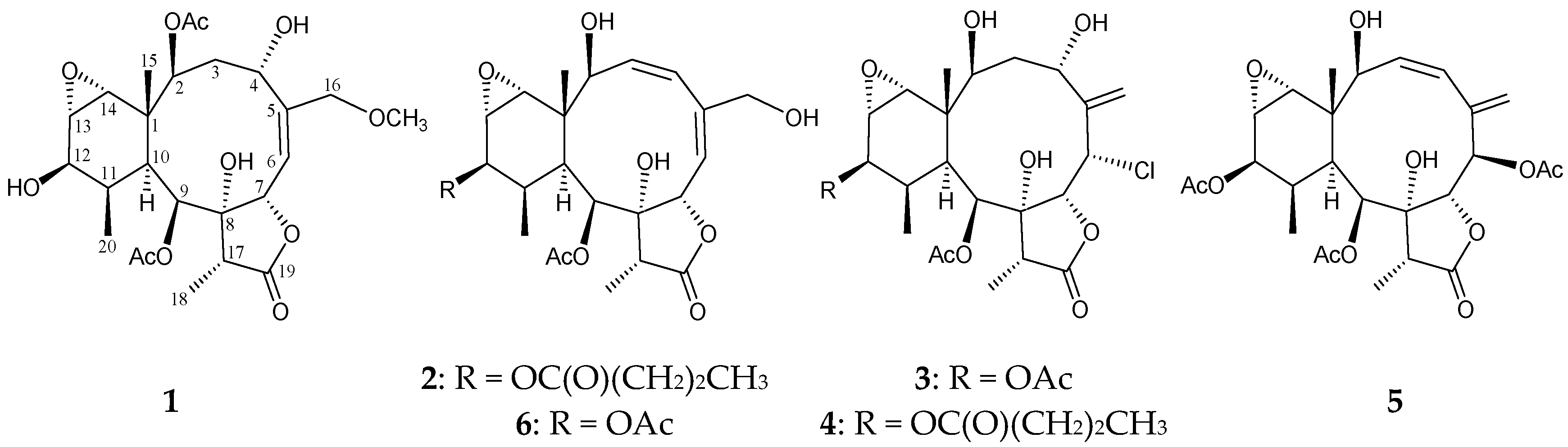
2. Results and Discussion
Briarenolide U (
1) was isolated as a white powder. The molecular formula of
1 was established as C
25H
36O
11 (eight degrees of unsaturation) from a sodium adduct at
m/
z 535 in the electrospray ionization mass spectrum (ESIMS) and further supported by the high-resolution electrospray ionization mass spectrum (HRESIMS) at
m/
z 535.21480 (calcd. for C
25H
36O
11 + Na, 535.21498). The IR spectrum of
1 showed bands at 3445, 1770 and 1733 cm
−1, consistent with the presence of hydroxy, γ-lactone and ester carbonyl groups. The
13C NMR and distortionless enhancement polarization transfer (DEPT) spectroscopic data showed that this compound has 25 carbons (
Table 1), including six methyls, two sp
3 methylenes, ten sp
3 methines, two sp
3 quaternary carbons, one sp
2 methine and four sp
2 quaternary carbons. From
1H and
13C NMR spectra (
Table 1),
1 was found to possess two acetoxy groups (δ
H 2.23, 2.10, each 3H × s; δ
C 21.9, 21.3, 2 × CH
3; 169.0, 172.6, 2 × acetate carbonyls), one γ-lactone moiety (δ
C 176.1, C-19) and a trisubstituted olefin (δ
H 5.66, 1H, dd,
J = 10.4, 1.6 Hz, H-6; δ
C 147.5, C-5; 116.7, CH-6). The presence of one disubstituted epoxy group was established from the signals of two oxymethines at δ
C 63.1 (CH-14) and 59.1 (CH-13) and further confirmed by the proton signals at δ
H 2.92 (1H, d,
J = 3.6 Hz, H-14) and 3.15 (1H, d,
J = 3.6 Hz, H-13). On the basis of the above unsaturation data,
1 was concluded to be a diterpenoid molecule possessing four rings.
Table 1.
1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data and 1H–1H COSY and HMBC correlations for briarane 1.
Table 1.
1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data and 1H–1H COSY and HMBC correlations for briarane 1.
