Total Synthesis and Structure-Activity Relationship of Glycoglycerolipids from Marine Organisms
Abstract
:1. Introduction
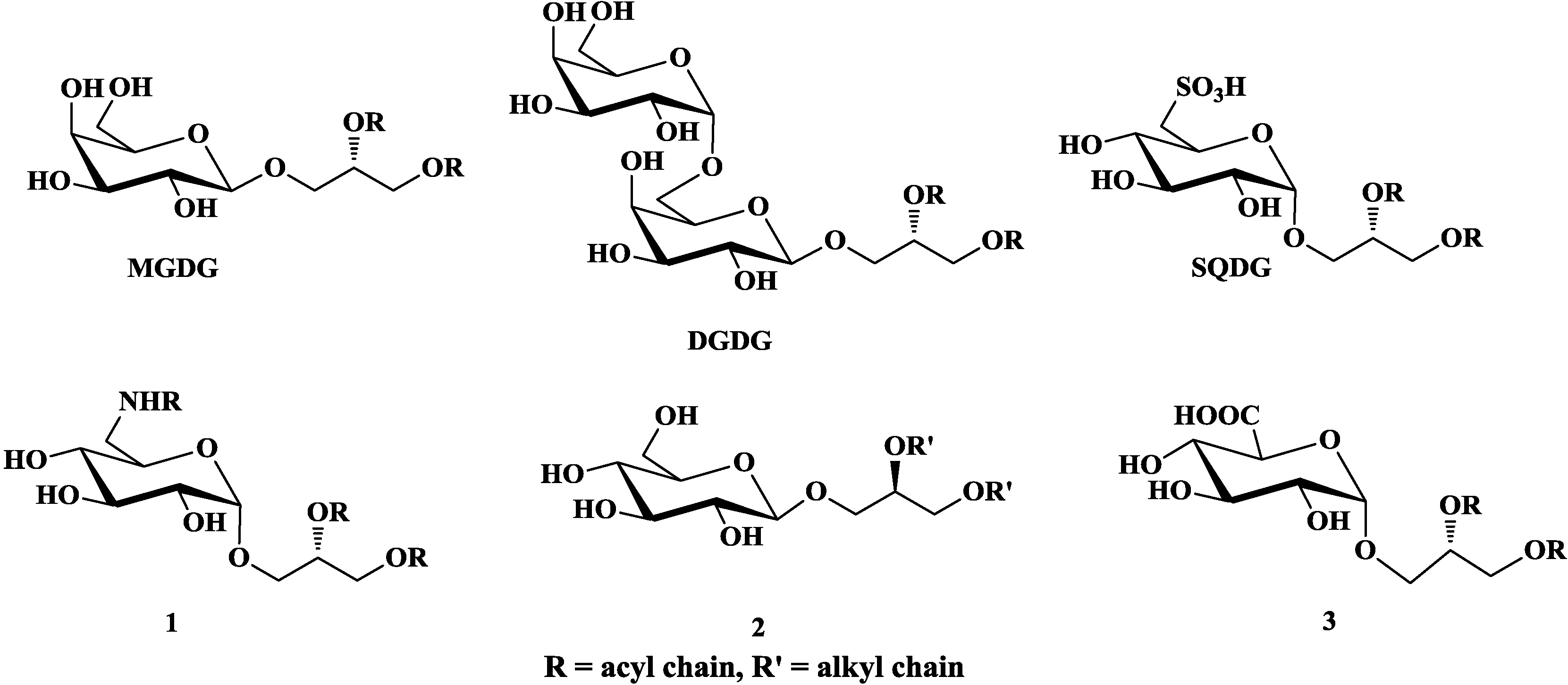
2. Bioactivities
2.1. Anti-Tumor Activity
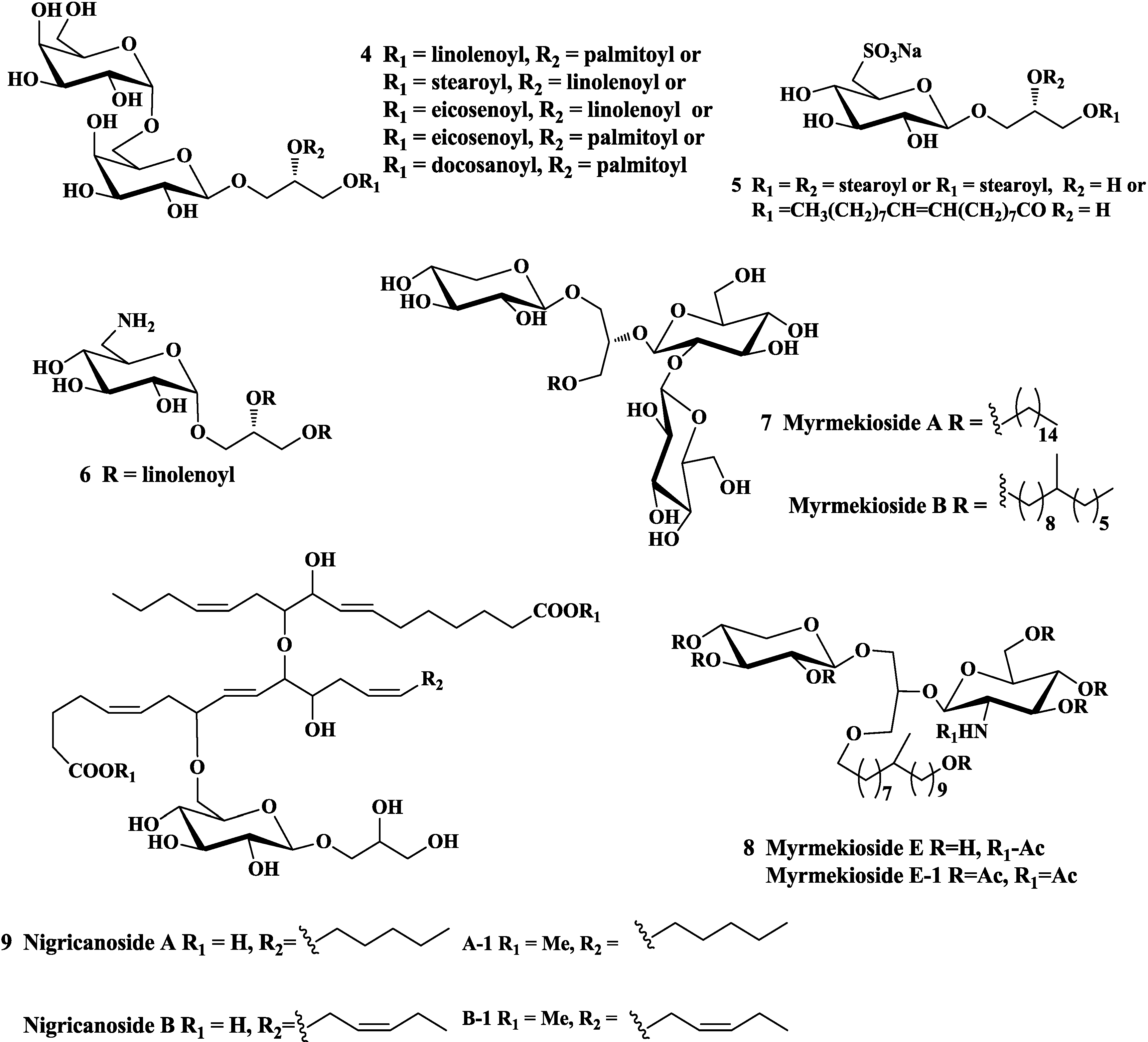
2.3. Anti-Inflammatory Activity
2.4. Other Activities

3. Total Synthesis
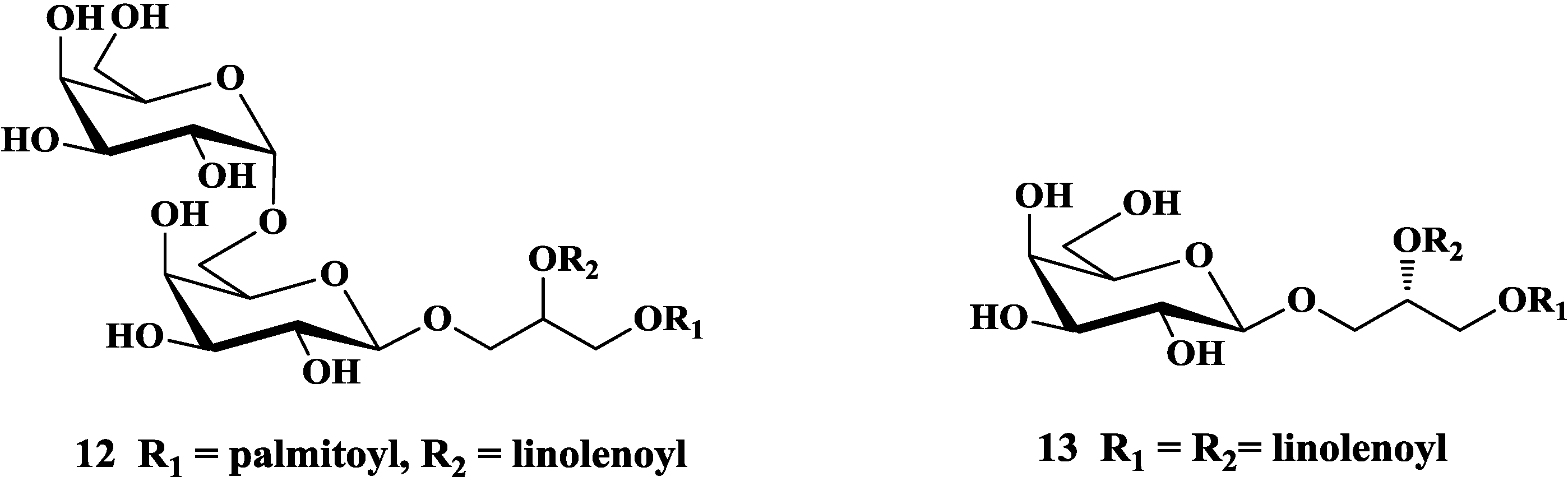
3.1. Total Synthesis of Mono- and Di-Glycosyldiacylglycerols
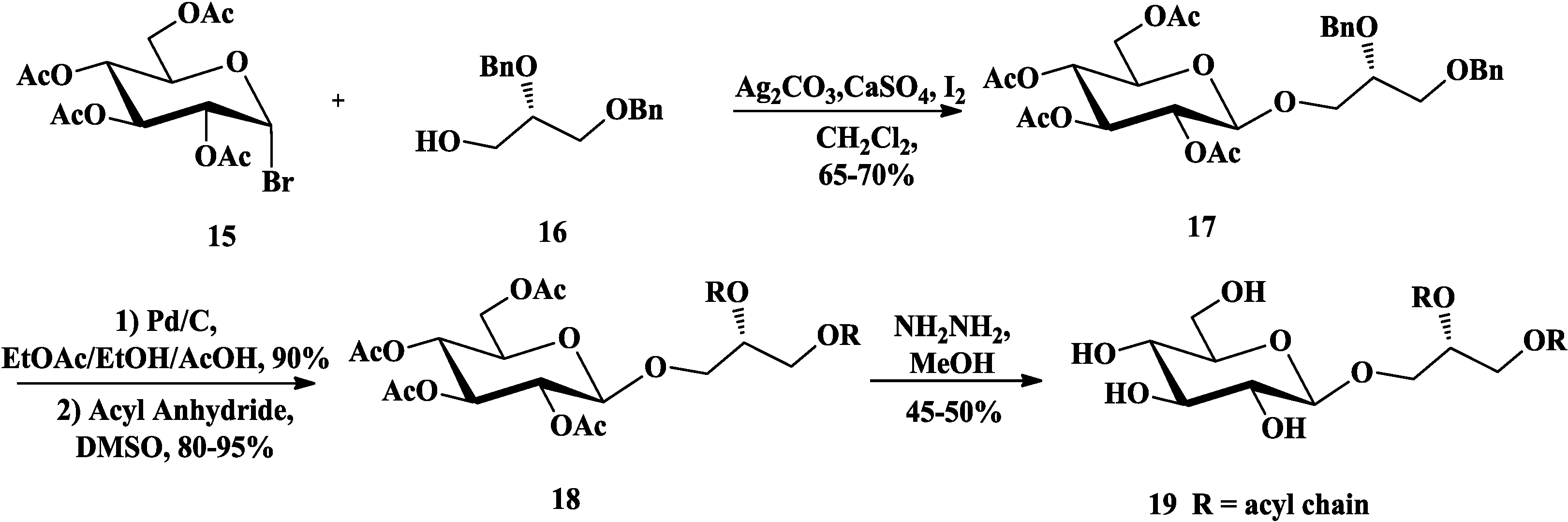
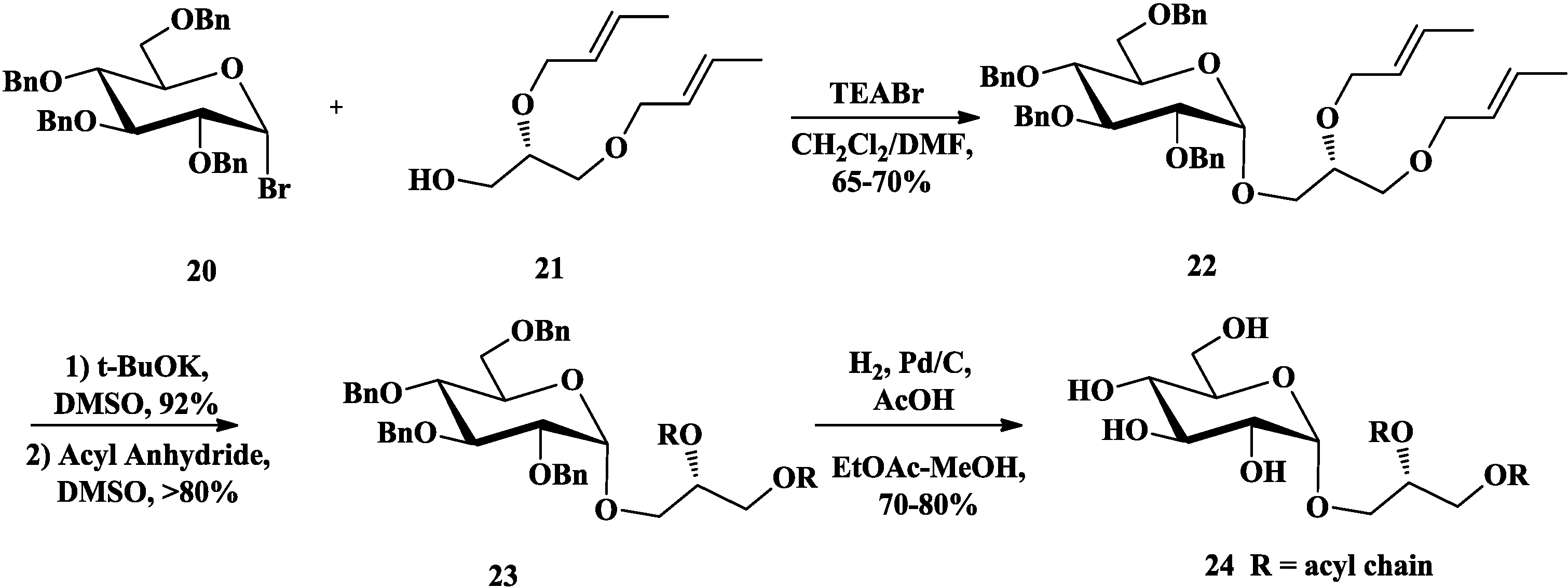

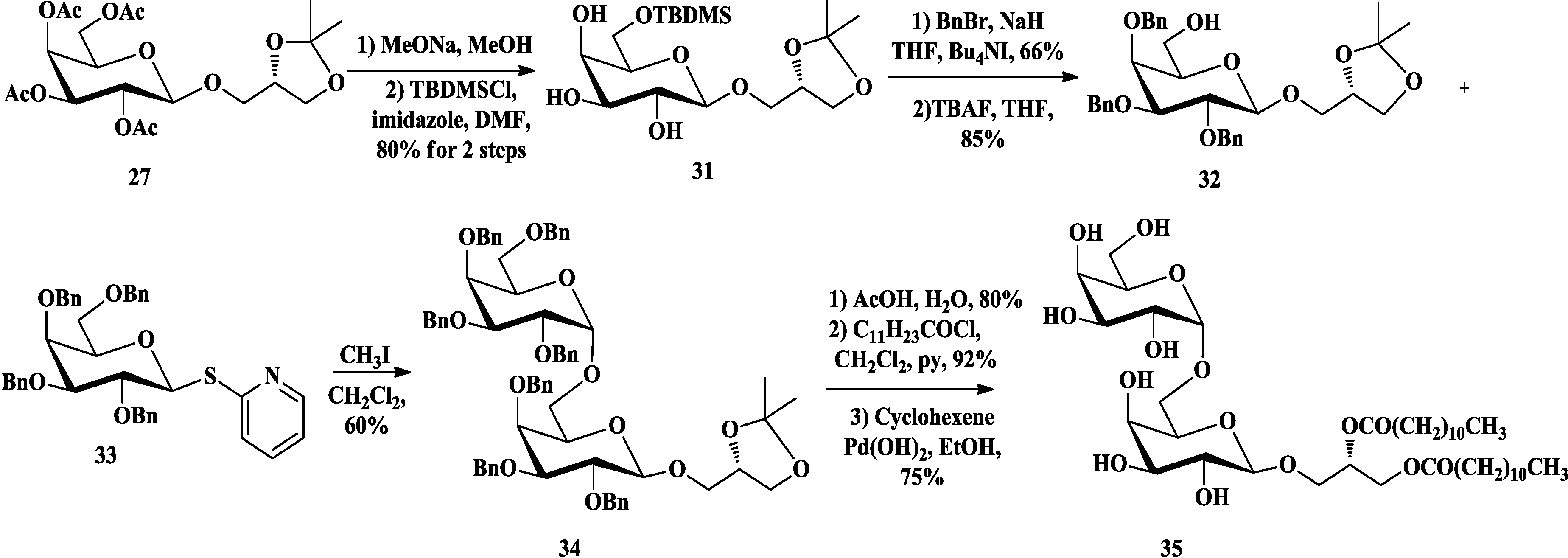
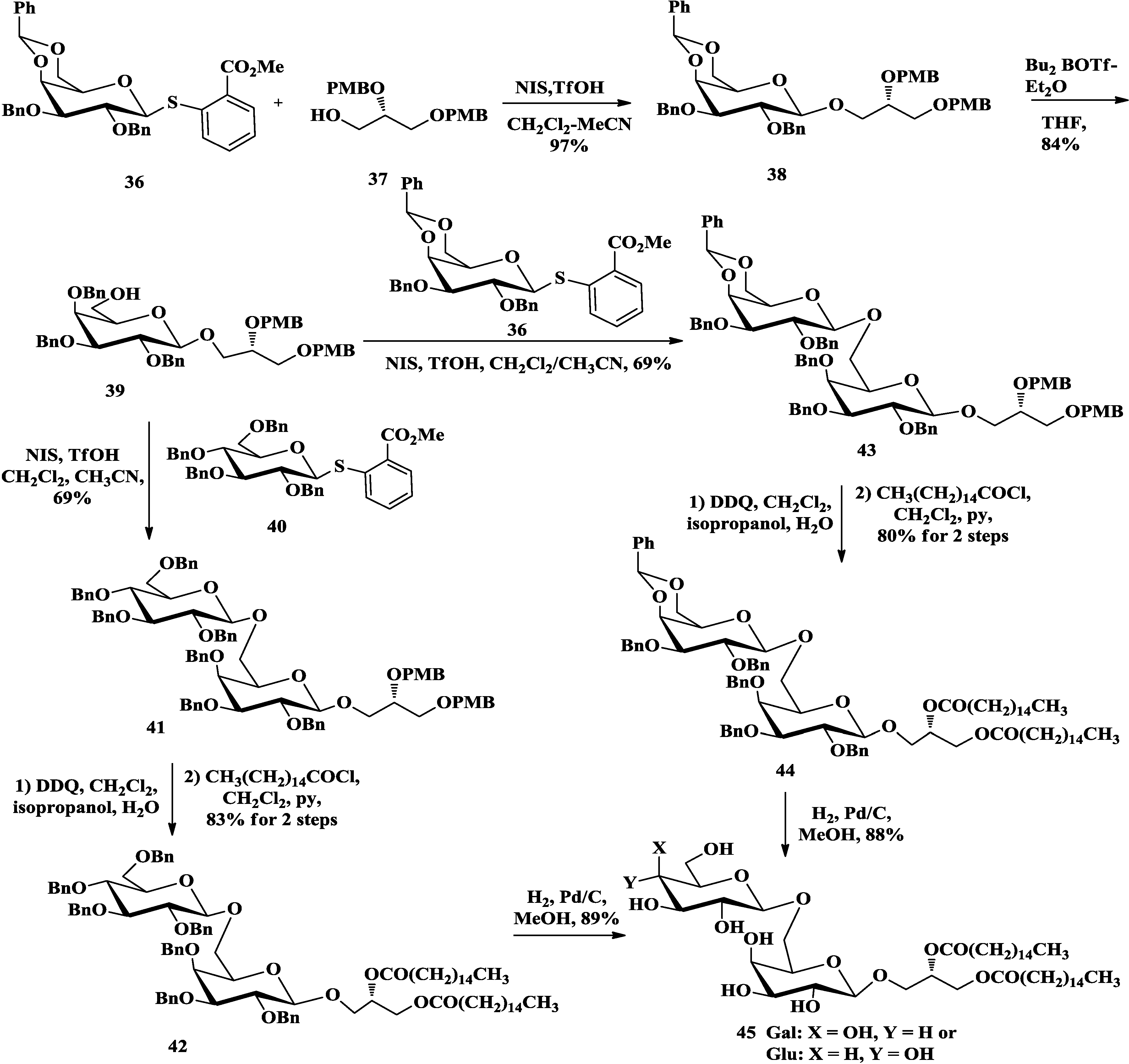
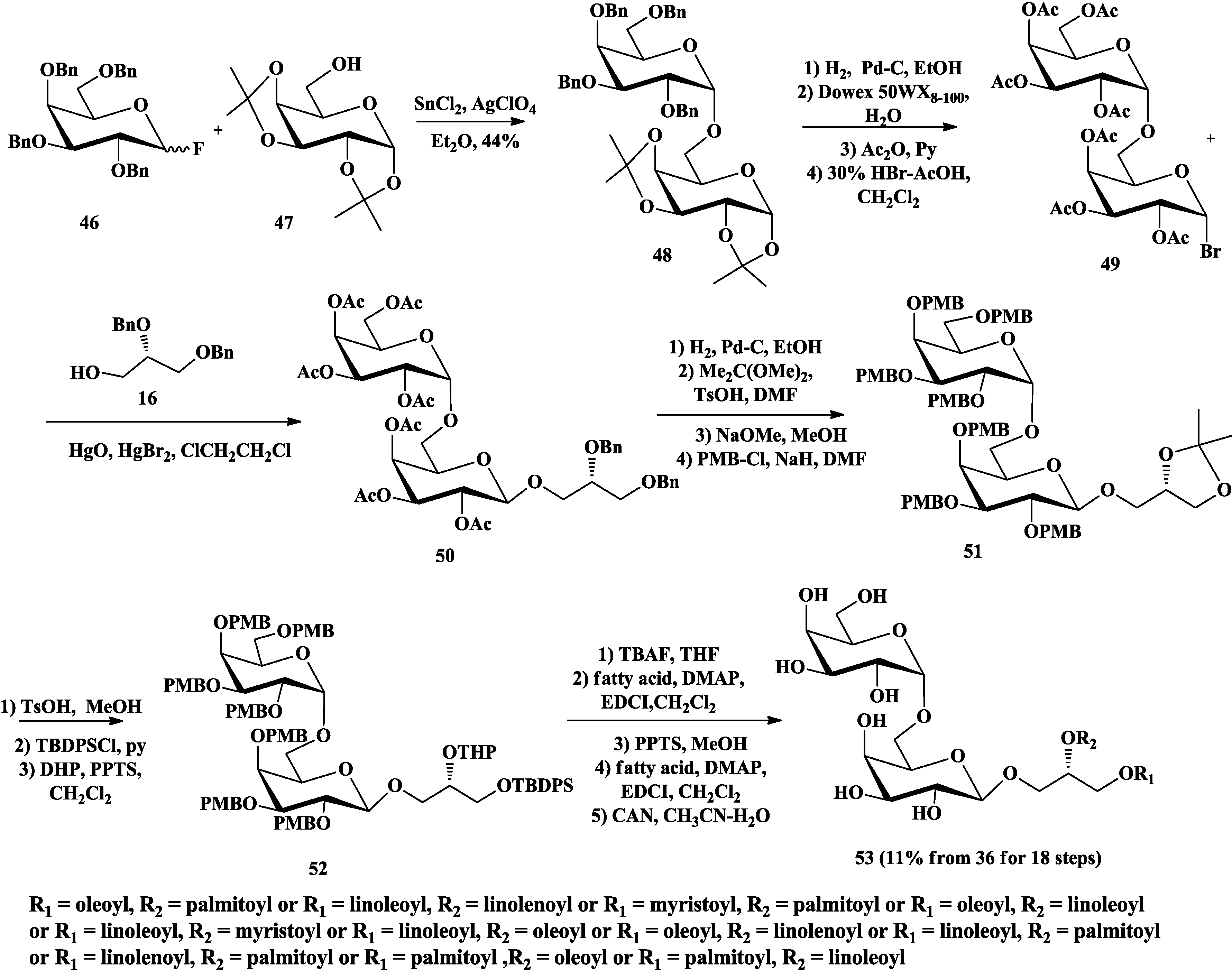
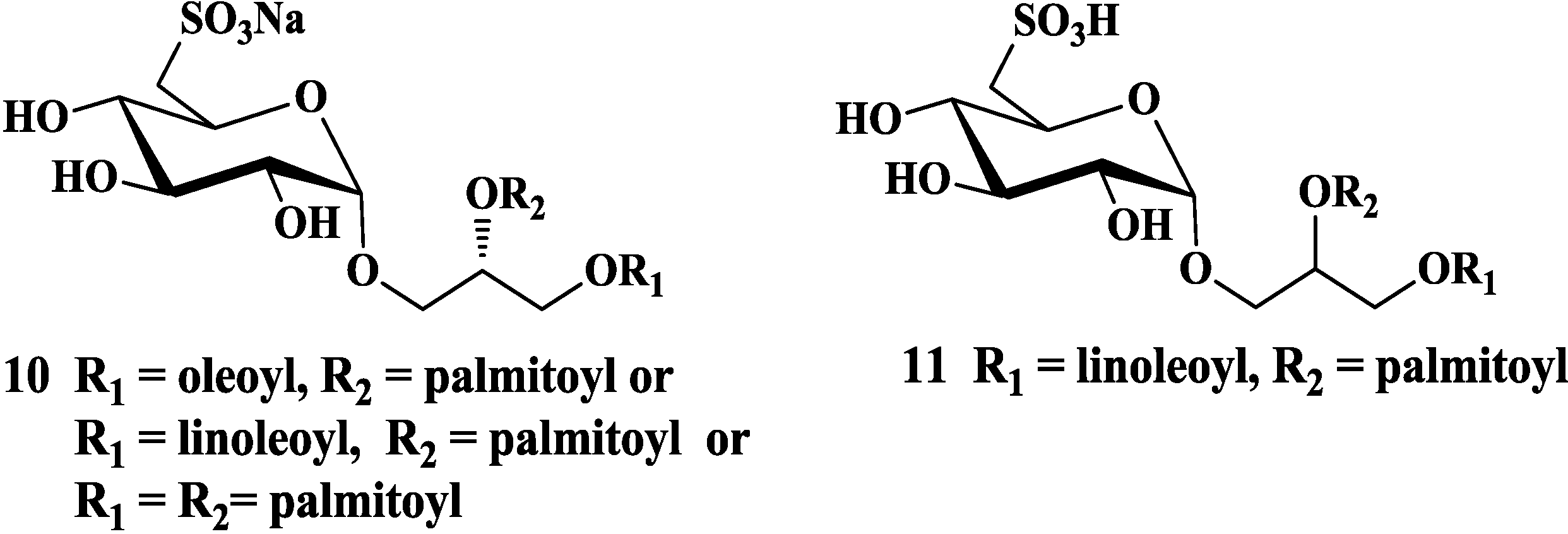
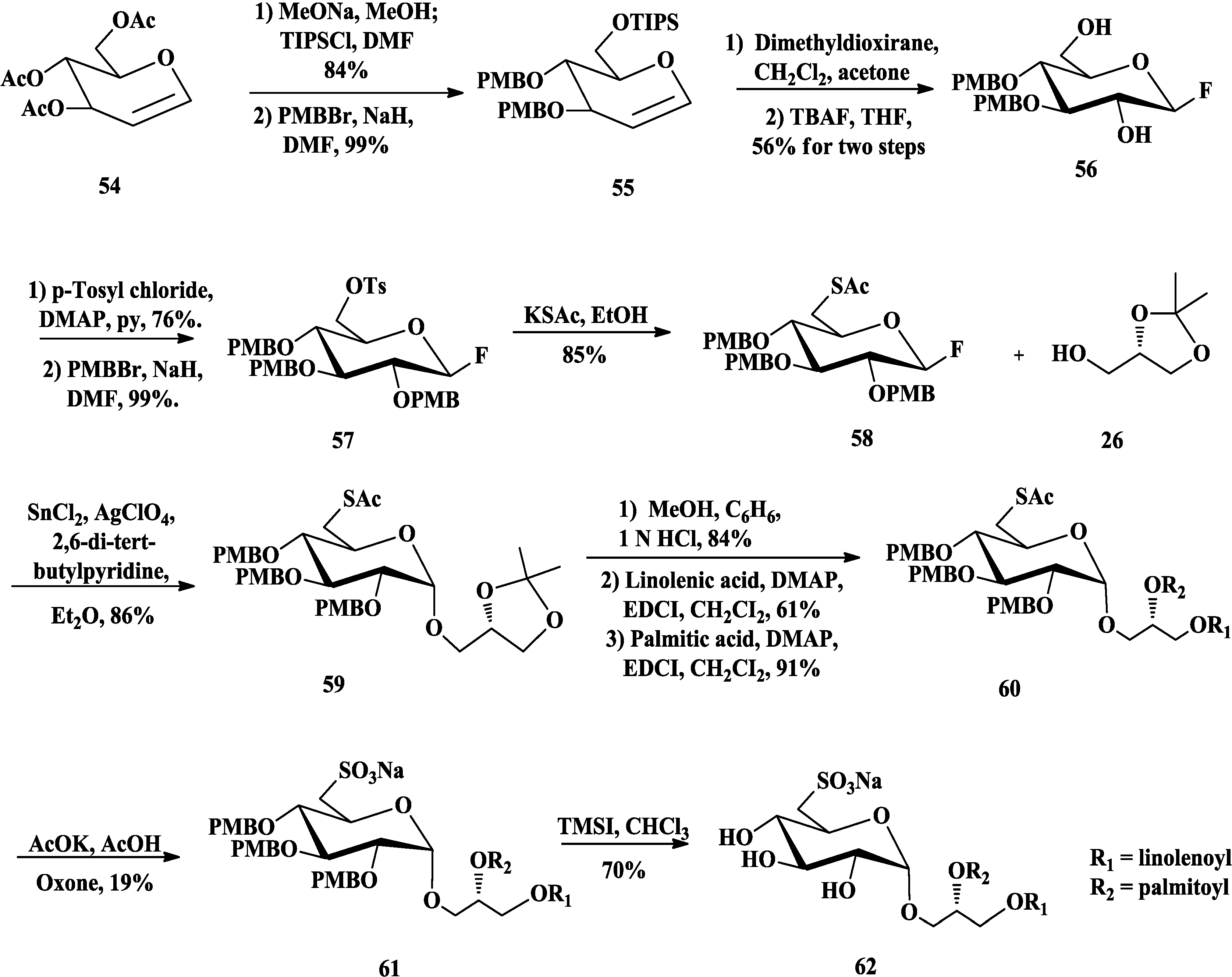
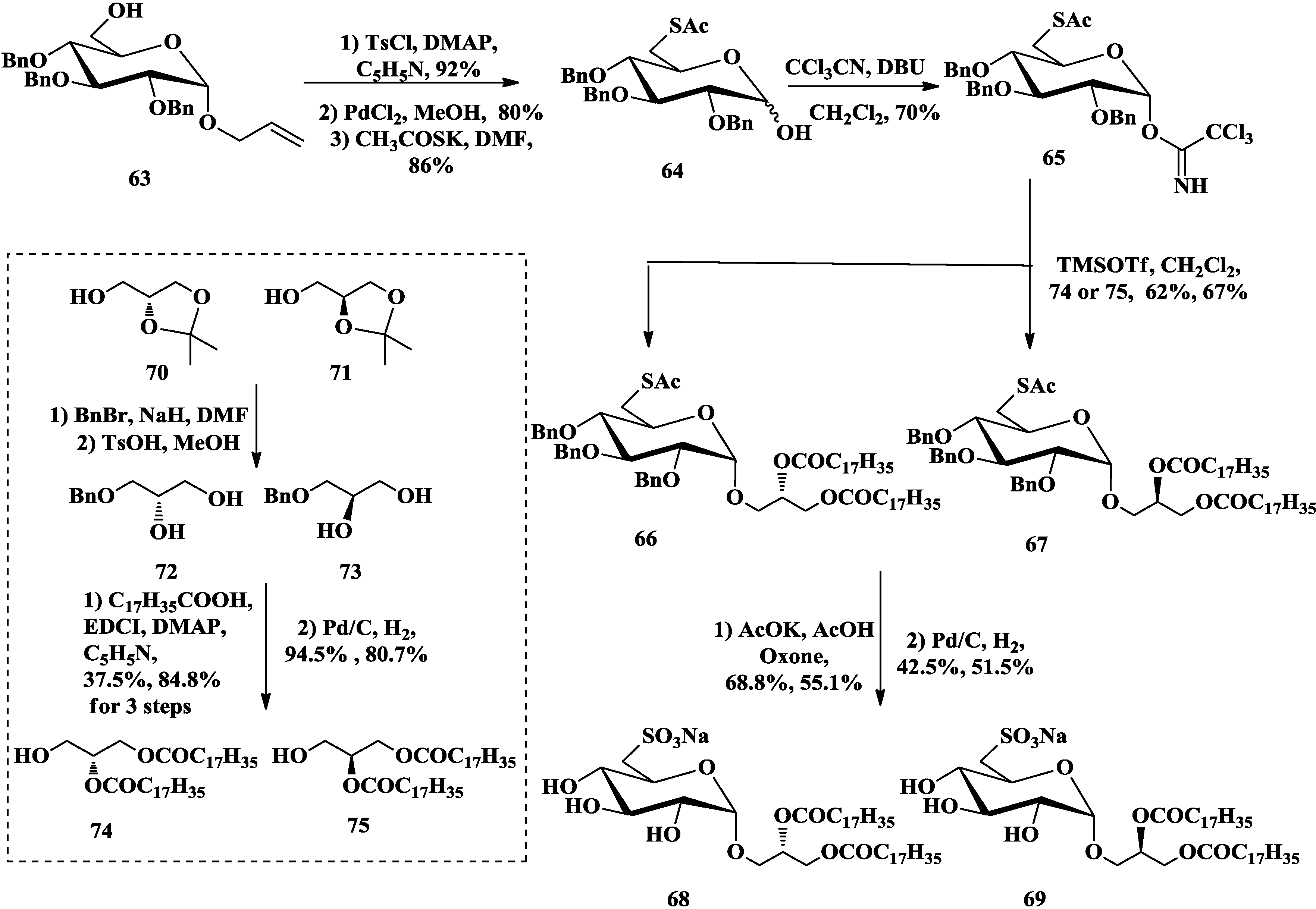
3.2. Total Synthesis of Sulfoquinovosylacylglycerols
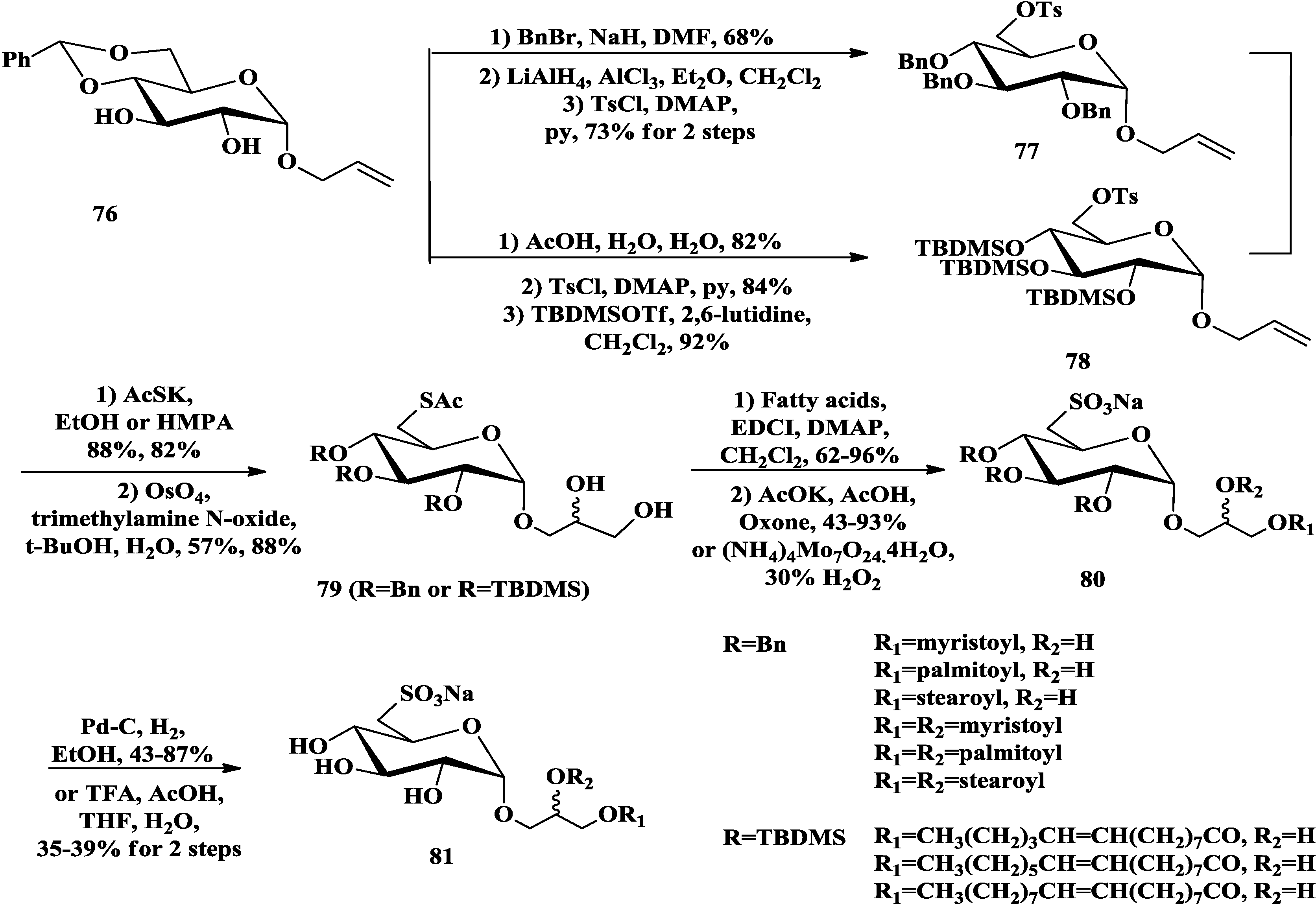
3.3. Total Synthesis of Aminoglycoglycerolipids
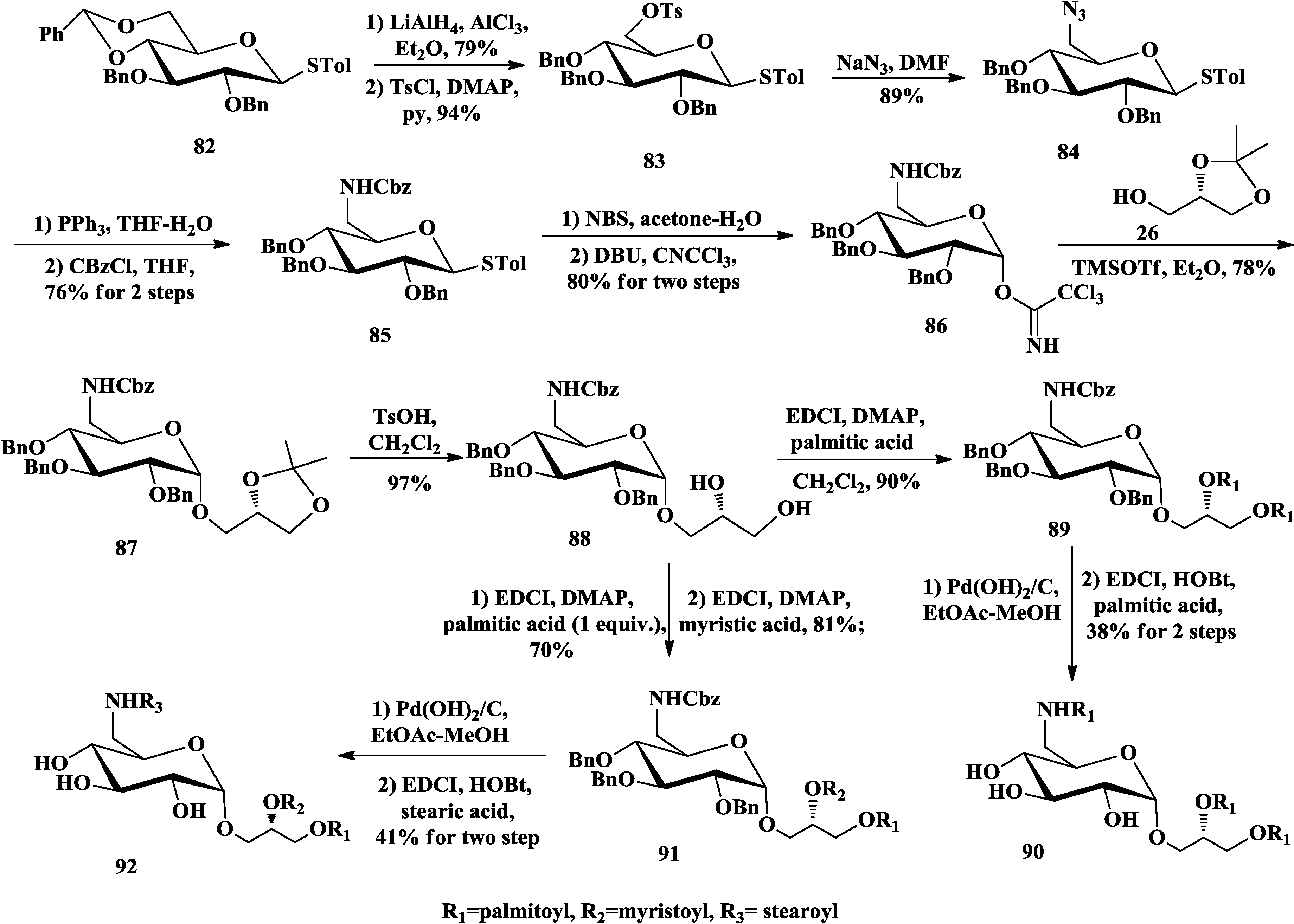
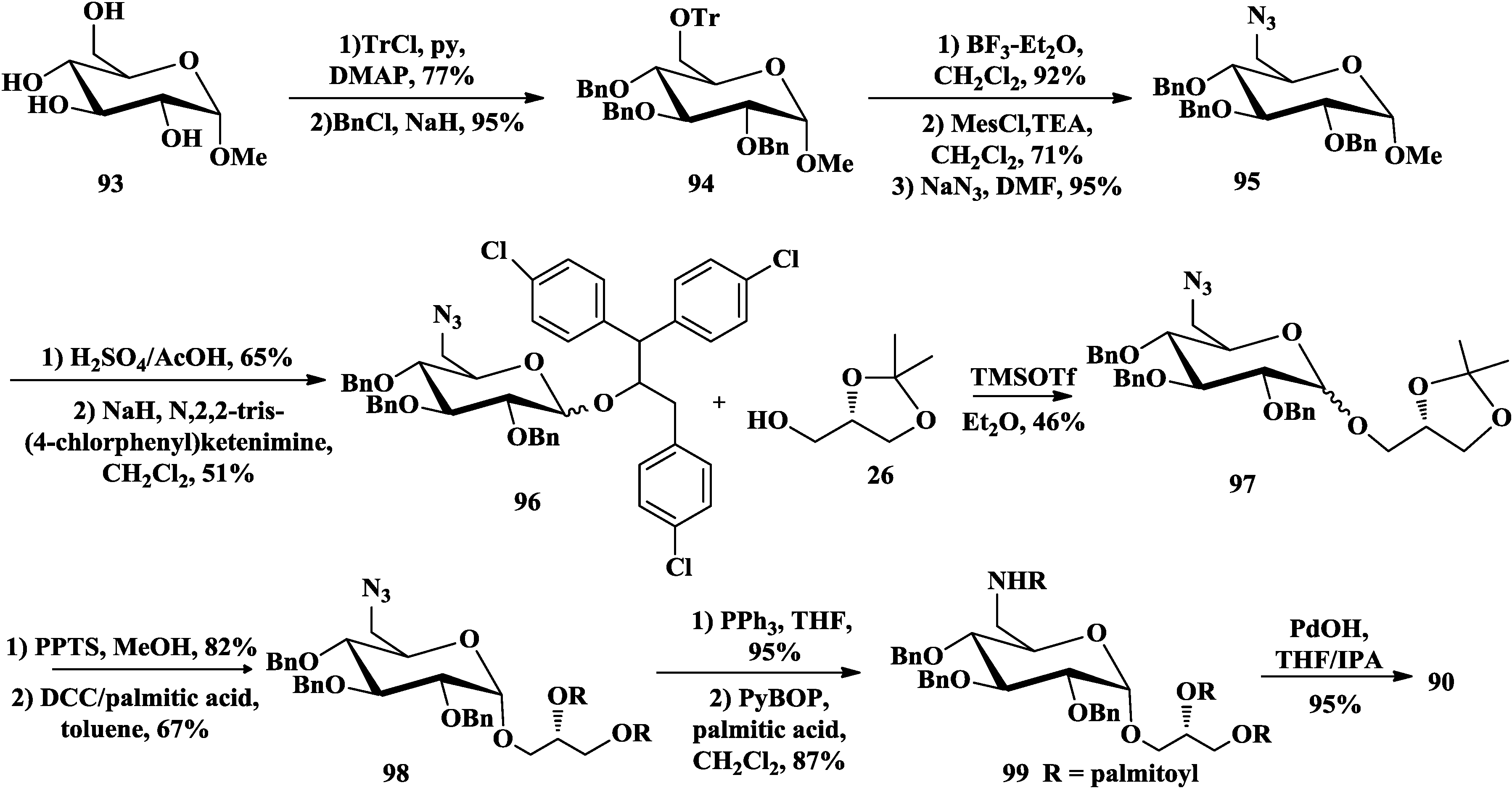
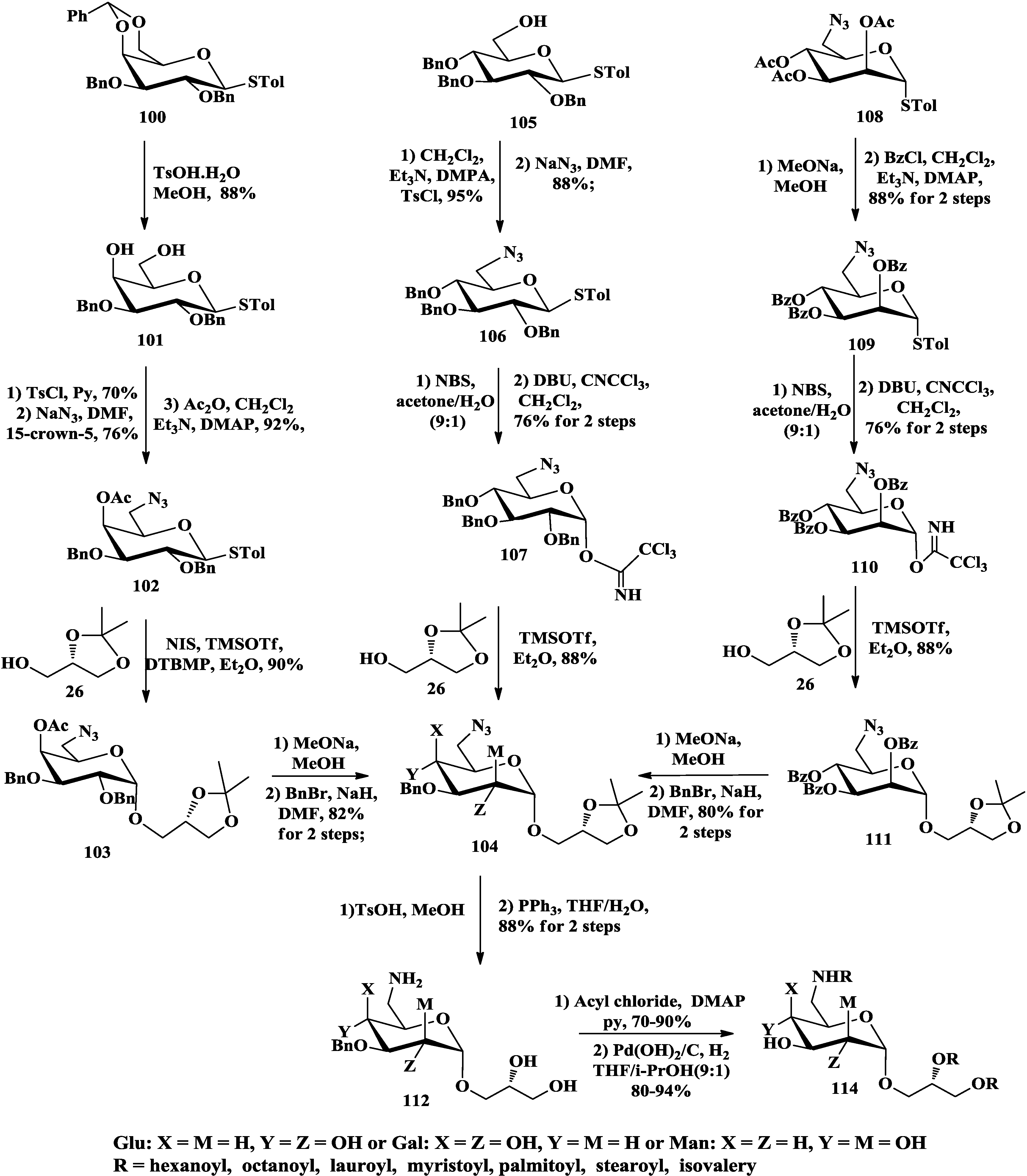
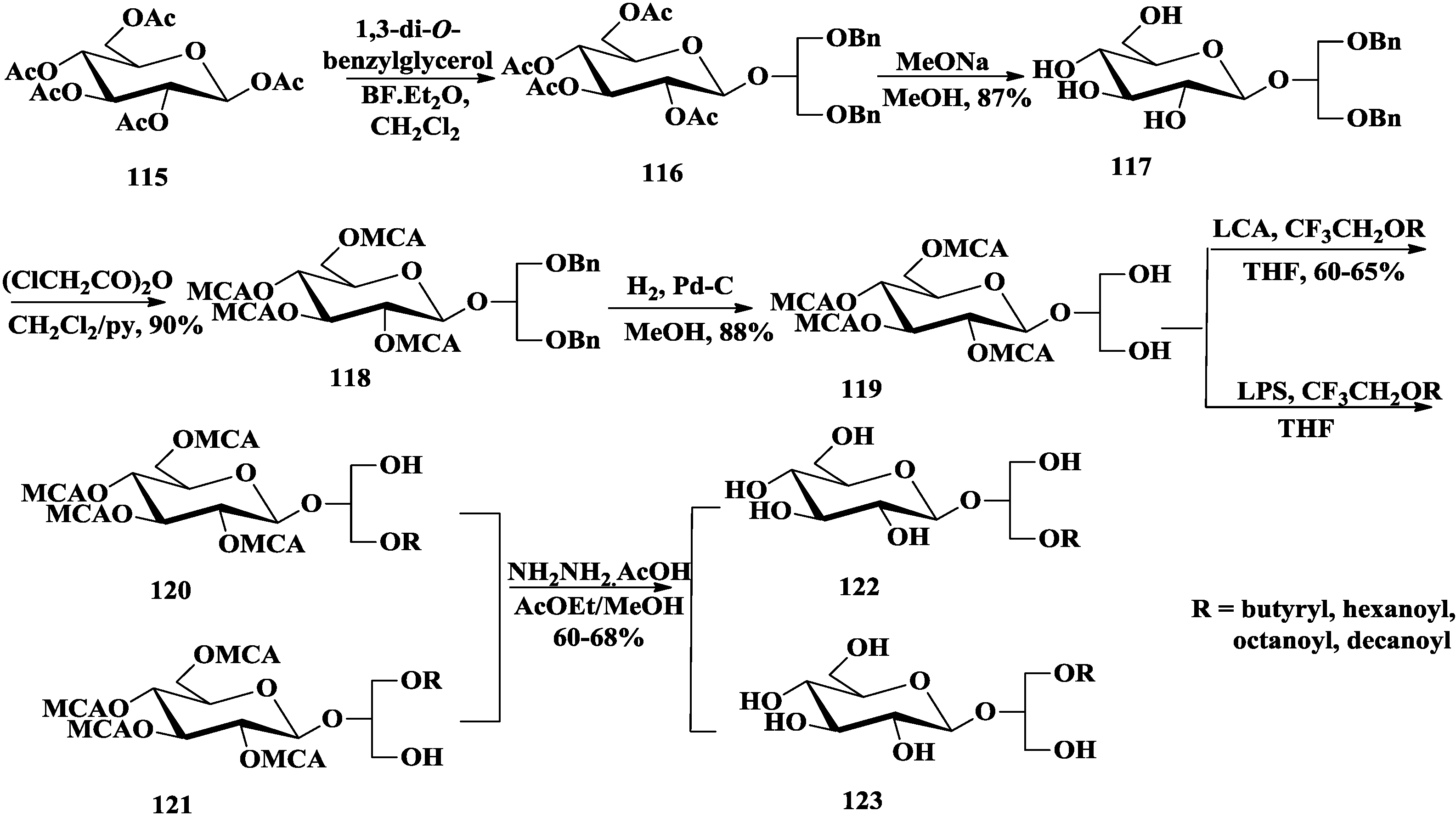
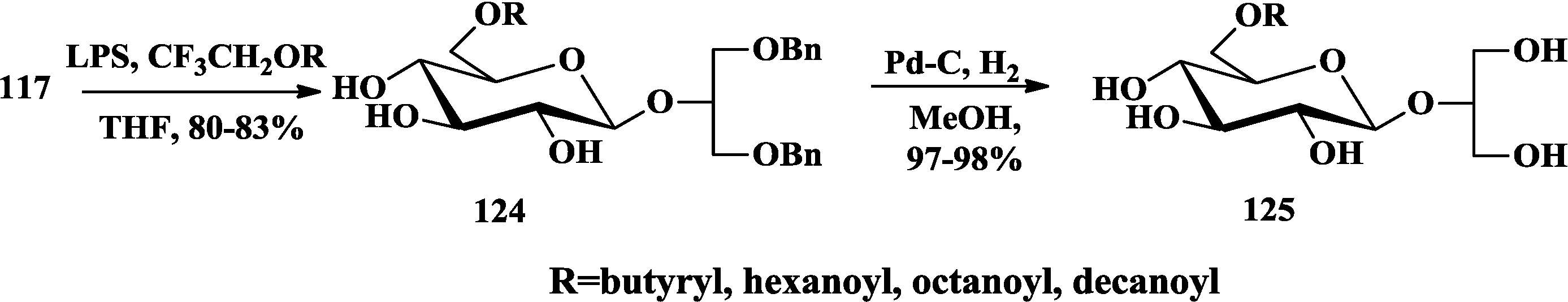
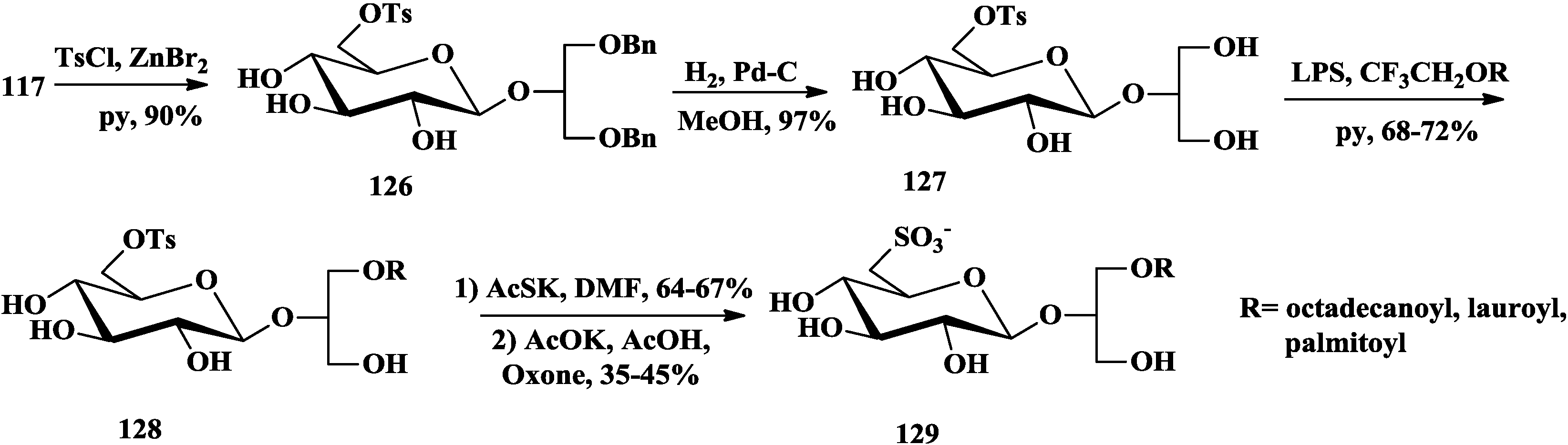
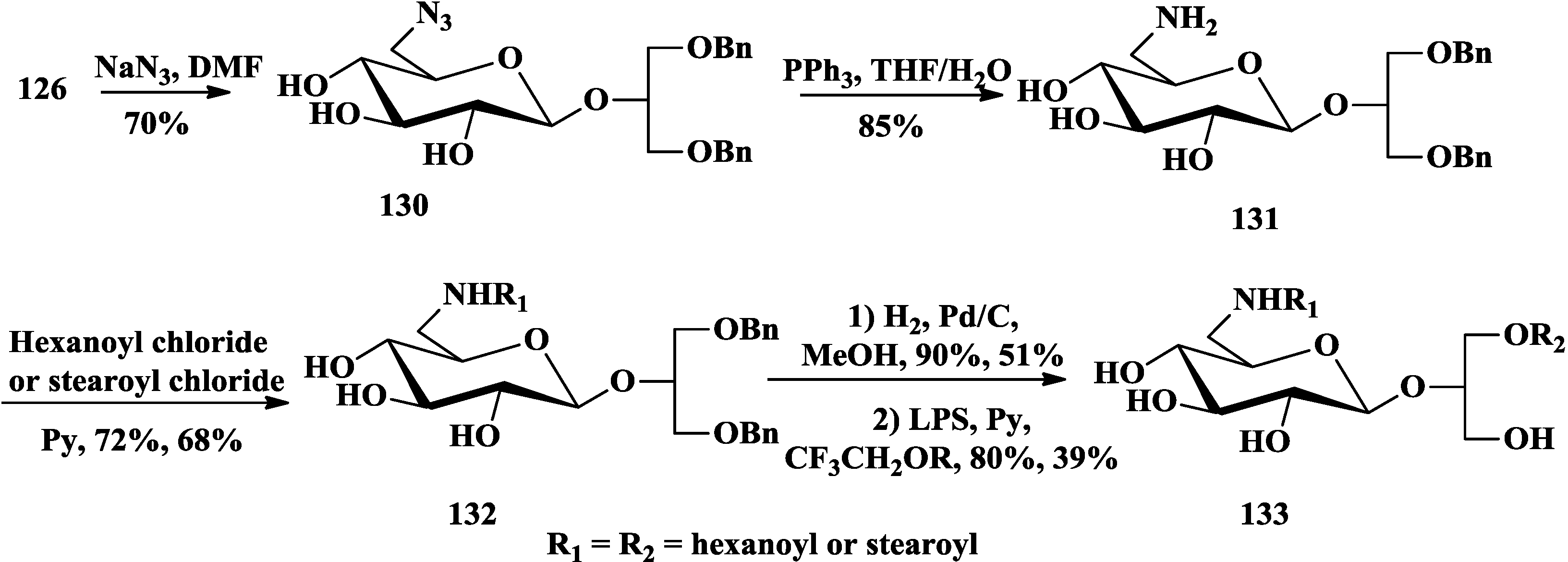
4. The Structure-Activity Relationship
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Kim, Y.; Kim, E.-H.; Lee, C.; Kim, M.-H.; Rho, J.-R. Two new monogalactosyl diacylglycerols from brown alga Sargassum thunbergii. Lipids 2007, 42, 395–399. [Google Scholar] [CrossRef]
- Illijas, M.I.; Indy, J.R.; Yasui, H.; Itabashi, Y. Lipid class and fatty acid composition of a little-known and rarely collected alga Exophyllum wentii Weber-van Bosse from Bali Island, Indonesia. J. Oleo Sci. 2009, 58, 103–110. [Google Scholar] [CrossRef]
- Al-Fadhli, A.; Wahidulla, S.; D’Souza, L. Glycolipids from the red alga Chondria armata (Kütz.) Okamura. Glycobiology 2006, 16, 902–915. [Google Scholar]
- Son, B.-W.; Cho, Y.-J.; Kim, N.-K.; Choi, H.-D. New glyceroglycolipids from the brown alga Sargassum thunbergii. Bull. Korean Chem. Soc. 1992, 13, 584–584. [Google Scholar]
- Kim, Y.; Choi, J.-S.; Hong, J.; Yoo, J.; Kim, M. Identification of acylated glycoglycerolipids from a cyanobacterium, Synechocystis sp., by tandem mass spectrometry. Lipids 1999, 34, 847–853. [Google Scholar] [CrossRef]
- Kim, Y.H.; Choi, J.-S.; Yoo, J.S.; Park, Y.-M.; Kim, M.S. Structural identification of glycerolipid molecular species isolated from cyanobacterium Synechocystis sp. PCC 6803 using fast atom bombardment tandem mass spectrometry. Anal. Biochem. 1999, 267, 260–270. [Google Scholar] [CrossRef]
- Marcolongo, G.; de Appolonia, F.; Venzo, A.; Berrie, C.P.; Carofiglio, T.; Ceschi Berrini, C. Diacylglycerolipids isolated from a thermophile cyanobacterium from the Euganean hot springs. Nat. Prod. Res. 2006, 20, 766–774. [Google Scholar] [CrossRef]
- Guschina, I.; Harwood, J. Algal lipids and effect of the environment on their biochemistry. In Lipids in Aquatic Ecosystems; Kainz, M., Brett, M.T., Arts, M.T., Eds.; Springer: New York, NY, USA, 2009; pp. 1–24. [Google Scholar]
- Hölzl, G.; Dörmann, P. Structure and function of glycoglycerolipids in plants and bacteria. Prog. Lipid Res. 2007, 46, 225–243. [Google Scholar] [CrossRef]
- Andersen, R.J.; Taglialatela-Scafati, O. Avrainvilloside, a 6-deoxy-6-aminoglucoglycerolipid from the green alga Avrainvillea nigricans. J. Nat. Prod. 2005, 68, 1428–1430. [Google Scholar] [CrossRef]
- Dai, J.Q.; Zhu, Q.X.; Zhao, C.Y.; Yang, L.; Li, Y. Glyceroglycolipids from Serratula strangulata. Phytochemistry 2001, 58, 1305–1309. [Google Scholar] [CrossRef]
