Paper Synthesis, Cytotoxicity and Apoptosis Induction in Human Tumor Cells by Galaxamide and Its Analogues
Abstract
:1. Introduction
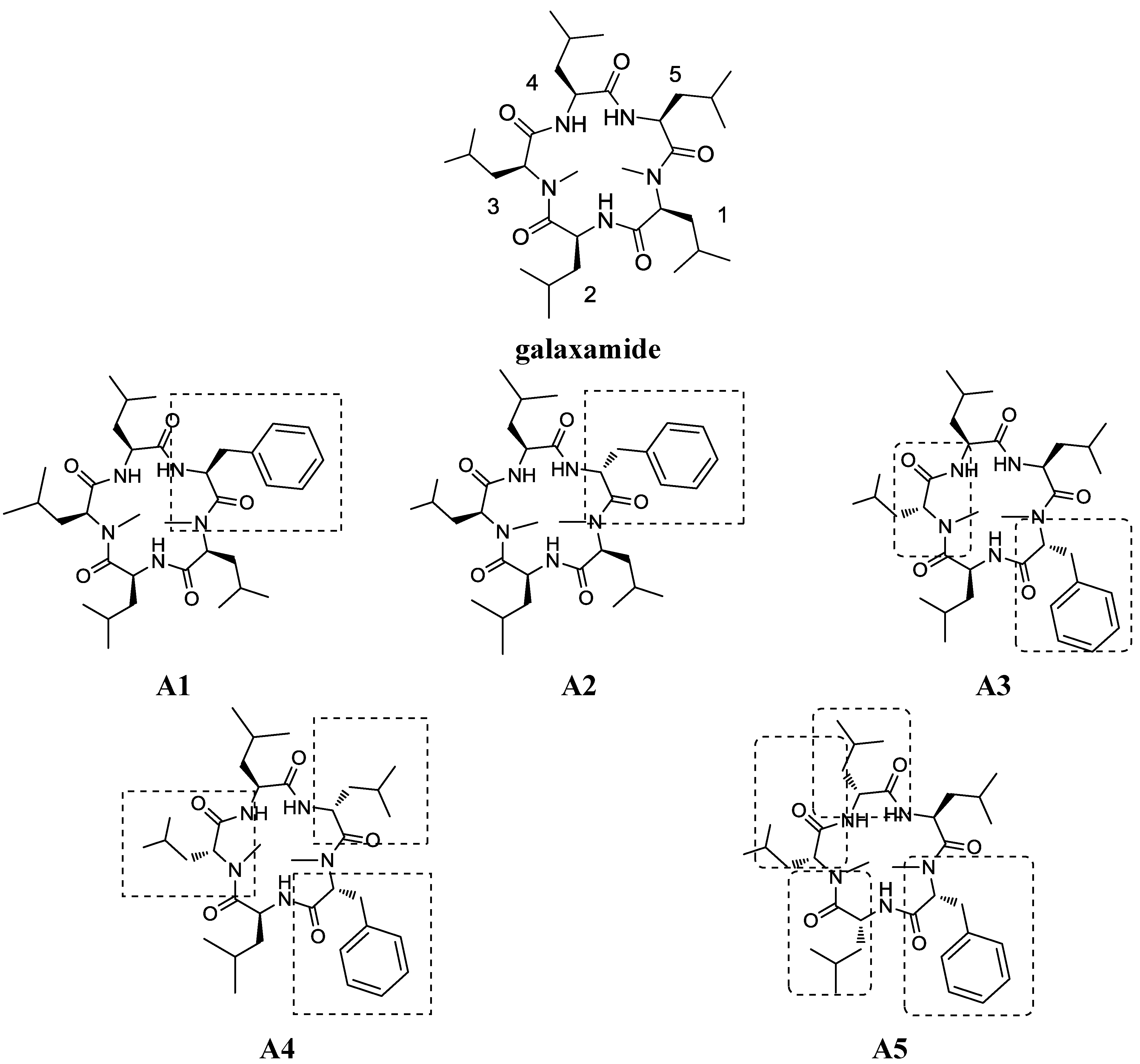
2. Results
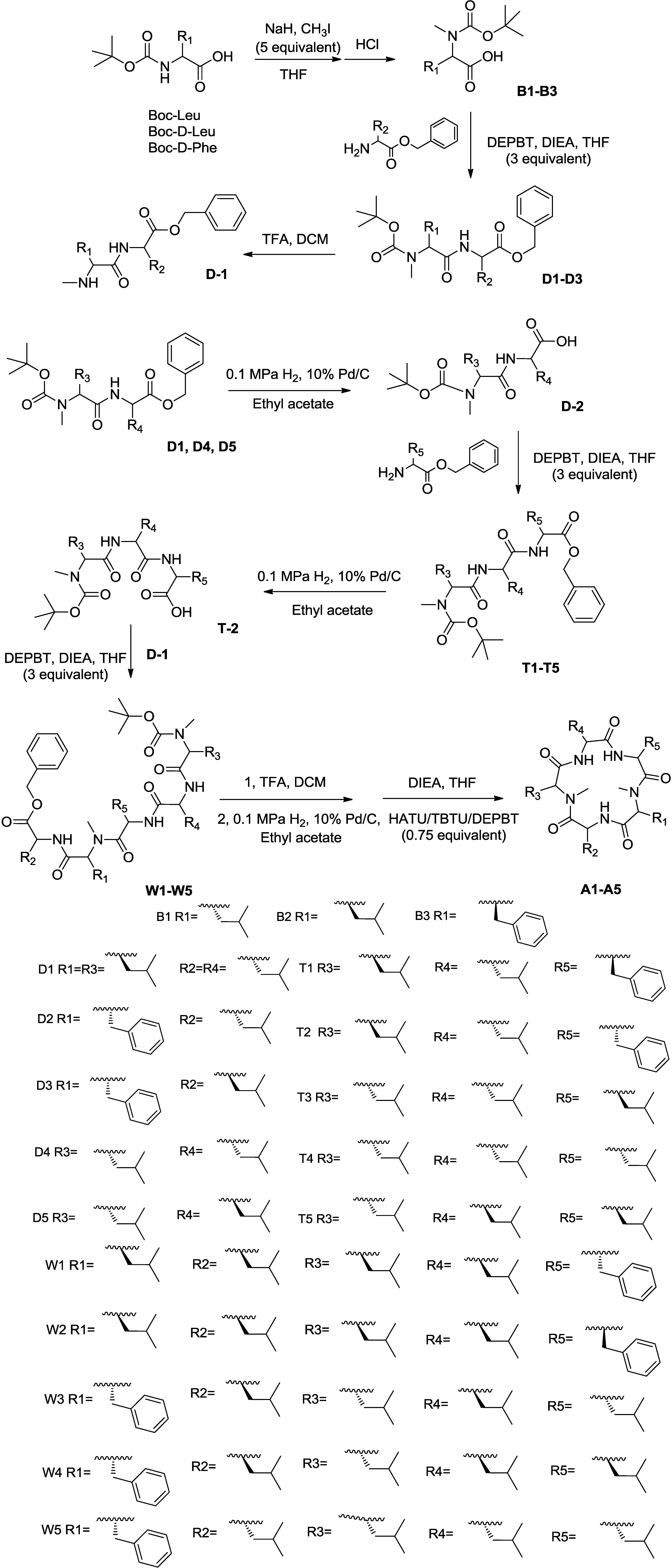
2.1. Chemistry
2.2. Biological Activity
2.2.1. Galaxamide and Its Analogues Inhibit the Proliferation of Different Cancer Cells
| Compound/Cell Line | HepG2 b | L02 b | SW480 b | U87 b | MCF-7 b |
|---|---|---|---|---|---|
| Galaxamide | 4.63 ± 0.14 | >40 | >40 | 10.61 ± 0.24 | 14.09 ± 1.35 |
| A1 | 4.11 ± 0.16 | >40 | 12.37 ± 1.01 | >40 | 9.40 ± 0.43 |
| A2 | 4.56 ± 0.20 | >40 | 5.75 ± 0.19 | >40 | 9.64 ± 0.35 |
| A3 | 5.58 ± 0.11 | >40 | 10.20 ± 0.15 | 20.16 ± 0.30 | 7.23 ± 0.27 |
| A4 | 6.27 ± 0.13 | >40 | 8.60 ± 0.08 | 2.82 ± 0.10 | 6.56 ± 0.18 |
| A5 | 1.46 ± 0.09 | 10.55 ± 0.60 | 5.10 ± 0.12 | 1.85 ± 0.11 | 4.18 ± 0.15 |
2.2.2. Effect of Galaxamide and A5 on the Cell Morphology and Nuclear Integrity
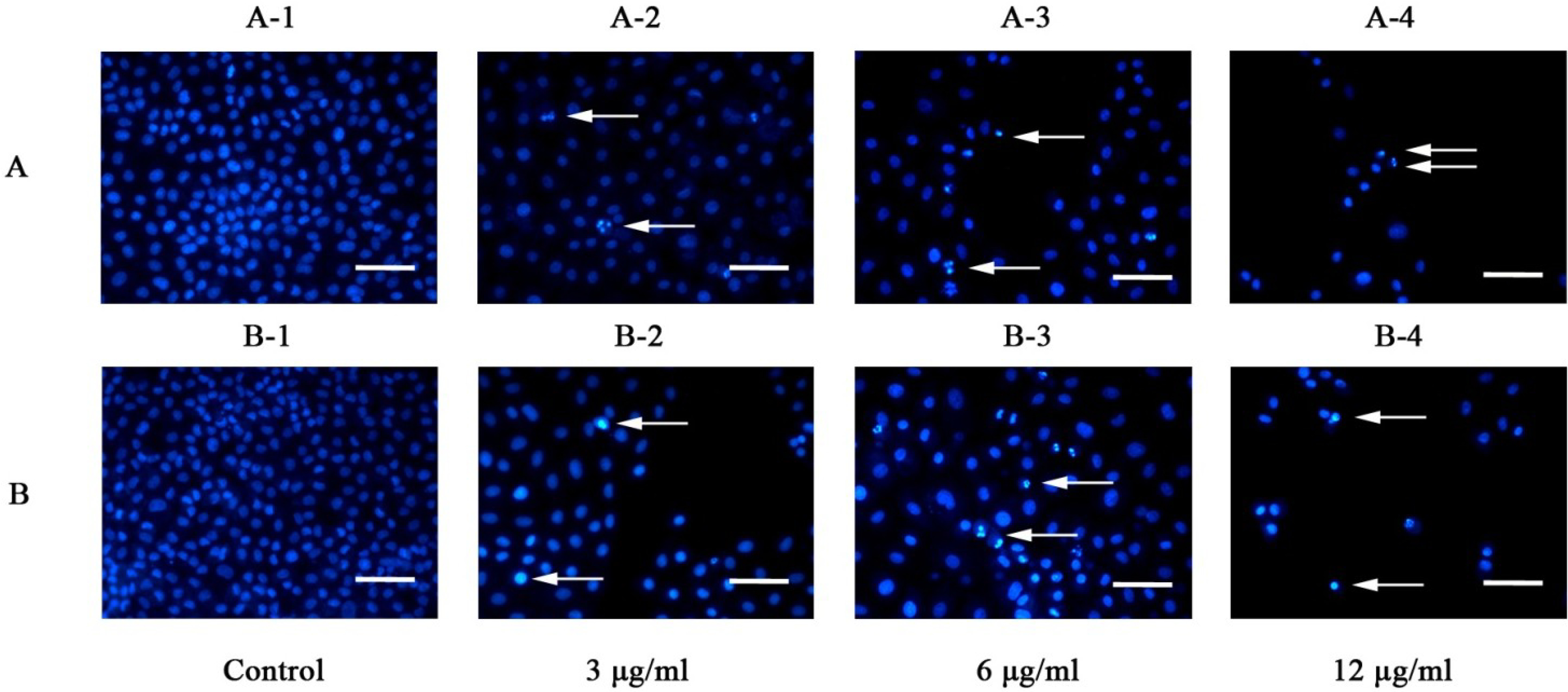
