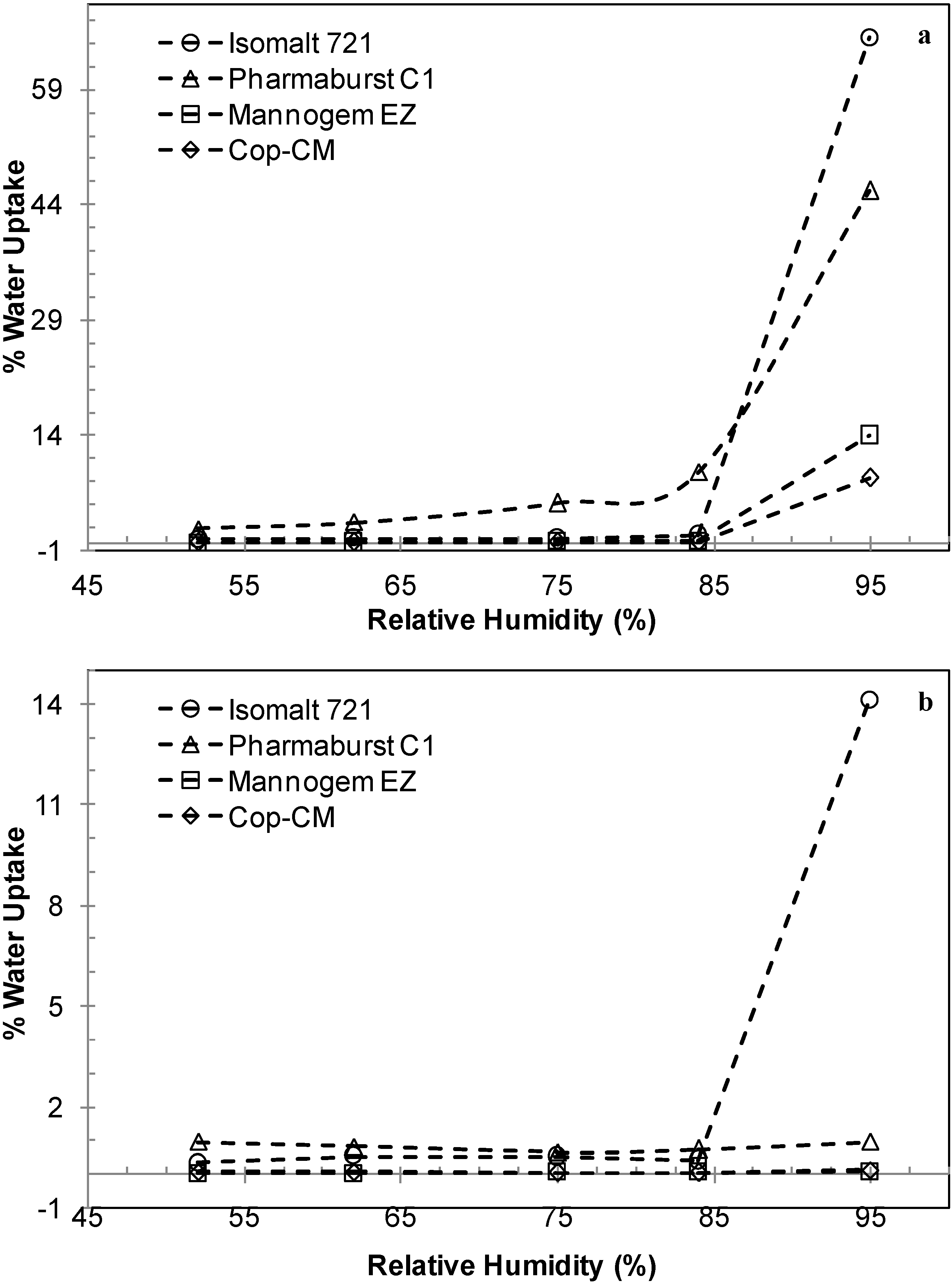
2.5. Hygroscopicity of Tablets Prepared from Cop–CM
2.6. Compression Profile of Tablet Prepared from Cop–CM
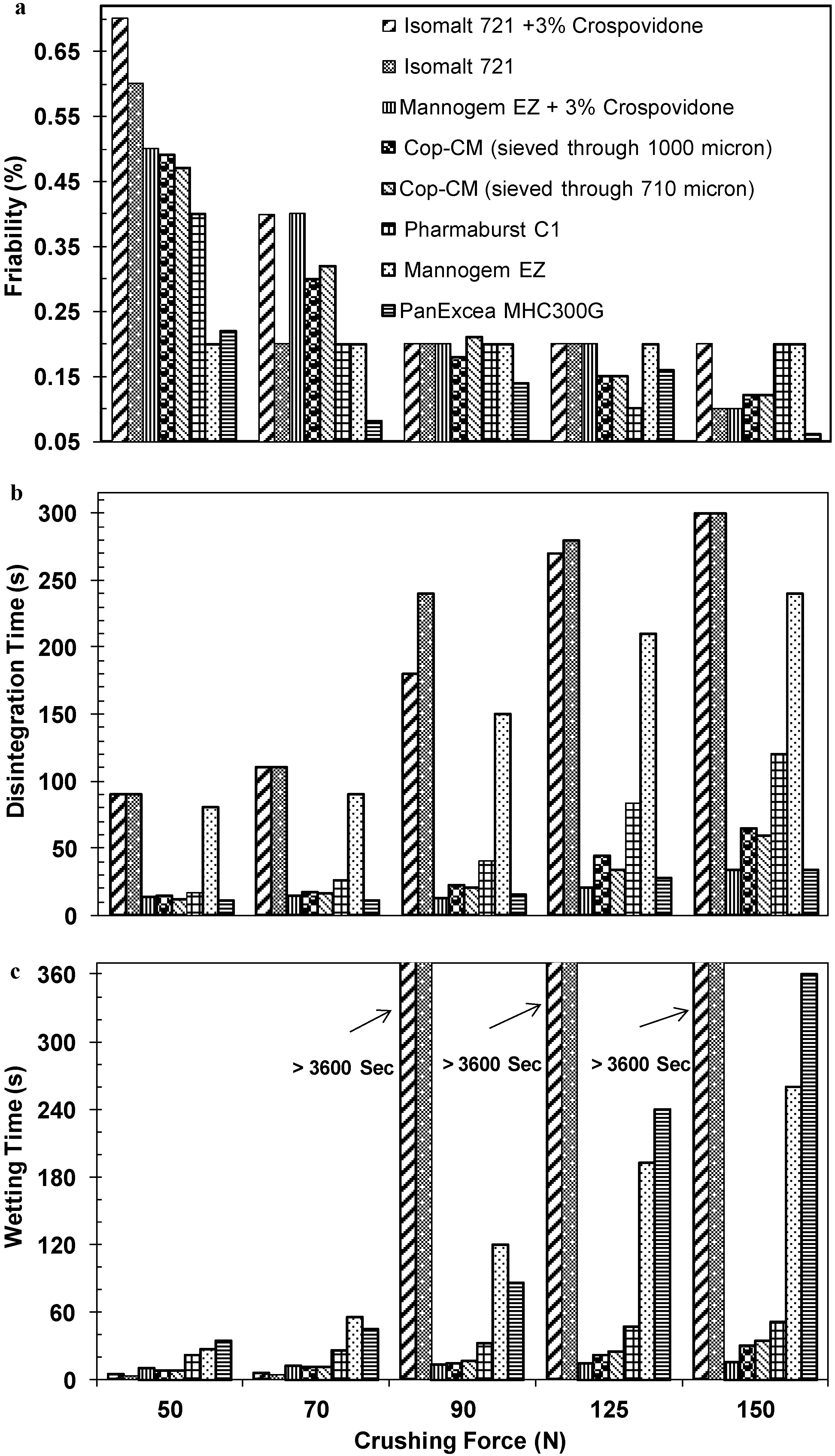
| Trade Name | Composition | Manufacturer | Advantages & Function |
|---|---|---|---|
| PanExcea MC200G | Mannitol (75%), calcium silicate (25%). Particle size: 50% (103 μm) | Avantor Performance Materials, Inc./Center Valley, PA, USA http://www.avantormaterials.com/ | High performance, rapid disintegration, direct compression excipient for oro-dissolving tablets formulation |
| Mannogem™ EZ | Spray dried direct compression mannitol Particle size: 60% (75–150 μm) | SPI Pharma™, Inc., New Castel, DE, USA http://www.spipharma.com | Assist in formulating difficult to use non-hygroscopic ODT containing fine drugs |
| Pharmaburst ™ C1 | Mannitol 84%, crospovidone 16%, silicon dioxide <1% | High compactibility, high loading in small diameter tablets, smooth mouth feel, rapid disintegration | |
| Isomalt galenIQ–721 | 1-O-d-glucopyranosyl-d-mannitol dehydrate and 6-O-d-glucopyranosyl-d-sorbitol (1:3) Particle size: 90% (360 μm), 50% (220 μm) | BENEO–Palatinit GmbH (Mannheim, Germany) http://www.beneo–palatinit.com/en/Pharma_Excipients/galenIQ/galenIQ_Grades/galenIQ721/ | Highly soluble agglomerated spherical isomalt for fast dissolving and very fast disintegrating direct compression tablet preparations |
2.7. Powder Compressibility
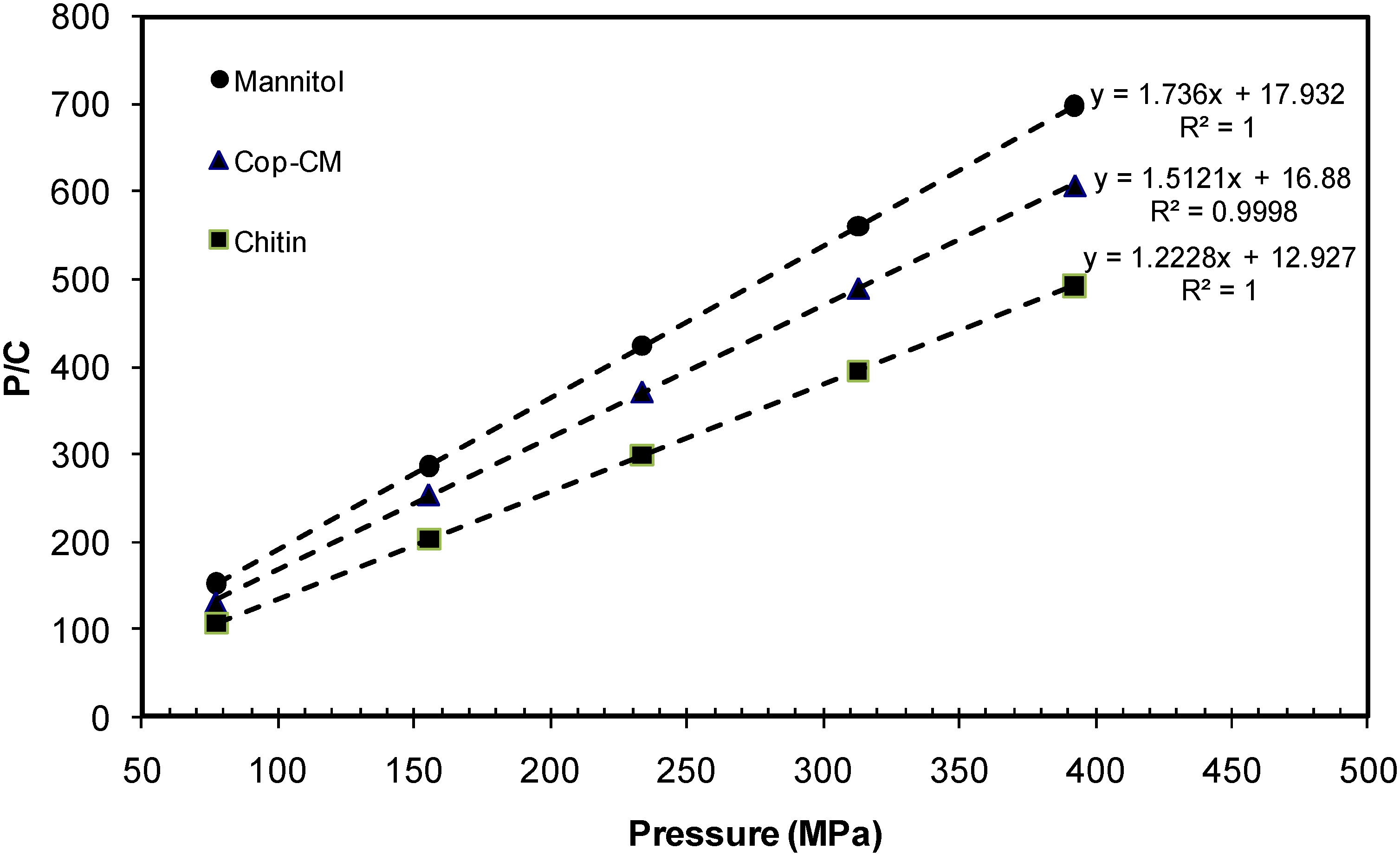