- Mishra, P.K.; Singh, N.; Ahmad, G.; Dube, A.; Maurya, R. Glycolipids and other constituents from Desmodium gangeticum with antileishmanial and immunomodulatory activities. Bioorg. Med. Chem. Lett. 2005, 15, 4543–4546. [Google Scholar] [CrossRef]
- Zhou, B.-N.; Tang, S.; Johnson, R.K.; Mattern, M.P.; Lazo, J.S.; Sharlow, E.R.; Harich, K.; Kingston, D.G.I. New glycolipid inhibitors of Myt1 kinase. Tetrahedron 2005, 61, 883–887. [Google Scholar] [CrossRef]
- Genin, E.; Njinkoue, J.-M.; Wielgosz-Collin, G.; Houssay, C.; Kornprobst, J.-M.; Debitus, C.; Bonin, M.; Micouin, L.; Boury-Esnault, N.; Hooper, J. Glycolipids from marine sponges: Monoglycosylceramides and alkyldiglycosylglycerols: Isolation, characterization and biological activity. Boll. Musei. Ist. Biol. Univ. Genova 2004, 68, 327–334. [Google Scholar]
- Farokhi, F.; Wielgosz-Collin, G.; Robic, A.; Debitus, C.; Malleter, M.; Roussakis, C.; Kornprobst, J.-M.; Barnathan, G. Antiproliferative activity against human non-small cell lung cancer of two O-alkyl-diglycosylglycerols from the marine sponges Myrmekioderma dendyi and Trikentrion laeve. Eur. J. Med. Chem. 2012, 49, 406–410. [Google Scholar] [CrossRef]
- Aoki, S.; Higuchi, K.; Kato, A.; Murakami, N.; Kobayashi, M. Myrmekiosides A and B, novel mono-O-alkyl-diglycosylglycerols reversing tumor cell morphology of ras-transformed cells from a marine sponge of Myrmekioderma sp. Tetrahedron 1999, 55, 14865–14870. [Google Scholar] [CrossRef]
- Williams, D.E.; Sturgeon, C.M.; Roberge, M.; Andersen, R.J. Nigricanosides A and B, antimitotic glycolipids isolated from the green alga Avrainvillea nigricans collected in dominica. J. Am. Chem. Soc. 2007, 129, 5822–5823. [Google Scholar] [CrossRef]
- Costantino, V.; Fattorusso, E.; Mangoni, A.; Aknin, M.; Fall, A.; Samb, A.; Miralles, J. An unusual ether glycolipid from the Senegalese sponge Trikentrion loeve Carter. Tetrahedron 1993, 49, 2711–2716. [Google Scholar] [CrossRef]
- Batrakov, S.G.; Nikitin, D.I.; Pitryuk, I.A. A novel glycolipid, 1,2-diacyl-3-α-d-glucuronopyranosyl-sn-glycerol taurineamide, from the budding seawater bacterium Hyphomonas jannaschiana. Biochim. Biophys. Acta 1996, 1302, 167–176. [Google Scholar] [CrossRef]
- Wolucka, B.A.; McNeil, M.R.; Kalbe, L.; Cocito, C.; Brennan, P.J. Isolation and characterization of a novel glucuronosyl diacylglycerol from Mycobacterium smegmatis. Biochim. Biophys. Acta 1993, 1170, 131–136. [Google Scholar] [CrossRef]
- Okazaki, Y.; Otsuki, H.; Narisawa, T.; Kobayashi, M.; Sawai, S.; Kamide, Y.; Kusano, M.; Aoki, T.; Hirai, M.Y.; Saito, K. A new class of plant lipid is essential for protection against phosphorus depletion. Nat. Commun. 2013, 4, 1510. [Google Scholar] [CrossRef]
- Morimoto, T.; Nagatsu, A.; Murakami, N.; Sakakibara, J.; Tokuda, H.; Nishino, H.; Iwashima, A. Anti-tumour-promoting glyceroglycolipids from the green alga, Chlorella vulgaris. Phytochemistry 1995, 40, 1433–1437. [Google Scholar] [CrossRef]
- Murakami, C.; Kumagai, T.; Hada, T.; Kanekazu, U.; Nakazawa, S.; Kamisuki, S.; Maeda, N.; Xu, X.; Yoshida, H.; Sugawara, F. Effects of glycolipids from spinach on mammalian DNA polymerases. Biochem. Pharmacol. 2003, 65, 259–267. [Google Scholar] [CrossRef]
- Chirasuwan, N.; Chaiklahan, R.; Kittakoop, P.; Chanasattru, W.; Ruengjitchatchawalya, M.; Tanticharoen, M.; Bunnag, B. Anti HSV-1 activity of sulphoquinovosyl diacylglycerol isolated from Spirulina platensis. Sci. Asia 2009, 35, 137–141. [Google Scholar]
- Loya, S.; Reshef, V.; Mizrachi, E.; Silberstein, C.; Rachamim, Y.; Carmeli, S.; Hizi, A. The inhibition of the reverse transcriptase of HIV-1 by the natural sulfoglycolipids from cyanobacteria: Contribution of different moieties to their high potency. J. Nat. Prod. 1998, 61, 891–895. [Google Scholar] [CrossRef]