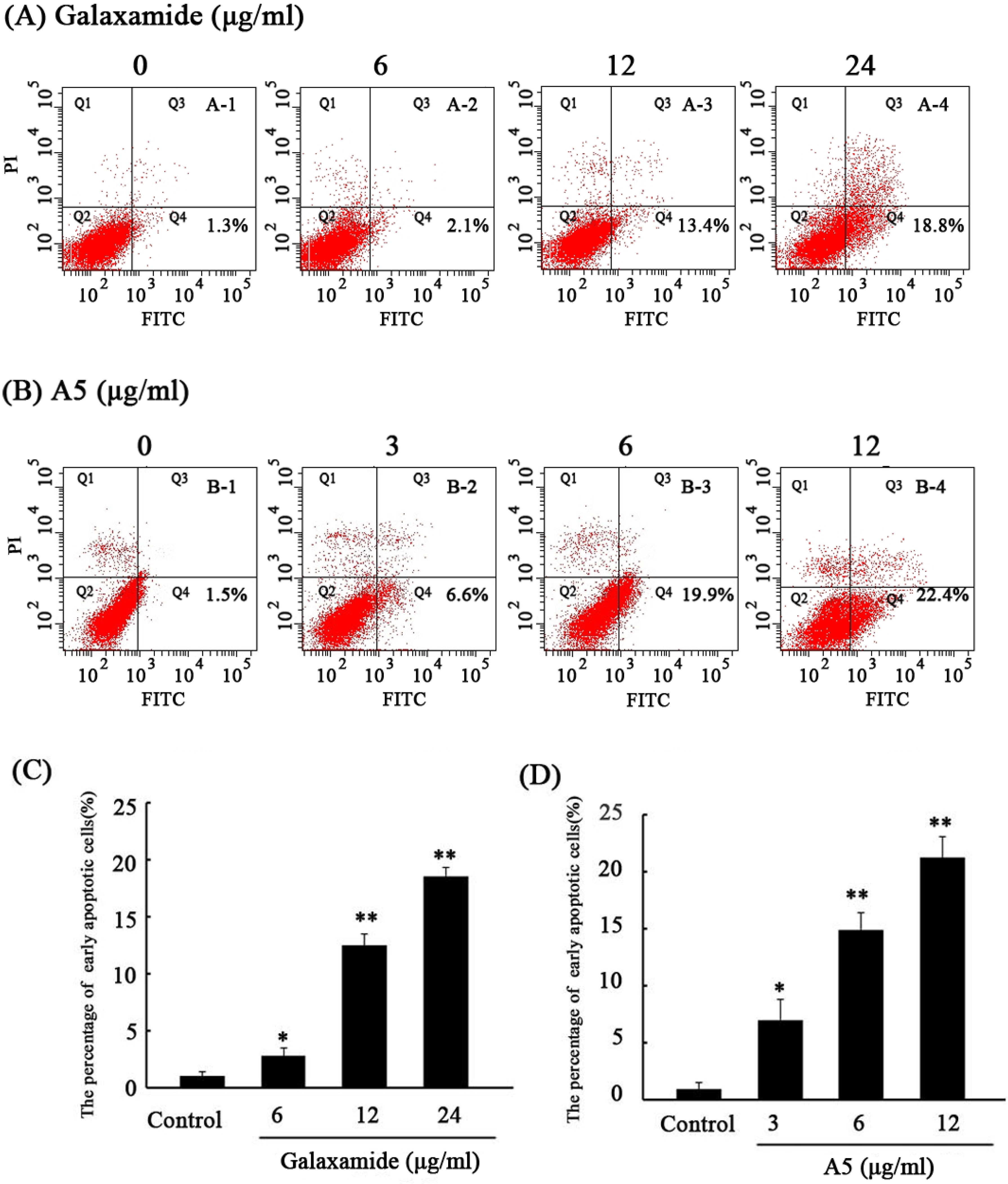
2.2.3. Galaxamide and A5 Induce HepG2 Apoptosis
2.2.4. Galaxamide and A5 Activate Caspase-3, Caspase-9 and PARP in HepG2
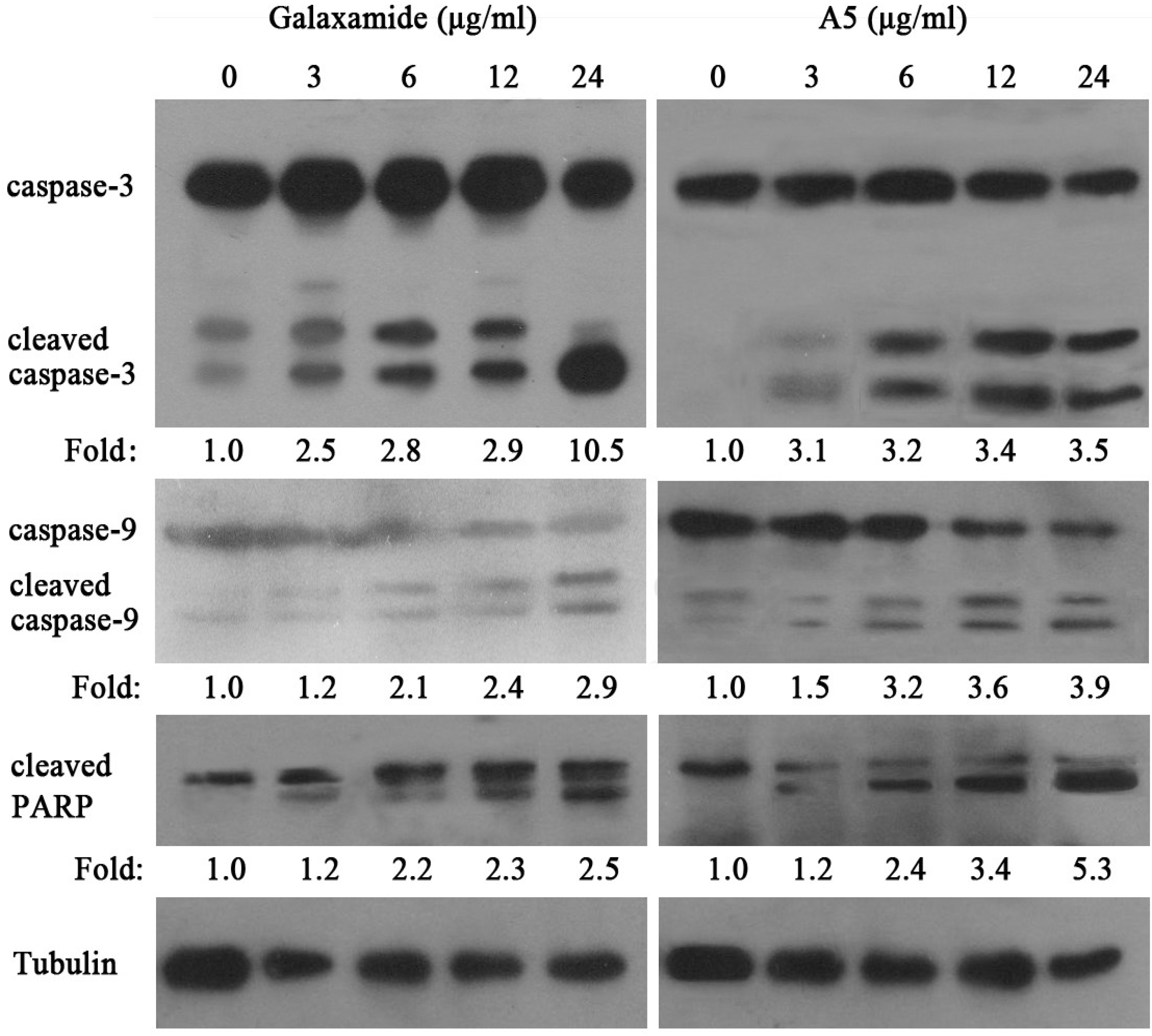
3. Discussion
4. Experimental Section
4.1. Chemistry
4.1.1. N-Boc-Me-Leu-Leu-OBn (D1)
4.1.2. N-Boc-Me-d-Phe-Leu-OBn (D2)
4.1.3. N-Boc-Me-d-Phe-d-Leu-OBn (D3)
4.1.4. N-Boc-Me-d-Leu-Leu-OBn (D4)
4.1.5. N-Boc-Me-d-Leu-d-Leu-OBn (D5)
4.1.6. N-Boc-Me-Leu-Leu-Phe-OBn (T1)
4.1.7. N-Boc-Me-Leu-Leu-d-Phe-OBn (T2)
4.1.8. N-Boc-Me-d-Leu-Leu-Leu-OBn (T3)
4.1.9. N-Boc-Me-d-Leu-Leu-d-Leu-OBn (T4)
4.1.10. N-Boc-Me-d-Leu-d-Leu-Leu-OBn (T5)
4.1.11. N-Boc-Me-Leu-Leu-Phe-Me-Leu-LeuOBn (W1)
4.1.12. N-Boc-Me-Leu-Leu-d-Phe-Me-Leu-Leu-OBn (W2)
4.1.13. N-Boc-Me-d-Leu-Leu-Leu-d-Phe-Leu-OBn (W3)
4.1.14. N-Boc-Me-d-Leu-Leu-d-Leu-d-Phe-Leu-OBn (W4)
4.1.15. N-Boc-Me-d-Leu-d-Leu-Leu-d-Phe-d-Leu-OBn (W5)
4.1.16. Cyclo(Phe-N-Me-Leu-Leu-N-Me-Leu-Leu) (A1)
4.1.17. Cyclo(d-Phe-N-Me-Leu-Leu-N-Me-Leu-d-Leu) (A2)