| Material | Kawakita Parameters | |||||
|---|---|---|---|---|---|---|
| Slope | Intercept | a | ab | b | 1 /b | |
| Mannitol | 1.736 | 17.932 | 0.576 | 0.0558 | 0.0968 | 10.330 |
| Chitin | 1.223 | 12.927 | 0.818 | 0.0774 | 0.0946 | 10.570 |
| Cop–CM | 1.512 | 16.880 | 0.661 | 0.0592 | 0.0896 | 11.164 |
2.8. Loading Capacity

2.9. Functionality
| Montelukast Sodium | |||
|---|---|---|---|
| Singulair [50] | FDT [51] | ODT [52] | ODT [Present work] |
| - Mannitol - Microcrystalline cellulose - Hydroxypropyl cellulose - Croscarmellose sodium - Aspartame - Red ferric oxide - Cherry flavor - Magnesium stearate | - Mannitol - Microcrystalline cellulose - Crospovidone * - Sodium starch glycolate * - Sodium saccharin - Mint flavor - Talc - Magnesium stearate | - Mannitol - Microcrystalline cellulose - Crospovidone or - Croscarmellose sodium - Aspartame - Talc - Magnesium stearate | - Mannitol + - Chitin + - Strawberry flavor - Aspartame - Sodium stearyl fumarate |
| Domperidone | |||
| Oroperidys [53] | FDT [54] | FDT [55] | FDT [56] |
| - Mannitol - Microcrystalline cellulose - Maltodextrin - Croscarmellose sodium - Glucose - Mint flavor - Peppermint gasoline - Anise oil - Gasoline mint - Levomentol - Glycyrrhizate ammonium - Gasoline cloves - Acesulfame potassium - Arabic gum - Sulphur dioxide - Magnesium stearate | - Mannitol - Microcrystalline cellulose - Sodium starch glycolate - Lactose - Talc - Magnesium stearate | - Mannitol - Microcrystalline cellulose - Sodium