| Position | δH (J in Hz) | δC, Multiple | 1H–1H COSY | HMBC |
|---|
| 1 | | 41.2, C | | |
| 2 | 4.70 ddd (2.4, 2.0, 2.0) | 77.6, CH | H2-3 | C-1, -4, -10, -15, acetate carbonyl |
| 3 | 3.11 ddd (15.2, 4.8, 2.0); 1.94 m | 40.2, CH2 | H-2, H-4 | C-1, -2, -4, -5 |
| 4 | 4.85 br s | 68.6, CH | H2-3, OH-4 | n. o. a |
| 5 | | 147.5, C | | |
| 6 | 5.66 dd (10.4, 1.6) | 116.7, CH | H-7, H2-16 | C-4, -16 |
| 7 | 5.02 d (10.4) | 75.9, CH | H-6 | C-5, -6 |
| 8 | | 82.2, C | | |
| 9 | 5.22 d (5.2) | 71.5, CH | H-10 | C-7, -8, -10, -11, acetate carbonyl |
| 10 | 1.81 dd (5.2, 2.8) | 37.3, CH | H-9, H-11 | C-1, -2, -8, -9, -11, -15, -20 |
| 11 | 1.97 m | 42.2, CH | H-10, H-12, H3-20 | n. o. |
| 12 | 3.71 d (4.4) | 70.2, CH | H-11 | C-13, -20 |
| 13 | 3.15 d (3.6) | 59.1, CH | H-14 | C-1 |
| 14 | 2.92 d (3.6) | 63.1, CH | H-13 | C-1, -10, -13, -15 |
| 15 | 1.21 s | 16.9, CH3 | | C-1, -2, -10, -14 |
| 16 | 4.36 br s | 73.2, CH2 | H-6 | C-4, -5, -6, methoxy carbon |
| 17 | 2.36 q (7.2) | 42.5, CH | H3-18 | C-8, -18, -19 |
| 18 | 1.16 d (7.2) | 6.5, CH3 | H-17 | C-8, -17, -19 |
| 19 | | 176.1, C | | |
| 20 | 1.07 d (7.2) | 8.7, CH3 | H-11 | C-10, -11, -12 |
| 2-OAc | | 172.6, C | | |
| | 2.10 s | 21.3, CH3 | | Acetate carbonyl |
| 9-OAc | | 169.0, C | | |
| | 2.23 s | 21.9, CH3 | | Acetate carbonyl |
| 16-OCH3 | 3.46 s | 58.9, CH3 | | C-16 |
| OH-4 | 3.99 d (10.4) | | H-4 | n. o. |
From the
1H–
1H correlation spectroscopy (COSY) spectrum of
1 (
Table 1), it was possible to establish the separate system that maps out the proton sequences from H-2/H
2-3/H-4, H-6/H-7 and H-9/H-10. These data, together with the heteronuclear multiple-bond coherence (HMBC) correlations between H-2/C-1, -4, -10; H
2-3/C-1, -2, -4, -5; H-6/C-4; H-7/C-5, -6; H-9/C-7, -8, -10; and H-10/C-1, -2, -8, -9, established the connectivity from C-1 to C-10 in the 10-membered ring (
Table 1). The methylcyclohexane ring, which is fused to the 10-membered ring at C-1 and C-10, was elucidated by the
1H–
1H COSY correlations between H-10/H-11/H-12, H-13/H-14, and H-11/H
3-20 and by the HMBC correlations between H-9/C-11; H-10/C-11, -20; H-12/C-13, -20; H-13/C-1; H-14/C-1, -10, -13 and H
3-20/C-10, -11, -12. The ring junction C-15 methyl group was positioned at C-1 from the HMBC correlations between H
3-15/C-1, -2, -10, -14 and H-2, H-10, H-14/C-15. The acetate esters at C-2 and C-9 were established by the correlations between H-2 (δ
H 4.70), H-9 (δ
H 5.22) and the acetate carbonyls at δ
C 172.6 and 169.0, respectively, in the HMBC spectrum of
1. The methoxy group at C-16 was confirmed by the HMBC correlations between the oxymethylene protons at δ
H 4.36 (H
2-16) and C-4 (δ
C 68.6), -5 (δ
C 147.5), -6 (δ
C 116.7) and an oxygenated methyl carbon at δ
C 58.9, and further confirmed by the allylic couplings between H
2-16 and H-6. The presence of a hydroxy group at C-4 was deduced from the
1H–
1H COSY correlation between a hydroxy proton (δ
H 3.99) and H-4 (δ
H 4.85). Thus, the remaining hydroxy groups had to be attached at C-8 and C-12 positions, respectively. These data, together with the
1H–
1H COSY correlation between H-17 and H
3-18 and the HMBC correlations between H-17/C-8, -18, -19 and H
3-18/C-8, -17, -19, were used to establish the molecular framework of
1.