- Bergé, J.P.; Debiton, E.; Dumay, J.; Durand, P.; Barthomeuf, C. In vitro anti-inflammatory and anti-proliferative activity of sulfolipids from the red alga Porphyridium cruentum. J. Agric. Food. Chem. 2002, 50, 6227–6232. [Google Scholar] [CrossRef]
- Shirahashi, H.; Murakami, N.; Watanabe, M.; Nagatsu, A.; Sakakibara, J.; Tokuda, H.; Nishino, H.; Iwashima, A. Isolation and identification of anti-tumor-promoting principles from the fresh-water cyanobacterium Phormidium tenue. Chem. Pharm. Bull. 1993, 41, 1664–1666. [Google Scholar] [CrossRef]
- Hou, C.-C.; Chen, Y.-P.; Wu, J.-H.; Huang, C.-C.; Wang, S.-Y.; Yang, N.-S.; Shyur, L.-F. A Galactolipid Possesses Novel Cancer Chemopreventive Effects by Suppressing Inflammatory Mediators and Mouse B16 Melanoma. Cancer Res. 2007, 67, 6907–6915. [Google Scholar] [CrossRef]
- Zhang, H.; Oh, J.; Jang, T.-S.; Min, B.S.; Na, M. Glycolipids from the aerial parts of Orostachys japonicus with fatty acid synthase inhibitory and cytotoxic activities. Food Chem. 2012, 131, 1097–1103. [Google Scholar] [CrossRef]
- Maeda, N.; Kokai, Y.; Ohtani, S.; Hada, T.; Yoshida, H.; Mizushina, Y. Inhibitory effects of preventive and curative orally administered spinach glycoglycerolipid fraction on the tumor growth of sarcoma and colon in mouse graft models. Food Chem. 2009, 112, 205–210. [Google Scholar] [CrossRef]
- Maeda, N.; Hada, T.; Yoshida, H.; Mizushina, Y. Inhibitory effect on replicative DNA polymerases, human cancer cell proliferation, and in vivo anti-tumor activity by glycolipids from spinach. Curr. Med. Chem. 2007, 14, 955–967. [Google Scholar] [CrossRef]
- Maeda, N.; Kokai, Y.; Ohtani, S.; Sahara, H.; Hada, T.; Ishimaru, C.; Kuriyama, I.; Yonezawa, Y.; Iijima, H.; Yoshida, H. Anti-tumor effects of the glycolipids fraction from spinach which inhibited DNA polymerase activity. Nutr. Cancer 2007, 57, 216–223. [Google Scholar]
- Maeda, N.; Matsubara, K.; Yoshida, H.; Mizushina, Y. Anti-cancer effect of spinach glycoglycerolipids as angiogenesis inhibitors based on the selective inhibition of DNA polymerase activity. Mini Rev. Med. Chem. 2011, 11, 32–38. [Google Scholar] [CrossRef]
- Shaikh, N.; Colombo, D.; Ronchetti, F.; Dangate, M. SQAGs: A stepping stone in the biotic world. C. R. Chim. 2013, 16, 850–862. [Google Scholar] [CrossRef]
- Ohta, K.; Mizushina, Y.; Hirata, N.; Takemura, M.; Sugawara, F.; Matsukage, A.; Yoshida, S.; Sakaguchi, K. Sulfoquinovosyldiacylglycerol, KM043, a new potent inhibitor of eukaryotic DNA polymerases and HIV-reverse transcriptase type 1 from a marine red alga, Gigartina tenella. Chem. Pharm. Bull. 1998, 46, 684–686. [Google Scholar] [CrossRef]
- Ohta, K.; Mizushina, Y.; Hirata, N.; Takemura, M.; Sugawara, F.; Matsukage, A.; Yoshida, S.; Sakaguchi, K. Action of a new mammalian DNA polymerase inhibitor, sulfoquinovosyldiacylglycerol. Biol. Pharm. Bull. 1999, 22, 111–116. [Google Scholar] [CrossRef]
- Mizushina, Y.; Watanabe, I.; Ohta, K.; Takemura, M.; Sahara, H.; Takahashi, N.; Gasa, S.; Sugawara, F.; Matsukage, A.; Yoshida, S. Studies on inhibitors of mammalian DNA polymerase α and β: Sulfolipids from a pteridophyte, Athyrium niponicum. Biochem. Pharmacol. 1998, 55, 537–541. [Google Scholar] [CrossRef]
- Kuriyama, I.; Musumi, K.; Yonezawa, Y.; Takemura, M.; Maeda, N.; Iijima, H.; Hada, T.; Yoshida, H.; Mizushina, Y. Inhibitory effects of glycolipids fraction from spinach on mammalian DNA polymerase activity and human cancer cell proliferation. J. Nutr. Biochem. 2005, 16, 594–601. [Google Scholar] [CrossRef]
- Aoki, S.; Ohta, K.; Yamazaki, T.; Sugawara, F.; Sakaguchi, K. Mammalian mitotic centromere-associated kinesin (MCAK). FEBS J. 2005, 272, 2132–2140. [Google Scholar] [CrossRef]