starch glycolate - Lactose - Crospovidone - Aspartame - Citric acid - Sodium bicarbonate - Maize starch - Starch 1500 - Colloidal anhydrous silica - Lemon flavor - Green lake color - Menthol - Magnesium stearate | - Mannitol - Microcrystalline cellulose - Camphor - Ispaghula husk - Povidone K30 - Crospovidone - Guar gum - Aspartame - Colloidal anhydrous silica - Orange flavor - Talc - Magnesium stearate |
| Material | Composition (% w/w) | |||||
| Montelukast | Domperidone | |||||
| Drug | 01.77 | 03.40 | ||||
| Cop–CM | 94.73 | 93.10 | ||||
| Strawberry powder flavor | 02.00 | 02.00 | ||||
| Aspartame | 00.50 | 00.50 | ||||
| Sodium stearyl fumarate | 01.00 | 01.00 | ||||
| Tablet Physical Properties | Tablet preparation process | |||||
| DC | RC | WG | DC | RC | WG | |
| Crushing force (N) | 70–80 | 60–70 | ||||
| Friability (%) | 0.25 | 0.32 | 0.30 | 0.18 | 0.29 | 0.37 |
| Disintegration time(s) | 20 | 30 | 20 | 26 | 20 | 28 |
2.10. Stability Studies
| Product | Compound/Limit | Percentage (w/w) | |||
|---|---|---|---|---|---|
| 25 °C/65%RH | 40 °C/75% RH | ||||
| Initial | 24 Months | 3 Months | 6 Months | ||
| Montelukast | Drug/90%–110% | 99.4 | 104.8 | 102.8 | 98.2 |
| Drug S–oxide/≤2.0% | 0.9 | 1.1 | 3.4 | 3.6 | |
| Drug cis–isomer/≤0.5% | 0.1 | 0.5 | 0.1 | 0.1 | |
| Any other ≤ 0.4% | 0.0 | 0.2 | 0.1 | 0.1 | |
| Total impurities ≤ 3.0% | 1.0 | 2.0 | 3.6 | 3.8 | |
| Domperidone | Drug/95%–105% | 100.1 | – | 100.7 | 101.4 |
