Phallusiasterols A and B: Two New Sulfated Sterols from the Mediterranean Tunicate Phallusia fumigata and Their Effects as Modulators of the PXR Receptor
Abstract
:1. Introduction
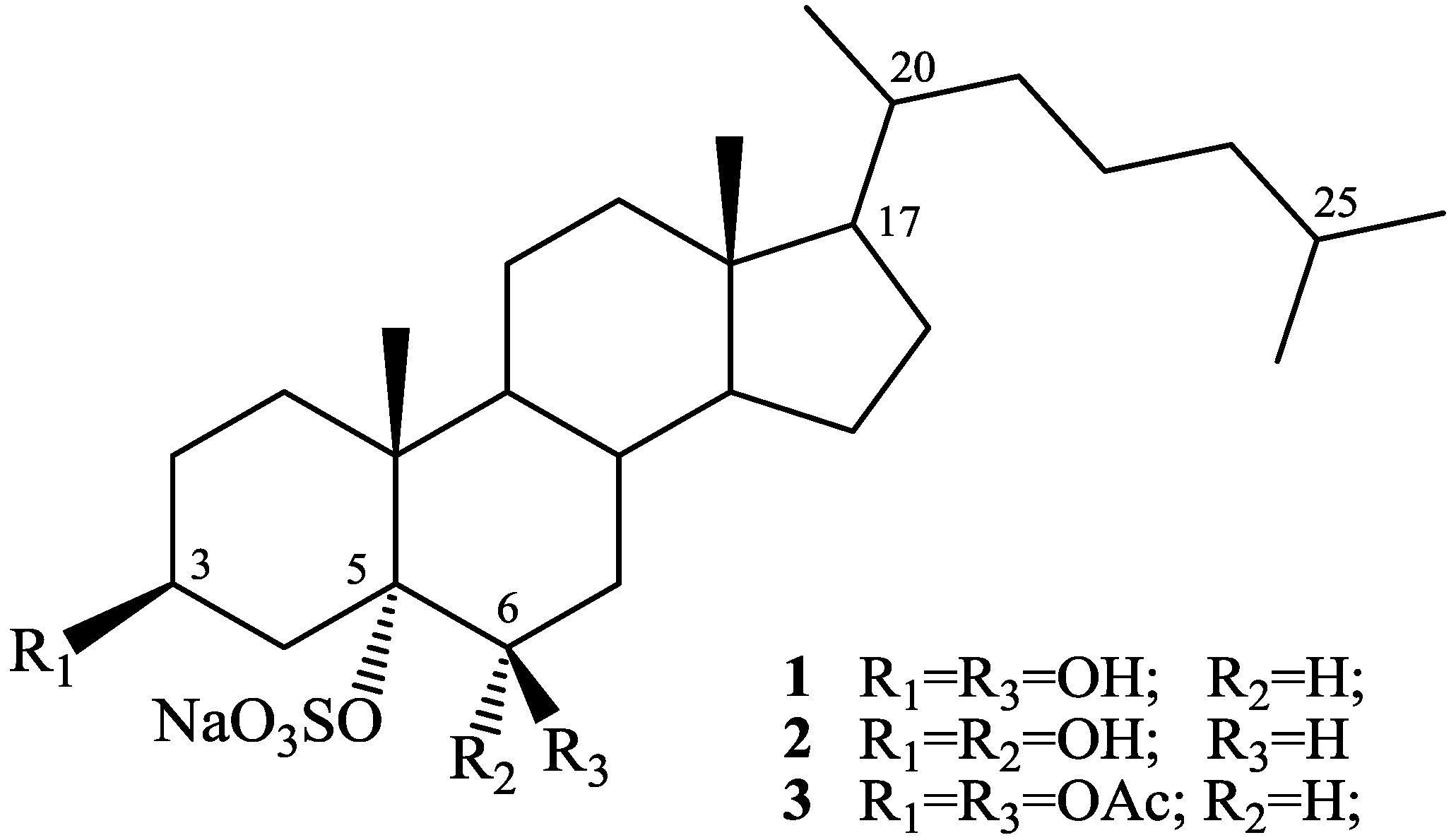
2. Results and Discussion
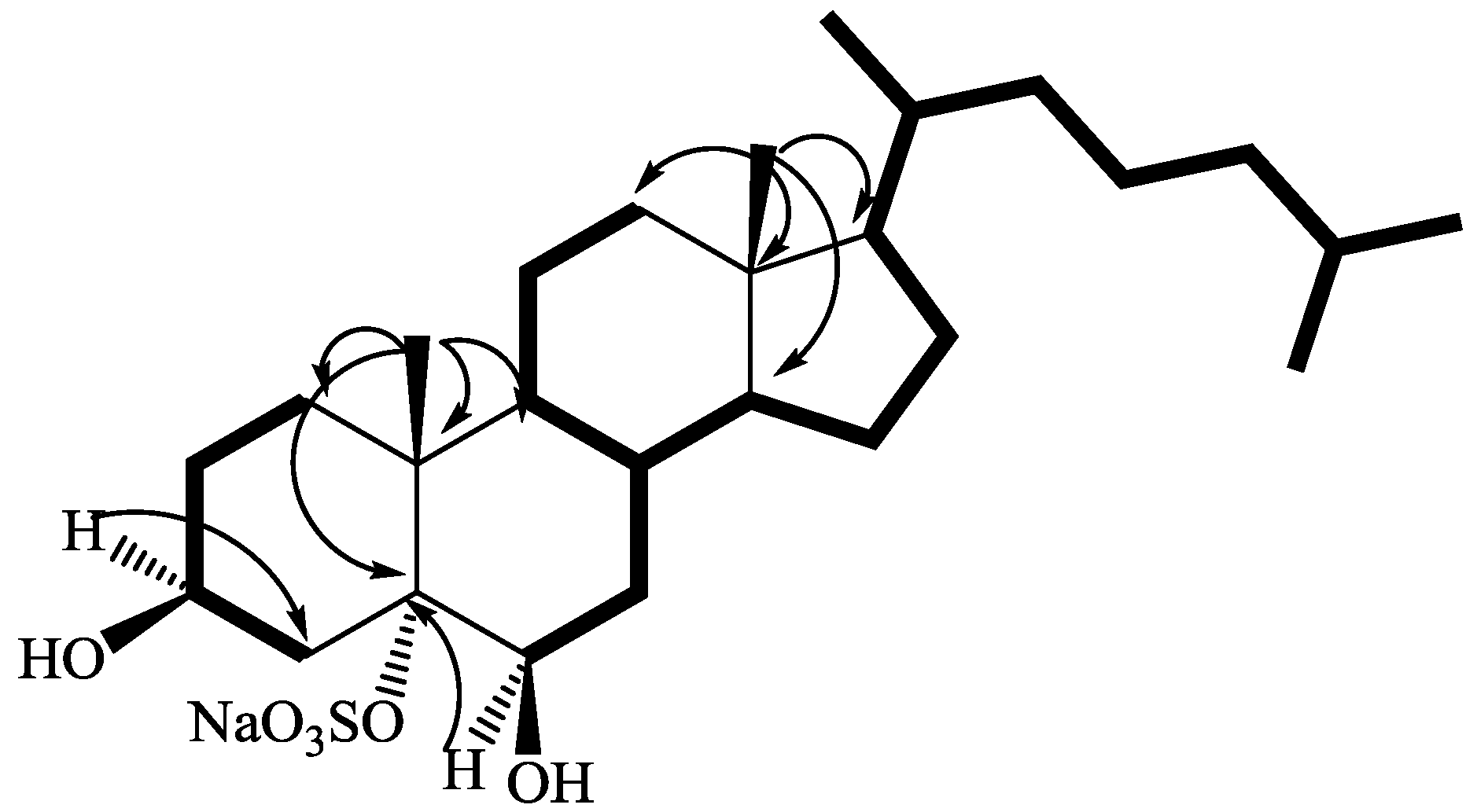
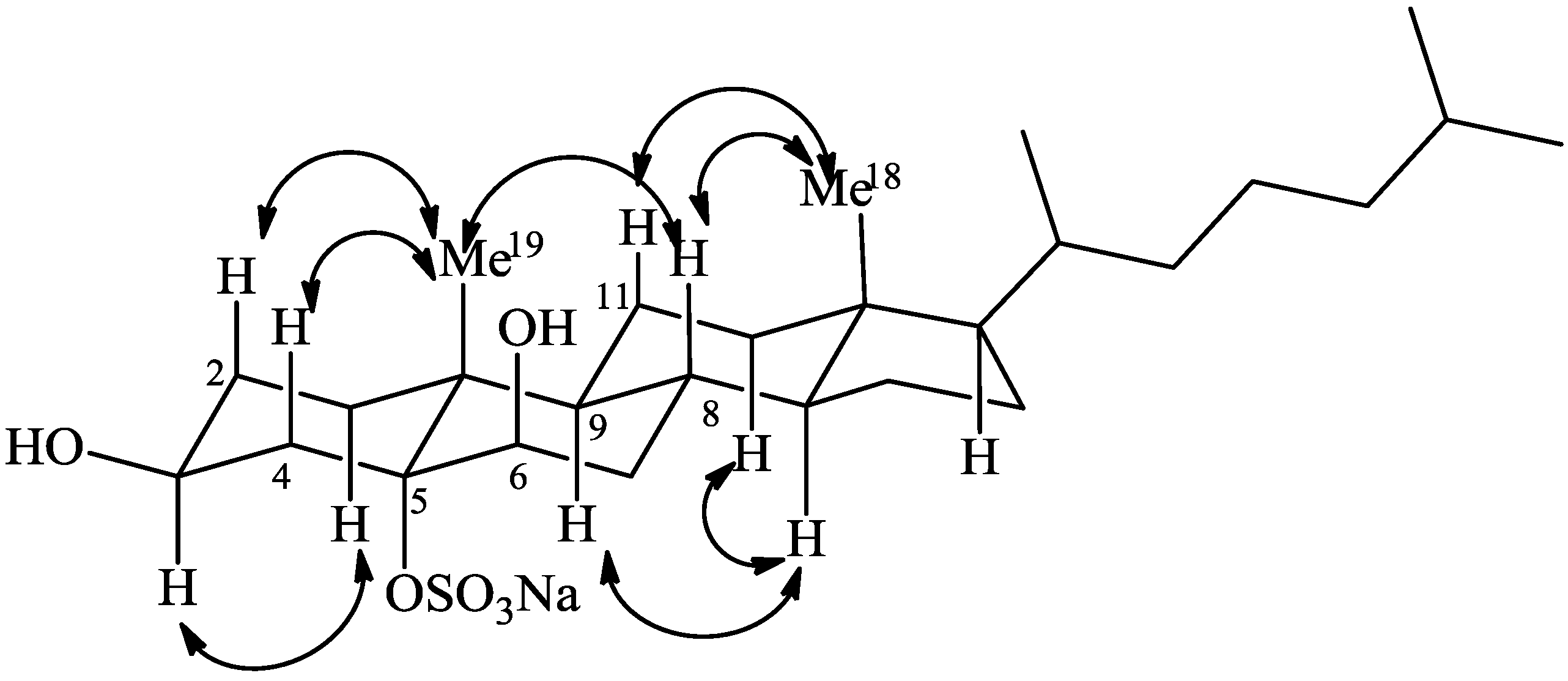
2.1. Isolation and Structure Elucidation
| Phallusiasterol A (1) | Phallusiasterol B (2) | ||||