- Sahara, H.; Hanashima, S.; Yamazaki, T.; Takahashi, S.; Sugawara, F.; Ohtani, S.; Ishikawa, M.; Mizushina, Y.; Ohta, K.; Shimozawa, K. Anti-tumor effect of chemically synthesized sulfolipids based on sea urchin’s natural sulfonoquinovosylmonoacylglycerols. Cancer Sci. 2002, 93, 85–92. [Google Scholar] [CrossRef]
- Sahara, H.; Ishikawa, M.; Takahashi, N.; Ohtani, S.; Sato, N.; Gasa, S.; Akino, T.; Kikuchi, K. In vivo anti-tumour effect of 3′-sulphonoquinovosyl 1′-monoacylglyceride isolated from sea urchin (Strongylocentrotus. intermedius) intestine. Br. J. Cancer 1997, 75, 324. [Google Scholar] [CrossRef]
- Mori, Y.; Sahara, H.; Matsumoto, K.; Takahashi, N.; Yamazaki, T.; Ohta, K.; Aoki, S.; Miura, M.; Sugawara, F.; Sakaguchi, K.; Sato, N. Downregulation of Tie2 gene by a novel antitumor sulfolipid, 3ulfolipid, ion of tiemonoacylglycerol, targeting angiogenesis. Cancer Sci. 2008, 99, 1063–1070. [Google Scholar] [CrossRef]
- Brastianos, H.C. Bioactive Natural Products from Nature. Ph.D. Thesis, The University of British Columbia, BC, Canada, 2007. [Google Scholar]
- Letourneux, Y.; Brunel, J.M.; Fernandez, R.; Dherbomez, M.; Debitus, C. Isolation and characterization of new tetrahydropyranyl substituted sesquiterpene and Myrmekiodermin glycolipid ether isolated from the marine sponge Myrmekioderma. Heterocycl. Commun. 2005, 11, 291–298. [Google Scholar]
- Gustafson, K.R.; Cardellina, J.H.; Fuller, R.W.; Weislow, O.S.; Kiser, R.F.; Snader, K.M.; Patterson, G.M.L.; Boyd, M.R. AIDS-antiviral sulfolipids from cyanobacteria (blue-green algae). J. Nat. Cancer Inst. 1989, 81, 1254–1258. [Google Scholar] [CrossRef]
- Reshef, V.; Mizrachi, E.; Maretzki, T.; Silberstein, C.; Loya, S.; Hizi, A.; Carmeli, S. New acylated sulfoglycolipids and digalactolipids and related known glycolipids from cyanobacteria with a potential to inhibit the reverse transcriptase of HIV-1. J. Nat. Prod. 1997, 60, 1251–1260. [Google Scholar] [CrossRef]
- Vo, T.-S.; Ngo, D.-H.; Ta, Q.V.; Kim, S.-K. Marine organisms as a therapeutic source against herpes simplex virus infection. Eur. J. Pharm. Sci. 2011, 44, 11–20. [Google Scholar] [CrossRef]
- Plouguerné, E.; de Souza, L.M.; Sassaki, G.L.; Cavalcanti, J.F.; Villela Romanos, M.T.; da Gama, B.A.; Pereira, R.C.; Barreto-Bergter, E. Antiviral Sulfoquinovosildiacylglycerols (SQDGs) from the Brazilian Brown Seaweed Sargassum vulgare. Mar. Drugs 2013, 11, 4628–4640. [Google Scholar] [CrossRef]
- Mattos, B.B.; Romanos, M.T.V.; de Souza, L.M.; Sassaki, G.; Barreto-Bergter, E. Glycolipids from macroalgae: Potential biomolecules for marine biotechnology? Braz. J. Pharmacogn. 2011, 21, 244–247. [Google Scholar]
- Bruno, A.; Rossi, C.; Marcolongo, G.; di Lena, A.; Venzo, A.; Berrie, C.P.; Corda, D. Selective in vivo anti-inflammatory action of the galactolipid monogalactosyldiacylglycerol. Eur. J. Pharmacol. 2005, 524, 159–168. [Google Scholar] [CrossRef]
- Máñez, S.; del Carmen Recio, M.; Gil, I.; Gómez, C.; Giner, R.-M.; Waterman, P.G.; Ríos, J.-L. A glycosyl analogue of diacylglycerol and other antiinflammatory constituents from Inula viscosa. J. Nat. Prod. 1999, 62, 601–604. [Google Scholar] [CrossRef]
- Larsen, E.; Kharazmi, A.; Christensen, L.P.; Christensen, S.B. An antiinflammatory galactolipid from rose hip (Rosa canina) that inhibits chemotaxis of human peripheral blood neutrophils in vitro. J. Nat. Prod. 2003, 66, 994–995. [Google Scholar] [CrossRef]
- Cateni, F.; Falsone, G.; Zilic, J.; Bonivento, P.; Zacchigna, M.; Žigon, D.; Sosa, S.; Altinier, G. Glyceroglycolipids from Euphorbia nicaeensis All. with antiinflamatory activity. Arkivoc 2004, 2004, 54–65. [Google Scholar]