| Any other/≤0.25% | 0.06 | – | 0.04 | 0.04 | |
| Total impurities/≤0.5% | 0.2 | – | 0.1 | 0.1 | |
3. Experimental Section
3.1. Materials
3.2. Methods
3.2.1. Preparation of co-Processed Mannitol-Chitin Excipient
3.2.2. Characterization of Co-Processed Chitin-Mannitol (Cop–CM)
3.2.3. Physical and Chemical Properties of Tablets Prepared from Cop–CM
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Jivraj, M.; Martini, L.G.; Thomson, C.M. An overview of the different excipients useful for the direct compression of tablets. Pharm. Sci. Tech. Today 2000, 3, 58–63. [Google Scholar] [CrossRef]
- Bhattacharyya, L.; Shuber, S.; Sheehan, S.; William, R. Excipients: background/Introduction. In Excipient Development for Pharmaceutical, Biotechnology, and Drug Delivery Systems; Katdare, A., Chaubal, M.V., Eds.; Informa Healthcare Inc.: New York, NY, USA, 2006; pp. 1–3. [Google Scholar]
- Jonwal, N.; Mane, P.; Mokati, S.; Meena, A. Preparation and in vitro evaluation of mouth dissolving tablets of domperidone. Int. J. Pharm. Pharm. Sci. 2010, 3, 975–1491. [Google Scholar]
- U.S. Department of Health and Human Services, Food and Drug Administration, Centre for Drug Evaluation and Research (CDER). Guidance for industry: Orally disintegrating tablets. 2007. Available online: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070578.pdf (accessed on 2 February 2015). [Google Scholar]
- Orodispersible tablets. In European Pharmacopeia, 8th ed.; Council of Europe: Strasbourge, France, 2014; Volume 1, p. 811.