4.1.18. Cyclo(Me-d-Leu-Leu-Leu-Me-d-Phe-Leu) (A3)
4.1.19. Cyclo(Me-d-Leu-Leu-d-Leu-Me-d-Phe-Leu) (A4)
4.1.20. Cyclo(Leu-d-N-Me-Phe-d-Leu-d-N-Me-Leu-d-Leu) (A5)
4.2. Antitumor Activity in Vitro
4.2.1. Cell Culture
4.2.2. Anti-Proliferative Activity Using MTT Assays
4.2.3. Cell Apoptosis Analysis
4.2.4. Observations of Nuclear Damage
4.2.5. Western Blot Analysis
4.2.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Donia, M.; Hamann, M.T. Marine natural products and their potential applications as anti-infective agents. Lancet Infect. Dis. 2003, 3, 338–348. [Google Scholar] [CrossRef]
- Chen, J.S. Marine drug research and development ramble. Chem. Rev. 1995, 95, 2115–2134. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2011, 28, 196–268. [Google Scholar] [CrossRef]
- Davies, J.S. The cyclization of peptides and depsipeptides. J. Peptide. Sci. 2003, 9, 471–501. [Google Scholar] [CrossRef]
- Xu, W.J.; Liao, X.J.; Xu, S.H.; Diao, J.Z. Isolation, structure determination, and synthesis of Galaxamide, a rare cytotoxic cyclic pentapeptide from a marine algae Galaxaura filamentosa. Org. Lett. 2008, 10, 4569–4572. [Google Scholar] [CrossRef]
- Davis, M.R.; Styers, T.J.; Rodriguez, R.A.; Pan, P.S.; Vasko, R.C.; McAlpine, S.R. Synthesis and cytotoxicity of a new class of potent decapeptide macrocycles. Org. Lett. 2008, 10, 177–180. [Google Scholar]
- Katerina, O.; Gerald, L.; David, V. Comprehensive study of Sansalvamide A analogues and their structure–activity relationships against drug-resistant colon cancer cell lines. J. Med. Chem. 2007, 51, 530–544. [Google Scholar]
- Sakurai, A.; Okumura, Y. Synthesis of viscumamide and its analogs. Chem. Soc. Jpn. 1979, 52, 540–543. [Google Scholar] [CrossRef]
- Cheung, S.T.; Benoiton, N.L. N-Methylamino acids in peptides synthesis. V. The synthesis of N-tert-butyloxycarbony1, N-methylamino acids by N-methylation. Can. J. Chem. 1977, 55, 906–910. [Google Scholar] [CrossRef]