- Matsufuji, M.; Nagamatsu, Y.; Yoshimoto, A. Protective effects of bacterial glyceroglycolipid M874B against cell death caused by exposure to heat and hydrogen peroxide. J. Biosci. Bioeng. 2000, 89, 345–349. [Google Scholar] [CrossRef]
- Matsufuji, M.; Taguchi, K.; Inagaki, M.; Higuchi, R.; Ohta, S.; Yoshimoto, A. Glyceroglycolipids preventing tert-butylhydroperoxide-induced cell death from Microbacterium sp. and Corynebacterium. aquaticum strains. J. Biosci. Bioeng. 2000, 89, 170–175. [Google Scholar] [CrossRef]
- Jiang, Z.; Du, Q. Glucose-lowering activity of novel tetrasaccharide glyceroglycolipids from the fruits of Cucurbita moschata. Bioorg. Med. Chem. Lett. 2011, 21, 1001–1003. [Google Scholar] [CrossRef]
- Karantonis, H.C.; Antonopoulou, S.; Demopoulos, C.A. Antithrombotic lipid minor constituents from vegetable oils. Comparison between olive oils and others. J. Agric. Food Chem. 2002, 50, 1150–1160. [Google Scholar] [CrossRef]
- Cateni, F.; Bonivento, P.; Procida, G.; Zacchigna, M.; Scialino, G.; Banfi, E. Chemoenzymatic synthesis and in vitro studies on the hydrolysis of antimicrobial monoglycosyl diglycerides by pancreatic lipase. Bioorg. Med. Chem. Lett. 2007, 17, 1971–1978. [Google Scholar] [CrossRef]
- Dörmann, P.; Benning, C. Galactolipids rule in seed plants. Trends Plant Sci. 2002, 7, 112–118. [Google Scholar] [CrossRef]
- Mannock, D.A.; Harper, P.E.; Gruner, S.M.; McElhaney, R.N. The physical properties of glycosyl diacylglycerols. Calorimetric, X-ray diffraction and Fourier transform spectroscopic studies of a homologous series of 1,2-di-O-acyl-3-O-(β-d-galactopyranosyl)-sn-glycerols. Chem. Phys. Lipids 2001, 111, 139–161. [Google Scholar] [CrossRef]
- Mannock, D.A.; Lewis, R.N.A.H.; McElhaney, R.N. An improved procedure for the preparation of 1,2-di-O-acyl-3-O-(β-d-glucopyranosyl)-sn-glycerols. Chem. Phys. Lipids 1987, 43, 113–127. [Google Scholar] [CrossRef]
- Mannock, D.A.; Lewis, R.N.A.H.; McElhaney, R.N. Physical properties of glycosyl diacylglycerols. 1. Calorimetric studies of a homologous series of 1,2-di-O-acyl-3-O-(α-d-glucopyranosyl)-sn-glycerols. Biochemistry 1990, 29, 7790–7799. [Google Scholar] [CrossRef]
- Janwitayanuchit, W.; Suwanborirux, K.; Patarapanich, C.; Pummangura, S.; Lipipun, V.; Vilaivan, T. Synthesis and anti-herpes simplex viral activity of monoglycosyl diglycerides. Phytochemistry 2003, 64, 1253–1264. [Google Scholar] [CrossRef]
- Cateni, F.; Bonivento, P.; Procida, G.; Zacchigna, M.; Favretto, L.G.; Scialino, G.; Banfi, E. Chemoenzymatic synthesis and antimicrobial activity evaluation of monogalactosyl diglycerides. Eur. J. Med. Chem. 2008, 43, 210–221. [Google Scholar] [CrossRef]
- Gordon, D.M.; Danishefsky, S.J. Synthesis of a cyanobacterial sulfolipid: confirmation of its structure, stereochemistry and anti-HIV-1 activity. J. Am. Chem. Soc. 1992, 114, 659–663. [Google Scholar] [CrossRef]
- Manzo, E.; Ciavatta, M.L.; Pagano, D.; Fontana, A. An efficient and versatile chemical synthesis of bioactive glyco-glycerolipids. Tetrahedron Lett. 2012, 53, 879–881. [Google Scholar] [CrossRef]
- Shingu, Y.; Nishida, Y.; Dohi, H.; Kobayashi, K. An easy access to halide ion-catalytic α-glycosylation using carbon tetrabromide and triphenylphosphine as multifunctional reagents. Org. Biomol. Chem. 2003, 1, 2518–2521. [Google Scholar] [CrossRef]
- Schmidt, R.R. New methods for the synthesis of glycosides and oligosaccharidess multifunctional reagents. An Koenigs-Knorr Method? Angew. Chem. Int. Ed. Engl. 1986, 25, 212–235. [Google Scholar] [CrossRef]