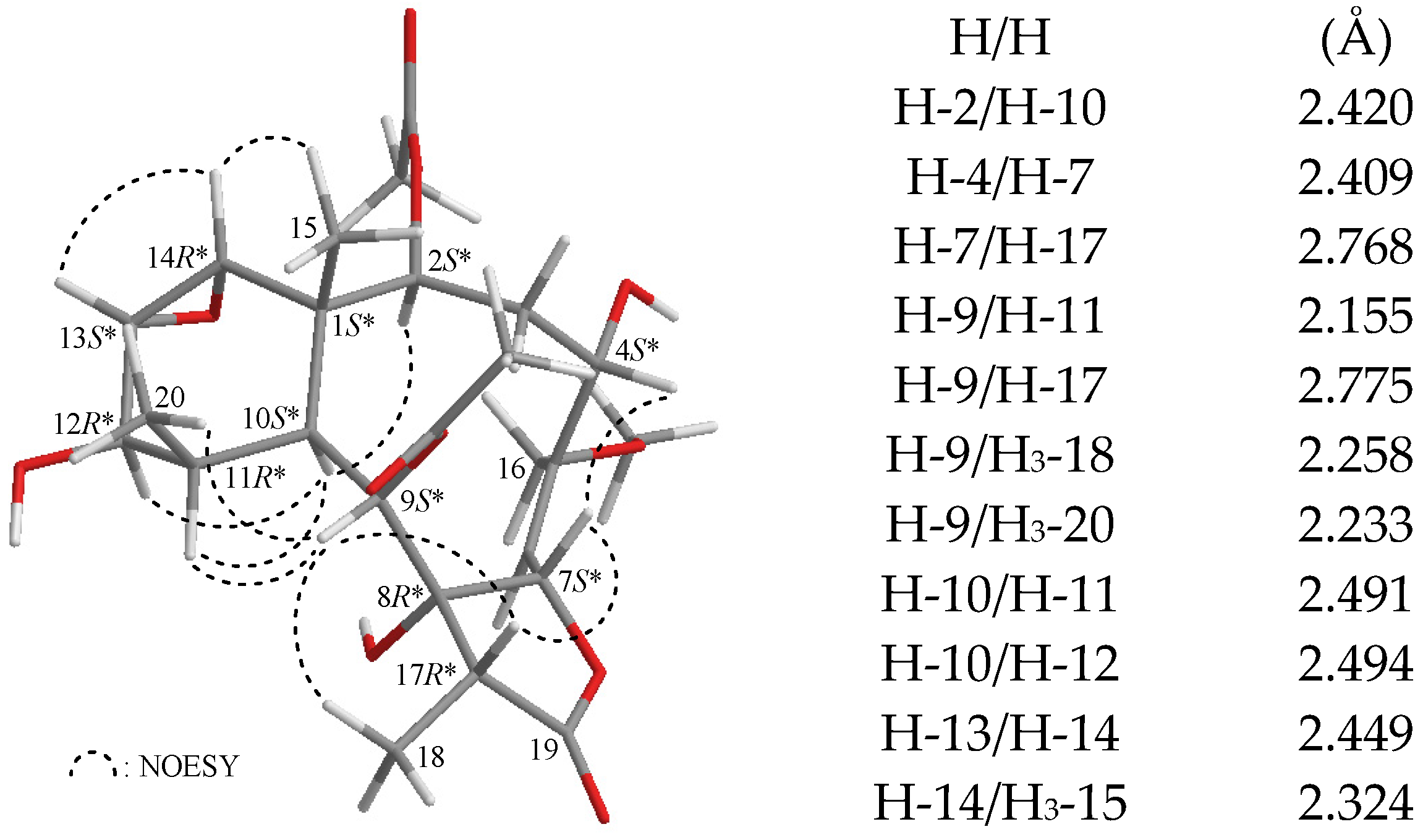
In all naturally-occurring briarane-type natural products, H-10 is
trans to the C-15 methyl group at C-1, and these two groups are assigned as α- and β-oriented, respectively, in briarane derivatives. The relative configuration of
1 was elucidated from the interactions observed in a nuclear Overhauser effect spectroscopy (NOESY) experiment and was found to be compatible with that of
1 offered by computer modeling (
Figure 2) [
7]. In the NOESY experiment of
1, the correlations of H-10 with H-2, H-11 and H-12, but not with H
3-15 and H
3-20, indicated that H-2, H-10, H-11, and H-12 were situated on the same face and were assigned as α protons, since the Me-15 and Me-20 are β-substituents at C-1 and C-11, respectively. H-14 showed correlations with H-13 and Me-15, but not with H-10, as well as a lack of coupling was detected between H-12 and H-13, indicating that the dihedral angle between H-12 and H-13 is approximately 90° and the 13,14-epoxy group has an α-orientation. H-9 was found to show responses to H-11, H-17, H
3-18, and H
3-20. From modeling analysis, H-9 was found to be close to H-11, H-17, H
3-18, and H
3-20 when H-9 was α-oriented. H-7 correlated with H-17, but not with H
3-18, indicating that H-7 and 8-hydroxy group were β- and α-oriented, respectively, in the γ-lactone moiety. Furthermore, H-4 correlated with H-7, but not with H-2, confirming the β-orientation for this proton. From the above evidence, the relative configuration of chiral carbons of
1 was assumed to be 1
S*, 2
S*, 4
S*, 7
S*, 8
R*, 9
S*, 10
S*, 11
R*, 12
R*, 13
S*, 14
R*, and 17
R*. Based on the above findings, the structure, including the relative configuration of
1, was fully determined.
Figure 2.
The computer-generated model of 1 using MM2 force field calculations and the calculated distances (Å) between selected protons with key NOESY correlations.
Figure 2.
The computer-generated model of 1 using MM2 force field calculations and the calculated distances (Å) between selected protons with key NOESY correlations.
Briarenolide V (
2) was isolated as a white powder and had a molecular formula of C
26H
36O
10 on the basis of HRESIMS at
m/
z 531.22025 (C
26H
36O
10 + Na, calcd. 531.22007). Carbonyl resonances in the
13C NMR spectrum of
2 (
Table 2) at δ
C 177.2, 173.6 and 170.1 revealed the presence of a γ-lactone and two other esters in
2. In the
1H NMR spectrum of
2 (
Table 2), a signal for one acetate methyl group was observed at δ
H 2.18 (3H, s). The additional acyl group was found to be an
n-butyrate group, which showed seven contiguous protons (δ
H 0.96, 3H, t,
J = 7.2 Hz; 1.66, 2H, sext,
J = 7.2 Hz; 2.35, 2H, t,
J = 7.2 Hz). The
13C NMR signal at δ
C 173.6 correlated with the methylene protons at δ
H 2.35 in the HMBC spectrum and was consequently assigned as the carbon atom of the
n-butyrate carbonyl.