- Li, H.T.; Jiang, X.H.; Ye, Y.H.; Fan, C.X.; Romoff, T.; Goodman, M. 3-(Diethoxyphosphoryloxy)-1,2,3-benzotriazin-4(3H)-one(DEPBT): A new coupling reagent with remarkable resistance to racemization. Org. Lett. 1999, 1, 91–93. [Google Scholar] [CrossRef]
- Zhu, J.D.; Ma, D.W. Total synthesis of microsclerodermin E. Angew. Chem. Int. Ed. Engl. 2003, 42, 5348–5351. [Google Scholar] [CrossRef]
- Belofsky, G.N.; Jensen, P.R.; Fenical, W. A new cytotoxic cyclic depsipeptide produced by a marine fungus of the genus Fusarium. Tetrahedron Lett. 1999, 40, 2913–2916. [Google Scholar] [CrossRef]
- Rodriguez, R.A.; Pan, P.S.; Pan, C.M.; Ravula, S.; Lapera, S.; Singh, E.K.; Styers, T.J.; Brown, J.D.; Cajica, J.; Parry, E.; et al. Synthesis of second-generation Sansalvamide A derivatives: Novel templates as potential antitumor agents. J. Org. Chem. 2007, 72, 1980–2002. [Google Scholar] [CrossRef]