- Redoulès, D.; Perié, J. Stereospecific synthesis of retinoic acid glucoconjugates, as pseudo-substrates of epidermal β-glucocerebrosidase. Tetrahedron Lett. 1999, 40, 4811–4814. [Google Scholar] [CrossRef]
- Pozsgay, V.; Kubler-Kielb, J.; Coxon, B.; Marques, A.; Robbins, J.B.; Schneerson, R. Synthesis and antigenicity of BBGL-2 glycolipids of Borrelia burgdorferi, the causative agent of Lyme disease. Carbohydr. Res. 2011, 346, 1551–1563. [Google Scholar] [CrossRef]
- Amara, S.; Lafont, D.; Fiorentino, B.; Boullanger, P.; Carrière, F.; de Caro, A. Continuous measurement of galactolipid hydrolysis by pancreatic lipolytic enzymes using the pH-stat technique and a medium chain monogalactosyl diglyceride as substrate. Biochim. Biophys. Acta 2009, 1791, 983–990. [Google Scholar] [CrossRef]
- Sias, B.; Ferrato, F.; Grandval, P.; Lafont, D.; Boullanger, P.; de Caro, A.; Leboeuf, B.; Verger, R.; Carrière, F. Human pancreatic lipase-related protein 2 is a galactolipase. Biochemistry 2004, 43, 10138–10148. [Google Scholar] [CrossRef]
- Lafont, D.; Carrière, F.; Ferrato, F.; Boullanger, P. Syntheses of an α-d-Gal-(1→6)-β-d-Gal diglyceride, as lipase substrate. Carbohydr. Res. 2006, 341, 695–704. [Google Scholar] [CrossRef]
- Schmidt, R.R.; Stumpp, M. Glycosylimidate, 8. Synthese von 1-Thioglycosiden. Liebigs Ann. Chem. 1983, 1983, 1249–1256. [Google Scholar] [CrossRef]
- Mereyala, H.B.; Reddy, G.V. Stereoselective synthesis of α-linked saccharides by use of per O-benzylated 2-pyridyl 1-thio hexopyranosides as glycosyl donors and methyl iodide as an activator. Tetrahedron 1991, 47, 6435–6448. [Google Scholar] [CrossRef]
- Miyachi, A.; Miyazaki, A.; Shingu, Y.; Matsuda, K.; Dohi, H.; Nishida, Y. Synthesis and absolute structures of Mycoplasma pneumoniae β-glyceroglycolipid antigens. Carbohydr. Res. 2009, 344, 36–43. [Google Scholar] [CrossRef]
- Dohi, H.; Nishida, Y.; Tanaka, H.; Kobayashi, K. O-Methoxycarbonylphenyl 1-thio-β-d-galactopyranoside, a non-malodorous thio glycosylation donor for the synthesis of globosyl α (1–4)-linkages. Synlett 2001, 2001, 1446–1448. [Google Scholar] [CrossRef]
- Tanaka, R.; Sakano, Y.; Nagatsu, A.; Shibuya, M.; Ebizuka, Y.; Goda, Y. Synthesis of digalactosyl diacylglycerols and their structure-inhibitory activity on human lanosterol synthase. Bioorg. Med. Chem. Lett. 2005, 15, 159–162. [Google Scholar] [CrossRef]
- Benson, A.; Daniel, H.; Wiser, R. A sulfolipid in plants. Proc. Natl. Acad. Sci. USA 1959, 45, 1582–1587. [Google Scholar] [CrossRef]
- Benning, C. Biosynthesis and function of the sulfolipid sulfoquinovosyl diacylglycerol. Ann. Rev. Plant. Biol. 1998, 49, 53–75. [Google Scholar] [CrossRef]
- Gigg, R.; Penglis, A.A.E.; Conant, R. Synthesis of 3-O-(6-deoxy-6-sulpho-α-d-glucopyranosyl)-1,2-di-O-hexadecanoyl-l-glycerol, “sulphoquinovosyl diglyceride”. J. Chem. Soc. Perkin Trans. 1 1980, 2490–2493. [Google Scholar] [CrossRef]
- Hanashima, S.; Mizushina, Y.; Yamazaki, T.; Ohta, K.; Takahashi, S.; Koshino, H.; Sahara, H.; Sakaguchi, K.; Sugawara, F. Structural determination of sulfoquinovosyldiacylglycerol by chiral syntheses. Tetrahedron Lett. 2000, 41, 4403–4407. [Google Scholar] [CrossRef]
- Fügedi, P.; Lipták, A.; Nánási, P.; Neszmélyi, A. Retention of the anomeric configuration in the imidate procedure: Synthesis of disaccharides containing α-l-rhamnopyranosyl and α-d-mannopyranosyl groups. Carbohydr. Res. 1982, 107, C5–C8. [Google Scholar] [CrossRef]
- Hanashima, S.; Mizushina, Y.; Yamazaki, T.; Ohta, K.; Takahashi, S.; Sahara, H.; Sakaguchi, K.; Sugawara, F. Synthesis of sulfoquinovosylacylglycerols, inhibitors of eukaryotic DNA polymerase α and β. Bioorg. Med. Chem. 2001, 9, 367–376. [Google Scholar] [CrossRef]