- Polyplasdone® crospovidone: Superdisintegrants for orally disintegrating and chewable tablets. Available online: http://www.anshulindia.com/pdfs/Polyplasdone%20for%20odt%20Lit.pdf (accessed on 3 February 2015).
- Deshpande, K.B.; Ganesh, N.S. Formulation and evaluation of orodispersible tablets of propranolol hydrochloride. Int. J. Res. Pharm. Biomed. Sci. 2011, 2, 529–534. [Google Scholar]
- Bandari, S.; Mittapalli, R.K.; Gannu, R.; Rao, Y.M. Orodispersible tablets: An overview. Asian J. Pharm. 2008, 2, 2–11. [Google Scholar] [CrossRef]
- Pfister, W.R.; Ghosh, T.K. Orally disintegrating tablets, products, technologies, and development issues Pharmaceutical Technology. Available online: http://www.pharmtech.com/node/238195?rel=canonical (accessed on 3 February 2015).
- Schiermeier, S.; Schmidt, P.C. Fast dispersible ibuprofen tablets. Eur. J. Pharm. Sci. 2002, 15, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Raymond, C.R.; Paul, J.S.; Siân, C.O. Handbook of Pharmaceutical Excipients, 5th ed.; Pharmaceutical Press: Greyslake, IL, USA; American Pharmacists Association: Washington, DC, USA, 2006. [Google Scholar]
- Mannogem® Mannitol. Available online: http://www.spipharma.com/product.php?id=15&prodtype=p (accessed on 3 February 2015).
- Dolson, L. Low Carb Diets: What are sugar alcohols? Comparisons and blood sugar impact. Available online: http://lowcarbdiets.about.com/od/whattoeat/a/sugaralcohols.htm (accessed on 3 February 2015).