Table 2.
1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data and 1H–1H COSY and HMBC correlations for briarane 2.
Table 2.
1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data and 1H–1H COSY and HMBC correlations for briarane 2.
| Position | δH (J in Hz) | δC, Multiple | 1H–1H COSY | HMBC |
|---|
| 1 | | 40.4, C | | |
| 2 | 4.11 d (10.4) | 75.9, CH | H-3 | C-1, -4, -15 |
| 3 | 5.80 dd (10.4, 10.4) | 135.9, CH | H-2, H-4 | C-5 |
| 4 | 6.31 d (10.4) | 125.5, CH | H-3 | n. o. a |
| 5 | | 145.3, C | | |
| 6 | 5.72 d (8.8) | 120.0, CH | H-7 | C-4, -16 |
| 7 | 5.23 d (8.8) | 79.9, CH | H-6 | n. o. |
| 8 | | 81.6, C | | |
| 9 | 5.15 d (6.8) | 70.1, CH | H-10 | C-8, -10, -11, -17, acetate carbonyl |
| 10 | 1.96 m | 37.4, CH | H-9, H-11 | C-1, -2, -8, -9, -11, -15, -20 |
| 11 | 2.07 m | 37.8, CH | H-10, H-12, H3-20 | C-10 |
| 12 | 4.73 d (4.4) | 71.8, CH | H-11 | C-13, C-1′ |
| 13 | 3.18 br s | 57.8, CH | | C-1 |
| 14 | 3.18 br s | 62.8, CH | | C-1, -15 |
| 15 | 1.13 s | 15.1, CH3 | | C-1, -2, -10, -14 |
| 16 | 4.29 br s | 63.6, CH2 | | n. o. |
| 17 | 2.28 q (7.2) | 43.4, CH | H3-18 | C-8, -18, -19 |
| 18 | 1.15 d (7.2) | 6.4, CH3 | H-17 | C-8, -17, -19 |
| 19 | | 177.2, C | | |
| 20 | 1.04 d (7.2) | 9.5, CH3 | H-11 | C-10, -11, -12 |
| 9-OAc | | 170.1, C | | |
| | 2.18 s | 21.8, CH3 | | Acetate carbonyl |
| 12-OC(O)CH2CH2CH3 | | | | |
| 1′ 2′ 3′ 4′ | | | | |
| 1′ | | 173.6, C | | |
| 2′ | 2.35 t (7.2) | 36.2, CH2 | H2-3′ | C-1′, -3′, -4′ |
| 3′ | 1.66 sext (7.2) | 18.3, CH2 | H2-2′, H3-4′ | C-1′, -2′, -4′ |
| 4′ | 0.96 t (7.2) | 13.7, CH3 | H2-3′ | C-2′, -3′ |
It was found that the NMR signals (
1H and
13C) of
2 were similar to those of a known briarane analogue, briaexcavatolide N (
6) [
8], except that the signals corresponding to an acetate group in
6 were replaced by signals for an
n-butyrate group in
2. The
n-butyrate ester was positioned at C-12 from an HMBC correlation between H-12 (δ
H 4.73) and the carbonyl carbon of the
n-butyrate (δ
C 173.6, C-1′) (
Table 2). The correlations from a NOESY experiment of
2 also showed that the stereochemistry of this metabolite is identical with that of
6 and the relative configuration of chiral carbons of
2 were assumed to be 1
S*, 2
S*, 7
S*, 8
R*, 9
S*, 10
S*, 11
R*, 12
R*, 13
S*, 14
R*, and 17
R*. Thus, briarenolide V (
2) was found to be the 12-
O-deacetyl-12-
O-
n-butyryl derivative of
6.
Briarenolide W (
3) had a molecular formula of C
24H
33ClO
10 as derived from a quasi-molecular ion at
m/
z 539 [M + Na]
+ in the ESIMS and from DEPT and
13C NMR spectra. Its IR bands indicated the presence of hydroxy (3461 cm
−1), γ-lactone (1778 cm
−1) and ester (1732 cm
−1) groups. The
1H NMR data of
3 (
Table 3) showed two acetyl singlets (δ
H 2.20, 2.12, each 3H × s), two methyl doublets (δ
H 1.14, 3H, d,
J = 7.2 Hz, H
3-18; 1.06, 3H, d,
J = 6.8 Hz, H
3-20) and a methyl singlet (δ
H 1.15, 3H, s, H
3-15), an exocyclic carbon-carbon double bond (δ
H 6.03, 2H, br s, H
2-16), three aliphatic methines (δ
H 1.95, 1H, m, H-10; 1.94, 1H, m, H-11; 2.38, 1H, q,
J = 7.2 Hz, H-17), one aliphatic methylene (δ
H 2.46, 1H, br d,
J = 16.4 Hz; 2.10, 1H, m, H
2-3), one chloromethine (δ
H 5.07, 1H, br s, H-6), seven oxymethines (δ
H 4.21, 1H, br s, H-2; 4.54, 2H, d,
J = 4.0 Hz, H-4 and H-12; 5.05, 1H, br s, H-7; 5.12, 1H, d,
J = 5.2 Hz, H-9; 3.21, 1H, d,
J = 3.2 Hz, H-13; 3.10, 1H, d,
J = 3.2 Hz, H-14) and one hydroxy proton (δ
H 3. 37, 1H, s, OH-8).