- Wessel, H.P. Use of trifluoromethanesulfonic acid in fischer glycosylations. J. Carbohydr. Chem. 1988, 7, 263–269. [Google Scholar] [CrossRef]
- Gupta, P.; Yadav, D.K.; Siripurapu, K.B.; Palit, G.; Maurya, R. Constituents of Ocimum sanctum with antistress activity. J. Nat. Prod. 2007, 70, 1410–1416. [Google Scholar] [CrossRef]
- Zou, Y.; Li, Y.; Kim, M.-M.; Lee, S.-H.; Kim, S.-K. Ishigoside, a new glyceroglycolipid isolated from the brown alga Isbige okamurae. Biotechnol. Bioprocess. Eng. 2009, 14, 20–26. [Google Scholar] [CrossRef]
- Al-Masoudi, N.A.L.; Tooma, N.J. Synthesis of 3-amino-3-deoxy-5-thio-d-allose and 3-amino-3-deoxy-1,2-O:5,6-S,O-di-isopropylidene-5-thio-α-d-glucofuranose. Carbohydr. Res. 1993, 239, 273–278. [Google Scholar] [CrossRef]
- Wu, H.-J.; Li, C.-X.; Song, G.-P.; Li, Y.-X. Synthesis of natural α-6-Dehydroxy-6-aminoglucoglycerolipids. Chin. J. Chem. 2008, 26, 1641–1646. [Google Scholar] [CrossRef]
- Göllner, C.; Philipp, C.; Dobner, B.; Sippl, W.; Schmidt, M. First total synthesis of 1,2-dipalmitoyl-3-(N-palmitoyl-6′-amino-6′-deoxy-α-d-glucosyl)-sn-glycerol—A glycoglycerolipid of a marine alga with a high inhibitor activity against human Myt1-kinase. Carbohydr. Res. 2009, 344, 1628–1631. [Google Scholar] [CrossRef]
- Li, C.; Sun, Y.; Zhang, J.; Zhao, Z.; Yu, G.; Guan, H. Synthesis of 6′-acylamido-6′-deoxy-α-d-galactoglycerolipids. Carbohydr. Res. 2013, 376, 15–23. [Google Scholar]
- Sun, Y.; Zhang, J.; Li, C.; Guan, H.; Yu, G. Synthesis of glycoglycerolipid of 1,2-dipalmitoyl-3-(N-palmitoyl-6′-amino-6′-deoxy-α-d-glucosyl)-sn-glycerol and its analogues, inhibitors of human Myt1-kinase. Carbohydr. Res. 2012, 355, 6–12. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Y.; Wang, W.; Zhang, X.; Li, C.; Guan, H. Synthesis and antiviral evaluation of 6′-acylamido-6′-deoxy-α-d-mannoglycerolipids. Carbohydr. Res. 2013, 381, 74–82. [Google Scholar] [CrossRef]
- Colombo, D.; Scala, A.; Taino, I.M.; Toma, L.; Ronchetti, F.; Tokuda, H.; Nishino, H.; Nagatsu, A.; Sakakibara, J. 1-O-, 2-O- and 3-O-β-glycosyl-sn-glycerols: Structure—Anti-tumor-promoting activity relationship. Bioorg. Med. Chem. Lett. 1996, 6, 1187–1190. [Google Scholar]
- Dangate, M.; Franchini, L.; Ronchetti, F.; Arai, T.; Iida, A.; Tokuda, H.; Colombo, D. Short regioselective chemoenzymatic synthesis and biological evaluation of 1-O-Acyl-2-O-(β-d-sulfoquinovopyranosyl)-sn-glycerols. Eur. J. Org. Chem. 2009, 2009, 6019–6026. [Google Scholar]
- Compostella, F.; Colombo, D.; Ferraboschi, P.; Scala, A.; Toma, L.; Ronchetti, F. Synthesis of isosteric analogues of acylglycosylglycerols active as chemoprevention agents. Eur. J. Org. Chem. 2002, 2002, 1429–1435. [Google Scholar]
- Colombo, D.; Gagliardi, C.; Vetro, M.; Ronchetti, F.; Takasaki, M.; Konoshima, T.; Suzuki, N.; Tokuda, H. New 6-amino-6-deoxy-glycoglycerolipids derived from 2-O-β-d-glucopyranosylglycerol: Insights into the structure-activity relationship of glycoglycerolipids as anti-tumor promoters. Carbohydr. Res. 2013, 373, 64–74. [Google Scholar] [CrossRef]
- Colombo, D.; Compostella, F.; Ronchetti, F.; Scala, A.; Toma, L.; Tokuda, H.; Nishino, H. Chemoenzymatic synthesis and antitumor promoting activity of 6′-and 3-esters of 2-O-β-d-glucosylglycerol. Bioorg. Med. Chem. 1999, 7, 1867–1871. [Google Scholar]
- Colombo, D.; Ronchetti, F.; Scala, A.; Taino, I.M.; Toma, L. A facile lipase catalyzed access to fatty acid monoesters of 2-O-β-d-glucosylglycerol. Tetrahedron 1996, 7, 771–777. [Google Scholar] [CrossRef]
- Colombo, D.; Ronchetti, F.; Scala, A.; Taino, I.M.; Albini, P.M.; Toma, L. Regio- and diastereoselective lipase-catalyzed preparation of acetylated 2-O-glucosylglycerols. Tetrahedron 1994, 5, 1377–1384. [Google Scholar] [CrossRef]