- Ghosh, A; Prasad, D. A review on new generation orodispersible tablets and its future prospective. Int. J. Pharm. Pharm. Sci. 2011, 3, 1–7. [Google Scholar]
- Erik, L.; Philippe, L.; Jose, L. Pulverulent Mannitol and Process for Preparing it. U.S. Patent 6743447, 1 June 2004. [Google Scholar]
- Debord, B.; Lefebvre, C.; Guyothermann, A.M.; Hubert, J.; Bouche, R.; Guyot, J.C. Study of different crystalline forms of mannitol: Comparative behavior under compression. Drug Dev. Ind. Pharm. 1987, 13, 1533–1546. [Google Scholar] [CrossRef]
- Patil, J.; Vishwajith, V.; Gopal, V. Formulation development and evaluation of chewable tablets containing non-sedating antihistamine. J. Pharm. Sci. Innov. 2012, 1, 112–117. [Google Scholar]
- Muzzarelli, R.A.; Tubertini, O. Chitin and chitosan as chromatographic supports and adsorbents for collection of metal ions from organic and aqueous solutions and sea-water. Talanta 1969, 16, 1571–1577. [Google Scholar] [CrossRef] [PubMed]
- Datta, P.K.; Basu, P.S.; Datta, T.K. Isolation and characterization of Vicia faba lectin affinity purified on chitin column. Prep. Biochem. 1984, 14, 373–387. [Google Scholar] [PubMed]
- Songkroah, C.; Nakbanpote, W.; Thiravetyan, P. Recovery of silver-thiosulphate complexes with chitin. Proc. Biochem. 2004, 39, 1553–1559. [Google Scholar] [CrossRef]
- Krajewska, B. Application of chitin- and chitosan-based materials for enzyme immobilizations: A review. Enzyme Microb. Tech. 2004, 35, 126–139. [Google Scholar] [CrossRef]
- Yusof, N.L.; Wee, A.; Lim, L.Y.; Khor, E. Flexible chitin films as potential wound-dressing materials: wound model studies. J. Biomed. Mater. Res. A 2003, 66, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Rathke, T.D.; Hudson, S.M. Review of chitin and chitosan as fiber and film formers. J. Macromol. Sci. C 1994, 34, 375–437. [Google Scholar] [CrossRef]
- Pierson, Y.; Chen, X.; Bobbink, F.D.; Zhang, J.; Yan, N. Acid-catalyzed chitin liquefaction in ethylene glycol. ACS Sustain. Chem. Eng. 2014, 2, 2081–2089. [Google Scholar] [CrossRef]
- Chen, X.; Chew, S.L.; Kerton, F.M.; Yan, N. Direct conversion of chitin into a N-containing furan derivative. Green Chem. 2014, 16, 2204–2212. [Google Scholar] [CrossRef]
- Bobbink, F.D.; Zhang, J.; Pierson, Y.; Chen, X.; Yan, N. Conversion of chitin derived N-acetyl-d-glucosamine (NAG) into polyols over transition metal catalysts and hydrogen in water. Green Chem. 2015, 17, 1024–1031. [Google Scholar] [CrossRef]
- Daraghmeh, N.H.; Chowdhry, B.Z.; Leharne, S.A.; Al Omari, M.M.; Badwan, A.A. Chitin. In Profiles of Drug Substances, Excipients and Related Methodology; Brittain, H., Ed.; Elsevier Inc.: New York, NY, USA, 2011; Volume 36, pp. 35–102. [Google Scholar]
- Muzzarellim, R.A.A. Chitin; Pergamon Press: Oxford, UK, 1977. [Google Scholar]
- Gupta, P.; Nachaegari, S.K.; Bansal, A.K. Improved Excipient Functionality by Co-Processing. In Excipient Development for Pharmaceutical, Biotechnology, and Drug Delivery Systems; Katdare, A., Chaubal, M.V., Eds.; Informa Healthcare Inc.: New York, NY, USA, 2006; pp. 109–124. [Google Scholar]
- Rashid, I.; Daraghmeh, N.; Al-Remawai, M.; Leharne, S.A.; Chowdhry, B.Z.; Badwan, A. Characterization of chitin-metal silicates as binding superdisintegrants. J. Pharm. Sci. 2009, 98, 4887–4901. [Google Scholar] [CrossRef] [PubMed]
- Rashid, I.; Al-Remawi, M.; Eftaiha, A.; Badwan, A. Chitin–silicon dioxide coprecipitate as a novel superdisintegrant. J. Pharm. Sci. 2008, 97, 4955–4969. [Google Scholar] [CrossRef] [PubMed]
- Daraghmeh, N.; Rashid, I.; Al Omari, M.M.H.; Leharne, S.A.; Chowdhry, B.Z.; Badwan, A. Preparation and characterization of a novel co-processed excipient of chitin and crystalline mannitol. AAPS Pharm. Sci. Tech. 2010, 11, 1558–1571. [Google Scholar] [CrossRef]