Table 3.
1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data and 1H–1H COSY and HMBC correlations for briarane 3.
Table 3.
1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data and 1H–1H COSY and HMBC correlations for briarane 3.
| Position | δH (J in Hz) | δC, Multiple | 1H–1H COSY | HMBC |
|---|
| 1 | | 40.4, C | | |
| 2 | 4.21 br s | 71.1, CH | H2-3 | C-1 |
| 3 | 2.46 br d (16.4); 2.10 m | 35.8, CH2 | H-2, H-4 | C-1 |
| 4 | 4.54 d (4.0) | 70.9, CH | H2-3, H2-16 | C-2, -5, -16 |
| 5 | | 143.6, C | | |
| 6 | 5.07 br s | 64.1, CH | H-7, H2-16 | n. o. a |
| 7 | 5.05 br s | 77.5, CH | H-6 | n. o. |
| 8 | | 83.9, C | | |
| 9 | 5.12 d (5.2) | 73.0, CH | H-10 | C-1, -7, -8, -10, -11, -17, acetate carbonyl |
| 10 | 1.95 m | 37.4, CH | H-9, H-11 | C-8 |
| 11 | 1.94 m | 39.5, CH | H-10, H-12, H3-20 | C-9 |
| 12 | 4.54 d (4.0) | 72.8, CH | H-11, H-13 | C-10, -13, -20, acetate carbonyl |
| 13 | 3.21 d (3.2) | 57.5, CH | H-12, H-14 | n. o. |
| 14 | 3.10 d (3.2) | 63.3, CH | H-13 | C-1 |
| 15 | 1.15 s | 16.7, CH3 | | C-1, -2, -10, -14 |
| 16 | 6.03 br s | 120.6, CH2 | H-4, H-6 | C-4, -5, -6 |
| 17 | 2.38 q (7.2) | 44.0, CH | H3-18 | C-8, -9, -18, -19 |
| 18 | 1.14 d (7.2) | 7.1, CH3 | H-17 | C-8, -17, -19 |
| 19 | | 174.7, C | | |
| 20 | 1.06 d (6.8) | 9.5, CH3 | H-11 | C-10, -11, -12 |
| 9-OAc | | 169.5, C | | |
| | 2.20 s | 21.9, CH3 | | Acetate carbonyl |
| 12-OAc | | 170.2, C | | |
| | 2.12 s | 21.0, CH3 | | Acetate carbonyl |
| OH-8 | 3.37 s | | | C-7, -8, -9 |
The planar structure of
3 was determined by 2D NMR studies. The
1H–
1H COSY experiment of
3 established the following correlations: H-2/H
2-3/H-4, H-6/H-7, H-9/H-10/H-11/H-12/H-13/H-14, H-17/H
3-18 and H-11/H
3-20 (
Table 3). These observations together with the HMBC correlations between H-2/C-1; H
2-3/C-1; H-4/ C-2, -5; H-9/C-1, -7, -8, -10, -11; H-10/C-8; H-11/C-9; H-12/C-10, -13; H-14/C-1; and OH-8/C-7, -8, -9, established the connectivity from C-1 to C-14 (
Table 3). The exocyclic carbon-carbon double bond at C-5 was elucidated by the HMBC correlations between H-4/C-16 and H
2-16/C-4, -5, -6, and further confirmed by the allylic couplings between H-4/H
2-16 and H-6/H
2-16. The intensity of [M + Na + 2] isotope peak observed in the ESIMS [(M + Na)/(M + 2 + Na) = 3:1] was strong evidence of the presence of a chlorine atom in
3. Consequently, the methine proton signal at δ
H 5.07 (1H, br s) was confidently assigned to H-6, which beared a chlorinated carbon (δ
C 64.1, CH-6), and was confirmed by the
1H–
1H COSY correlations between H-6/7 and H-6/16 (by allylic coupling); and by the HMBC correlations between H
2-16/C-4, -5, -6. C-15 methyl group was positioned at C-1 from the HMBC correlations between H
3-15/C-1, -2, -10, -14. Furthermore, seven oxymethine protons were observed at δ
H 5.12, 5.05, 4.54, 4.54, 4.21, 3.21, 3.10, were
1J-correlated to the carbons δ
C 73.0, 77.5, 72.8, 70.9, 71.1, 57.5, 63.3, and assigned to C-9, -7, -12, -4, -2, -13, -14, respectively. In addition, the presence of two acetate esters at C-9 and C-12 was established by the correlations between H-9 (δ
H 5.12), H-12 (δ
H 4.54) and the acetate carbonyls at δ
C 169.5 and 170.2, respectively, observed in the HMBC spectrum of
3. The relative stereochemistry of
3 was elucidated from the NOE interactions observed in a NOESY experiment. Due to the α-orientation of H-10, the ring junction C-15 methyl group should be β-oriented as no correlation was observed between H-10 and H
3-15. The correlations between H-14/H
3-15 and H-13/H-14, indicated the β-orientations of H-13 and H-14. In addition, the NOE correlations between H-10/H-2, OH-8, H-9, H-11, H-12, H
3-18, suggested the α-orientation of these protons (H-2, H-9, H-10, OH-8, H-11, H-12 and H
3-18) and H-17 is β-oriented. Furthermore, H-7 showed correlations with H-17 and H-6, suggesting that these protons are on the β face of
3. Based on the above findings, the configurations of all chiral centers of
3 were assigned as 1
S*, 2
S*, 4
S*, 6
S*, 7
R*, 8
R*, 9
S*, 10
S*, 11
R*, 12
R*, 13
S*, 14
R*, and 17
R*.