- Dangate, M.; Franchini, L.; Ronchetti, F.; Arai, T.; Iida, A.; Tokuda, H.; Colombo, D. 2-O-β-d-Glucopyranosyl-sn-glycerol based analogues of sulfoquinovosyldiacylglycerols (SQDG) and their role in inhibiting Epstein-Barr virus early antigen activation. Bioorg. Med. Chem. 2009, 17, 5968–5973. [Google Scholar] [CrossRef]
- Colombo, D.; Compostella, F.; Ronchetti, F.; Scala, A.; Toma, L.; Mukainaka, T.; Nagatsu, A.; Konoshima, T.; Tokuda, H.; Nishino, H. Inhibitory effects of monoacylated 2-O-β-galactosylglycerols on Epstein–Barr virus activation: the significant role of the hexanoyl chain. Cancer Lett. 1999, 143, 1–4. [Google Scholar] [CrossRef]
- Colombo, D.; Scala, A.; Taino, I.M.; Toma, L.; Ronchetti, F.; Tokuda, H.; Nishino, H.; Nagatsu, A.; Sakakibara, J. Inhibitory effects of fatty acid monoesters of 2-O-β-d-glucosylglycerol on Epstein-Barr virus activation. Cancer Lett. 1998, 123, 83–86. [Google Scholar] [CrossRef]
- Colombo, D.; Compostella, F.; Ronchetti, F.; Scala, A.; Tokuda, H.; Nishino, H. Diesters of glycosylglycerols active in cancer chemoprevention. Eur. J. Med. Chem. 2001, 36, 691–695. [Google Scholar]
- Colombo, D.; Franchini, L.; Toma, L.; Ronchetti, F.; Tanaka, R.; Takayasu, J.; Nishino, H.; Tokuda, H. Cyclic and branched acyl chain galactoglycerolipids and their effect on anti-tumor-promoting activity. Eur. J. Med. Chem. 2006, 41, 1456–1463. [Google Scholar]
- Colombo, D.; Compostella, F.; Ronchetti, F.; Scala, A.; Toma, L.; Tokuda, H.; Nishino, H. Glycoglycerolipid analogues active as anti-tumor-promoters: The influence of the anomeric configuration. Eur. J. Med. Chem. 2000, 35, 1109–1113. [Google Scholar]
- Mizushina, Y.; Tanaka, N.; Yagi, H.; Kurosawa, T.; Onoue, M.; Seto, H.; Horie, T.-I.; Aoyagi, N.; Yamaoka, M.; Matsukage, A. Fatty acids selectively inhibit eukaryotic DNA polymerase activities in vitro. Biochim. Biophys. Acta 1996, 1308, 256–262. [Google Scholar] [CrossRef]
- Mizushina, Y.; Yoshida, S.; Matsukage, A.; Sakaguchi, K. The inhibitory action of fatty acids on DNA polymerase β. Biochim. Biophys. Acta 1997, 1336, 509–521. [Google Scholar] [CrossRef]
- Hanashima, S.; Mizushina, Y.; Ohta, K.; Yamazaki, T.; Sugawara, F.; Sakaguchi, K. Structure-activity relationship of a novel group of mammalian DNA polymerase inhibitors, synthetic sulfoquinovosylacylglycerols. Cancer Sci. 2000, 91, 1073–1083. [Google Scholar] [CrossRef]
- Murakami, C.; Yamazaki, T.; Hanashima, S.; Takahashi, S.; Ohta, K.; Yoshida, H.; Sugawara, F.; Sakaguchi, K.; Mizushina, Y. Structure-function relationship of synthetic sulfoquinovosyl-acylglycerols as mammalian DNA polymerase inhibitors. Arch. Biochem. Biophys. 2002, 403, 229–236. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, J.; Li, C.; Yu, G.; Guan, H. Total Synthesis and Structure-Activity Relationship of Glycoglycerolipids from Marine Organisms. Mar. Drugs 2014, 12, 3634-3659. https://doi.org/10.3390/md12063634
Zhang J, Li C, Yu G, Guan H. Total Synthesis and Structure-Activity Relationship of Glycoglycerolipids from Marine Organisms. Marine Drugs. 2014; 12(6):3634-3659. https://doi.org/10.3390/md12063634
Chicago/Turabian StyleZhang, Jun, Chunxia Li, Guangli Yu, and Huashi Guan. 2014. "Total Synthesis and Structure-Activity Relationship of Glycoglycerolipids from Marine Organisms" Marine Drugs 12, no. 6: 3634-3659. https://doi.org/10.3390/md12063634
APA StyleZhang, J., Li, C., Yu, G., & Guan, H. (2014). Total Synthesis and Structure-Activity Relationship of Glycoglycerolipids from Marine Organisms. Marine Drugs, 12(6), 3634-3659. https://doi.org/10.3390/md12063634