- Kablan, T; Clément, Y. Bi, Y.; Françoise, K.A.; Mathias, O.K. Determination and modelling of moisture sorption isotherms of chitosan and chitin. Acta Chim. Slov. 2008, 55, 677–682. [Google Scholar]
- Silva, S.S.; Duarte, A.R.C.; Carvalho, A.P.; Mano, J.F.; Reis, R.L. Green processing of porous chitin structures for biomedical applications combining ionic liquids and supercritical fluid technology. Acta Biomater. 2011, 7, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Kharade, S.; Bhutkar, M.A. Novel superdisintegrants interpolymeric chitosan-alginate complex and chitin in the formulation of orodispersible tablets. Int. J. Pharm. Res. Dev. 2013, 5, 87–94. [Google Scholar]
- Glenn, T.C.; Bruno, C.H. A Comparison of Physical and Mechanical Properties of Common Tableting Diluents. In Excipient Development for Pharmaceutical, Biotechnology, and Drug Delivery Systems; Katdare, A., Chaubal, M.V., Eds.; Informa Healthcare Inc.: New York, NY, USA, 2006; pp. 127–151. [Google Scholar]
- Khinchi, M.P.; Gupta, M.K.; Bhandari, A.; Sharma, N.; Agarwal, D. Design and development of orally disintegrating tablets of famotidine prepared by direct compression method using different superdisintegrants. J. App. Pharm. Sci. 2011, 1, 50–58. [Google Scholar]
- Nyström, C.; Alderborn, G.; Duberg, M.; Karehill, P.G. Bonding surface area and bonding mechanism-two important factors for the understanding of powder compactibility. Drug Dev. Ind. Pharm. 1993, 19, 2143–2196. [Google Scholar]
- Alderborn, G.; Börjesson, E.; Glazer, M.; Nyström, C. Studies on direct compression of tablets. XIX: The effect of particle size and shape on the mechanical strength of sodium bicarbonate tablets. Acta Pharm. Suec. 1988, 25, 31–40. [Google Scholar] [PubMed]
- Juppo, A.M. Change in porosity parameters of lactose, glucose and mannitol granules caused by low compression force. Int. J. Pharm. 1996, 130, 149–157. [Google Scholar] [CrossRef]
- Bhowmik, D.; Chiranjib, B.; Krishnakanth, P.; Chandira, R.M. Fast dissolving tablet: An overview. J. Chem. Pharm. Res. 2009, 1, 163–177. [Google Scholar]
- Disintegration, Friability of Uncoated Tablets, Resistance to Crushing of Tablets. In British Pharmacopeia; The Stationary Office: London, UK, 2014; Volume V, pp. A333–A378, A493, A495.
- Kawakita, K.; Lüdde, K.H. Some considerations on powder compression equations. Powder Technol. 1071, 4, 61–68. [Google Scholar] [CrossRef]
- Shivanand, P.; Sprockel, O.L. Compaction behaviour of cellulose polymers. Powder Technol. 1992, 69, 177–184. [Google Scholar] [CrossRef]
- Lin, C.; Cham, T. Compression behaviour and tensile strength of heat-treated polyethylene glycols. Int. J. Pharm. 1995, 118, 169–179. [Google Scholar] [CrossRef]
- Nordström, J.; Klevan, I.; Alderborn, G. A particle rearrangement index based on the Kawakita powder compression equation. J. Pharm. Sci. 2008, 98, 1053–1063. [Google Scholar] [CrossRef]
- Adetunji, O.A.; Odeniyi, M.A.; Itiola, O.A. Compression, mechanical and release properties of chloroquine phosphate tablets containing corn and trifoliate yam starches as binders. Trop. J. Pharm. Res. 2006, 5, 589–596. [Google Scholar]
- Martins, E.; Christiana, I.; Olobayo, K. Effect of grewia gum on the mechanical properties of paracetamol tablet formulations. African J. Pharm. Pharmacol. 2008, 2, 1–6. [Google Scholar]
- Zhang, Y.; Law, Y.; Chakrabarti, S. Physical properties and compact analysis of commonly used direct compression binders. AAPS Pharm. Sci. Tech. 2005, 4. Article 62. [Google Scholar]
- U.S. Food and Drug Administration. Singulair® (montelukast sodium) Tablets, Chewable Tablets, and Oral Granules. Available online: http://www.accessdata.fda.gov (accessed on 29 January 2015).