Briarenolide X (
4), C
26H
37ClO
10 (HRESIMS,
m/
z 567.19687, calcd. for C
26H
37ClO
10 + Na, 567.19675), was recognized as a 6-chlorinated briarane diterpenoid closely related to
3 from their NMR data (
Table 3 and
Table 4). Both briaranes
3 and
4 have identical substituents: secondary hydroxy groups at C-2 and C-4; an exocyclic methylene at C-5; a chloride atom at C-6; a tertiary hydroxy group at C-8; a secondary acetate at C-9. They also have the C-13/14 epoxy group in common. While briarane
3 showed the presence of a secondary acetate at C-12 of the methylcyclohexane ring,
4 showed an
n-butyrate at this position. The
1H and
13C NMR data assignments of briarenolide X (
4) were made in comparison with the values of
3. The position of the
n-butyrate group at C-12 was corroborated by an HMBC correlation observed between
n-butyrate carbonyl carbon at δ
C 172.7 and the proton at δ
H 4.58 (H-12) (
Table 4). The other HMBC correlations observed fully supported the location of functional groups, and hence briarenolide X (
4) was assigned as the structure
4 with the same relative stereochemistry as in briarane
3 because for the chiral carbons that
4 has in common with
3, the
1H and
13C NMR chemical shifts and proton coupling constants matched well. Based on the above findings, the chiral carbons of
4 were assigned as 1
S*, 2
S*, 4
S*, 6
S*, 7
R*, 8
R*, 9
S*, 10
S*, 11
R*, 12
R*, 13
S*, 14
R*, and 17
R*.
Briarenolide Y (
5) was obtained as a white powder and the molecular formula for
5 was determined to be C
26H
34O
11 (10 degrees of unsaturation) was confirmed by HRESIMS at
m/
z 545.19918 (calcd. for C
26H
34O
11 + Na, 545.19933). Comparison of the
1H and DEPT spectra with the molecular formula indicated that there must be two exchangeable protons, requiring the presence of two hydroxy groups. The IR spectrum showed bands at 3445, 1770, and 1732 cm
−1, consistent with the presence of hydroxy, γ-lactone and ester groups. From the
13C NMR data of
5 (
Table 5), the presence of one disubstituted olefin and one exocyclic olefin were deduced from the signals at δ
C 138.1 (C-5), 134.7 (CH-3), 126.0 (CH-4), 122.2 (CH
2-16) and further supported by four olefin proton signals at δ
H 6.07 (1H, d,
J = 12.0 Hz, H-4), 5.80 (1H, dd,
J = 12.0, 9.2 Hz, H-3), 5.63 (1H, s, H-16), and 5.50 (1H, s, H-16) in the
1H NMR spectrum of
5 (
Table 5). Four carbonyl resonances appeared at δ
C 175.1, 170.6, 170.4, and 170.1 confirming the presence of a γ-lactone and three ester groups in
5; three acetate methyls (δ
H 2.18, 2.13 and 2.09, each 3H × s) were also observed. So from the NMR data, six degrees of unsaturation were accounted for, and therefore
5 must be tetracyclic. The presence of one epoxide was elucidated from the signals of two oxymethines at δ
C 62.6 (CH-14) and 57.8 (CH-13) and further confirmed by the proton signals at δ
H 3.17 (1H, d,
J = 3.6 Hz, H-14) and 3.25 (1H, d,
J = 3.6 Hz, H-13). In addition, one methyl singlet, two methyl doublets, three aliphatic methine protons, four oxymethine protons, were observed in the
1H NMR spectrum of
5.
Table 4.
1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data and 1H–1H COSY and HMBC correlations for briarane 4.
Table 4.
1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data and 1H–1H COSY and HMBC correlations for briarane 4.