- Martin, S.J.; Green, D.R. Apoptosis and cancer: The failure of controls on cell death and cell survival. Crit. Rev. Oncol. Hematol. 1995, 8, 137–153. [Google Scholar] [CrossRef]
- Gajate, C.; An, F.; Mollinedo, F. Rapid and selective apoptosis in human leukemic cells induced by aplidine through a Fas/CD95- and mitochondrial-mediated mechanism. Clin. Cancer Res. 2003, 9, 1535–1545. [Google Scholar]
- Baker, M.A.; Grubb, D.R.; Lawen, A. Didemnin B induces apoptosis in proliferating but not resting peripheral blood mononuclear cells. Apoptosis 2002, 7, 407–412. [Google Scholar] [CrossRef]
- Joseph, B.K.; Mark, N.P.; Leslie, D.A.; Veronica, C.A.; Jeanette, R.M.; Shelli, R.M. Synthesis and evaluation of biotinylated sansalvamide A analogs and their modulation of Hsp90. Bioorg. Med. Chem. Lett. 2011, 21, 4716–4719. [Google Scholar] [CrossRef]
- Pan, G.; O’Rourke, K.; Chinnaiyan, A.M.; Gentz, R.; Ebner, R.; Ni, J.; Dixit, V.M. The receptor for the cytotoxic ligand TRAIL. Science 1997, 276, 111–113. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xiao, X.; Liao, X.; Qiu, S.; Liu, Z.; Du, B.; Xu, S. Paper Synthesis, Cytotoxicity and Apoptosis Induction in Human Tumor Cells by Galaxamide and Its Analogues. Mar. Drugs 2014, 12, 4521-4538. https://doi.org/10.3390/md12084521
Xiao X, Liao X, Qiu S, Liu Z, Du B, Xu S. Paper Synthesis, Cytotoxicity and Apoptosis Induction in Human Tumor Cells by Galaxamide and Its Analogues. Marine Drugs. 2014; 12(8):4521-4538. https://doi.org/10.3390/md12084521
Chicago/Turabian StyleXiao, Xi, Xiaojian Liao, Shaoling Qiu, Zihao Liu, Bin Du, and Shihai Xu. 2014. "Paper Synthesis, Cytotoxicity and Apoptosis Induction in Human Tumor Cells by Galaxamide and Its Analogues" Marine Drugs 12, no. 8: 4521-4538. https://doi.org/10.3390/md12084521
APA StyleXiao, X., Liao, X., Qiu, S., Liu, Z., Du, B., & Xu, S. (2014). Paper Synthesis, Cytotoxicity and Apoptosis Induction in Human Tumor Cells by Galaxamide and Its Analogues. Marine Drugs, 12(8), 4521-4538. https://doi.org/10.3390/md12084521