- Mahesh, E.; Kiran Kumar, G.B.; Ahmed, M.G.; Kiran Kumar, P. Formulation and evaluation of montelukast sodium fast dissolving tablets. Asian J. Biomed. Pharm. Sci. 2012, 2, 75–82. [Google Scholar]
- Chhajed, M.; Tiwari, D.; Malve, A.; Godhwani, T.; Chhajed, A.; Shrivastava, A.K. Formulation development and evaluation of montelukast sodium orodispersible tablets: A new trend in asthma treatmentInt. J. Pharm. Res. Sci. 2012, 1, 127–139. [Google Scholar]
- Oroperidys 10 mg cp Orodispers, Vidal Homepage. Available online: http://www.vidal.fr/Medicament/oroperidys-75053.htm (accessed on 29 January 2015).
- Parmar, R.B.; Baria, A.H.; Tank, H.M.; Faldu, S.D. Formulation and evaluation of domperidone fast dissolving tablets. Int. J. Pharm. Tech. Res. 2009, 1, 483–487. [Google Scholar]
- Islam, A.; Haider, S.S.; Reza, M.S. Formulation and evaluation of orodispersible tablet of domperidone. Dhaka Univ. J. Pharm. Sci. 2011, 10, 117–122. [Google Scholar]
- Sutradhar, K.B.; Akhter, D.T.; Uddin, R. Formulation and evaluation of taste masked oral dispersible tablets of domperidone using sublimation method. Int. J. Pharm. Pharm. Sci. 2012, 4, 727–732. [Google Scholar]
- Validation of Compendia Procedures <1225>. In United States Pharmacopeia and National Formulary (USP37-NF32); US Pharmacopoeia Convention: Rockville, MD, USA, 2014; Volume I, pp. 1157–1162.
- Validation of Analytical Procedures: Text and Methodology, European Medicines Agency. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002662.pdf (accessed on 3 February 2015).
- Al Omari, M.M.; Zoubi, R.M.; Hasan, E.I.; Khader, T.Z.; Badwan, A.A. Effect of light and heat on the stability of montelukast in solution and in its solid state. J. Pharm. Biomed. Anal. 2007, 45, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Weast, R.C. Handbook of Chemistry and Physics, 55th ed.; CRC Press: Boca Raton, FL, USA, 1974–1975; p. E-46. [Google Scholar]
- Dissolution <711>. In United States Pharmacopeia and National Formulary (USP37-NF32); US Pharmacopoeia Convention: Rockville, MD, USA, 2014; Volume 1, pp. 344–351.
- Dissolution Method; U.S. Food and Drug Administration: Rockville, MD, USA. Available online: http://www.accessdata.fda.gov/scripts/cder/dissolution/dsp_SearchResults_Dissolutions.cfm?PrintAll=1 (accessed on 3 February 2015).
- Domperidone Tablets. In British Pharmacopeia; The Stationary Office: London, UK, 2014; Volume III, p. 469.
- Badwan, A.A.; The Jordanian Pharmaceutical Manufacturing Co. (JPM), Amman, Jordan. Unpublished work. 2014.
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daraghmeh, N.; Chowdhry, B.Z.; Leharne, S.A.; Al Omari, M.M.H.; Badwan, A.A. Co-Processed Chitin-Mannitol as a New Excipient for Oro-Dispersible Tablets. Mar. Drugs 2015, 13, 1739-1764. https://doi.org/10.3390/md13041739
Daraghmeh N, Chowdhry BZ, Leharne SA, Al Omari MMH, Badwan AA. Co-Processed Chitin-Mannitol as a New Excipient for Oro-Dispersible Tablets. Marine Drugs. 2015; 13(4):1739-1764. https://doi.org/10.3390/md13041739
Chicago/Turabian StyleDaraghmeh, Nidal, Babur Z. Chowdhry, Stephen A. Leharne, Mahmoud M. H. Al Omari, and Adnan A. Badwan. 2015. "Co-Processed Chitin-Mannitol as a New Excipient for Oro-Dispersible Tablets" Marine Drugs 13, no. 4: 1739-1764. https://doi.org/10.3390/md13041739
APA StyleDaraghmeh, N., Chowdhry, B. Z., Leharne, S. A., Al Omari, M. M. H., & Badwan, A. A. (2015). Co-Processed Chitin-Mannitol as a New Excipient for Oro-Dispersible Tablets. Marine Drugs, 13(4), 1739-1764. https://doi.org/10.3390/md13041739