| Position | δH (J in Hz) | δC, Multiple | 1H–1H COSY | HMBC |
|---|
| 1 | | 40.4, C | | |
| 2 | 4.20 br s | 71.4, CH | H2-3 | n. o. a |
| 3 | 2.45 br d (16.4); 2.13 m | 37.4, CH2 | H-2, H-4 | n. o. |
| 4 | 4.54 br d (5.2) | 70.5, CH | H2-3, H2-16 | n. o. |
| 5 | | 143.8, C | | |
| 6 | 5.06 br s | 64.1, CH | H2-16 | C-7 |
| 7 | 5.05 br s | 77.5, CH | | n. o. |
| 8 | | 83.8, C | | |
| 9 | 5.13 d (5.2) | 73.2, CH | H-10 | C-7, -8, -11, -17, acetate carbonyl |
| 10 | 1.98 m | 37.6, CH | H-9 | n. o. |
| 11 | 1.96 m | 39.7, CH | H-12, H3-20 | n. o. |
| 12 | 4.58 d (4.4) | 72.5, CH | H-11 | C-1′ |
| 13 | 3.21 d (3.6) | 57.3, CH | H-14 | n. o. |
| 14 | 3.09 d (3.6) | 63.2, CH | H-13 | C-13 |
| 15 | 1.16 s | 16.6, CH3 | | C-1, -2, -10, -14 |
| 16 | 6.03 d (2.0); 6.02 br s | 120.6, CH2 | H-4, H-6 | C-4, -5, -6 |
| 17 | 2.39 q (7.2) | 44.1, CH | H3-18 | C-8, -18, -19 |
| 18 | 1.14 d (7.2) | 7.1, CH3 | H-17 | C-8, -17, -19 |
| 19 | | 174.4, C | | |
| 20 | 1.06 d (7.2) | 9.5, CH3 | H-11 | C-10, -11, -12 |
| 9-OAc | | 169.5, C | | |
| | 2.20 s | 21.9, CH3 | | Acetate carbonyl |
| 12-OC(O)CH2CH2CH3 | | | | |
| 1′ 2′ 3′ 4′ | | | | |
| 1′ | | 172.7, C | | |
| 2′ | 2.35 t (7.2) | 36.2, CH2 | H2-3′ | C-1′, -3′, -4′ |
| 3′ | 1.69 sext (7.2) | 18.4, CH2 | H2-2′, H3-4′ | C-1′, -2′, -4′ |
| 4′ | 0.98 t (7.2) | 13.7, CH3 | H2-3′ | C-2′, -3′ |
| OH-8 | 3.35 s | | | C-8, -9 |
The gross structure of
5 was determined by 2D NMR studies.
1H NMR coupling information in the
1H–
1H COSY spectrum of
5 enabled identification of the C-2/-3/-4, C-6/-7, C-9/-10/-11/-12, C-13/-14 and C-11/20 units. From these data and the HMBC correlations (
Table 5), the connectivity from C-1 to C-14 and C-11 to C-20 could be established. One exocyclic double bond at C-5 was confirmed by the allylic coupling between H-4/H
2-16 and H-6/H
2-16 in the
1H–
1H COSY spectrum and by the HMBC correlations between H
2-16/C-4, -5, -6; H-4/C-16; and H-6/C-16. The ring junction C-15 methyl group was positioned at C-1 from the HMBC correlations between H-2/C-15, H-10/C-15, H-14/C-15, and H
3-15/C-1, -2, -10, -14. Furthermore, the acetate esters positioned at C-6, C-9, and C-12 were established by the correlations between δ
H 5.73 (H-6), 5.26 (H-9), 4.63 (H-12) and the acetate carbonyls appearing at δ
C 170.1, 170.4, and 170.6, respectively. Thus, the remaining hydroxy group had to be positioned at C-8. These data, together with the HMBC correlations between H-9/C-17; H-17/C-8, -9, -18, -19; and H
3-18/C-8, -17, -19, unambiguously established the molecular framework of
5.
Table 5.
1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data and 1H–1H COSY and HMBC correlations for briarane 5.
Table 5.
1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data and 1H–1H COSY and HMBC correlations for briarane 5.
| Position | δH (J in Hz) | δC, Multiple | 1H–1H COSY | HMBC |
|---|
| 1 | | 41.4, C | | |
| 2 | 5.08 d (9.2) | 71.9, CH | H-3 | C-1, -3, -4, -14, -15 |
| 3 | 5.80 dd (12.0, 9.2) | 134.7, CH | H-2, H-4 | n. o. a |
| 4 | 6.07 d (12.0) | 126.0, CH | H-3, H2-16 | C-2, -3, -16 |
| 5 | | 138.1, C | | |
| 6 | 5.73 d (9.6) | 75.5, CH | H-7, H2-16 | C-4, -5, -7, -16, acetate carbonyl |
| 7 | 4.67 d (9.6) | 81.6, CH | H-6 | C-6 |
| 8 | | 80.4, C | | |
| 9 | 5.26 d (7.6) | 69.9, CH | H-10 | C-7, -8, -10, -11, -17, acetate carbonyl |
| 10 | 2.15 m | 36.5, CH | H-9, H-11 | C-1, -2, -8, -9, -11, -15, -20 |
| 11 | 2.04 m | 37.3, CH | H-10, H-12, H3-20 | C-12 |
| 12 | 4.63 d (4.4) | 72.7, CH | H-11 | C-10, -13, -14, -20, acetate carbonyl |
| 13 | 3.25 d (3.6) | 57.8, CH | H-13 | n. o. |
| 14 | 3.17 d (3.6) | 62.6, CH | H-14 | C-1, -10, -15 |
| 15 | 1.07 s | 14.8, CH3 | | C-1, -2, -10, -14 |
| 16 | 5.63 s; 5.50 s | 122.2, CH2 | H-4, H-6 | C-4, -5, -6 |
| 17 | 2.46 q (7.2) | 45.0, CH | H3-18 | C-8, -9, -18, -19 |
| 18 | 1.14 d (7.2) | 6.2, CH3 | H-17 | C-8, -17, -19 |
| 19 | | 175.1, C | | |
| 20 | 1.04 d (7.2) | 9.3, CH3 | H-11 | C-10, -11, -12 |
| 6-OAc | | 170.1, C | | |
| | 2.09 s | 21.3, CH3 | | Acetate carbonyl |
| 9-OAc | | 170.4, C | | |
| | 2.18 s | 21.9, CH3 | | Acetate carbonyl |
| 12-OAc | | 170.6, C | | |
| | 2.13 s | 21.1, CH3 | | Acetate carbonyl |
The relative stereochemistry of
5 was elucidated from the NOESY interactions observed in a NOESY experiment (
Figure 3) and by the vicinal
1H–
1H coupling constants analysis. In the NOESY experiment of
5, H-10 gives correlations to H-2, H-9, H-11 and H-12, but not with H
3-15 and H
3-20, indicating that H-2, H-9, H-10, H-11, and H-12 are located on the same face of the molecule and assigned as α-protons, since C-15 and C-20 methyls are β-substituents at C-1 and C-11, respectively. The C-15 methyl protons were found to exhibit a response with H-14 and H-14 correlated with H-13 showing that the C-13/14 epoxy group was α-oriented. It was found that H-17 showed correlations with H-7 and H-9. Consideration of molecular models revealed that H-17 is reasonably close to H-7 and H-9 when H-17 and H-7 are β-oriented and H-9 and 8-hydroxy group are placed on the α face. H-7 showed a correlation with H-4, but not with H-6, and a large coupling constant (
J = 9.2 Hz) was detected between H-6 and H-7, indicating that the dihedral angle between H-6 and H-7 is approximately 180°, and H-6 was α-oriented. The
cis geometry of C-3/4 double bond was indicated by a correlation between H-3 (δ
H 5.80) and H-4 (δ 6.07) and confirmed by a 12.0 Hz coupling constant between these two olefin protons. Based on the consideration of a 3D model of
5, and the chiral centers for briarane
5 are assigned as 1
S*, 2
S*, 6
R*, 7
S*, 8
R*, 9
S*, 10
S*, 11
R*, 12
R*, 13
S*, 14
R*, and 17
R*.
In
in vitro anti-inflammatory activity tests, the upregulation of the pro-inflammatory iNOS and COX-2 protein expression of LPS-stimulated RAW264.7 macrophage cells was evaluated using immunoblot analysis. At a concentration of 10 μM, briarenolides U–Y (
1–
5) were found to significantly reduce the levels of iNOS to 41.9%, 47.3%, 50.1%, 66.2%, and 54.3%, respectively, and these five compounds were also found to significantly reduce the levels of COX-2 to 26.1%, 35.6%, 58.1%, 67.2%, and 55.4%, respectively, relative to the control cells stimulated with LPS only (
Figure 4).
Figure 3.
The computer-generated model of 5 using MM2 force field calculations and the calculated distances (Å) between selected protons with key NOESY correlations.
Figure 3.
The computer-generated model of 5 using MM2 force field calculations and the calculated distances (Å) between selected protons with key NOESY correlations.
Figure 4.
Effects of compounds briarenolides U–Y (1–5) on pro-inflammatory iNOS and COX-2 protein expression in the LPS-stimulated murine macrophage cell line RAW264.7. (A) The relative density of iNOS immunoblot; (B) the relative density of COX-2 immunoblot. The relative intensity of the LPS-stimulated group was taken to be 100%. Band intensities were quantified by densitometry and are indicated as the percent change relative to that of the LPS-stimulated group. Briarenolides U–Z (1–5) and dexamethasone (Dex) significantly inhibited LPS-induced iNOS and COX-2 protein expression in macrophages. The experiments were repeated three times (* p < 0.05, significantly different from the LPS-stimulated group).
Figure 4.
Effects of compounds briarenolides U–Y (1–5) on pro-inflammatory iNOS and COX-2 protein expression in the LPS-stimulated murine macrophage cell line RAW264.7. (A) The relative density of iNOS immunoblot; (B) the relative density of COX-2 immunoblot. The relative intensity of the LPS-stimulated group was taken to be 100%. Band intensities were quantified by densitometry and are indicated as the percent change relative to that of the LPS-stimulated group. Briarenolides U–Z (1–5) and dexamethasone (Dex) significantly inhibited LPS-induced iNOS and COX-2 protein expression in macrophages. The experiments were repeated three times (* p < 0.05, significantly different from the LPS-stimulated group).
| | iNOS | Cox-2 |
|---|
| | Expression (% of LPS) | Expression (% of LPS) |
|---|
| Control | 7.76 ± 1.99 | 2.2 ± 1.0 |
| LPS | 100 ± 0 | 100 ± 0 |
| U (1) | 41.9 ± 15.0 | 26.1 ± 7.7 |
| V (2) | 47.3 ± 16.5 | 35.6 ± 8.3 |
| W (3) | 50.1 ± 9.3 | 58.1 ± 8.1 |
| X (4) | 66.2 ± 6.7 | 67.2 ± 9.9 |
| Y (5) | 54.3 ± 9.6 | 55.4 ± 16.2 |
| Dexamethasone a | 26.7 ± 14.1 | 10.1 ± 6.8 |