|---|---|---|---|---|---|
| Pos. | δC | δH (mult., J in Hz) | HMBC | δC | δH (mult., J in Hz) |
| 1α/ax | 34.4 | 1.90 (dt,13.4, 4.2) | 2, 5, 10, 19 | 33.8 | 2.10, m |
| 1β/eq | 1.56, m | 2, 3, 5, 10, 19 | 1.50, m | ||
| 2α/eq | 31.8 | 2.20, m | 1, 3, 10 | 32.5 | 2.22, m |
| 2β/ax | 2.01 a | 1, 3, 9 | 1.97, m | ||
| 3α/ax | 67.2 | 4.79, m | 1, 2, 4 | 67.4 | 4.72, m |
| 4α/eq | 43.2 | 2.51 (dd, 13.6, 4.5) | 2, 3, 5, 6, 10 | 43.3 | 2.30 (dd, 13.0, 4.0) |
| 4β/ax | 3.10, m | 2, 3 | 2.81, m | ||
| 5 | 87.8 | - | - | 75.8 | - |
| 6α | 75.3 | 4.33 (bs) | 4, 5, 8, 10 | 66.1 | 4.34, (d, 5.5) |
| 7α/ax | 35.1 | 1.88 a | 5, 6, 8, 9 | 35.7 | 1.93, m |
| 7β/eq | 2.21, m | 8, 14 | 2.45, m | ||
| 8β/ax | 31.1 | 2.05 (qd, 11.6, 4.3) | 7, 9, 14 | 30.9 | 1.99 (qd, 11.0, 3.4) |
| 9α/ax | 46.9 | 1.75, (ddd, 13.6, 11.1, 3.6) | 8, 10, 11, 19 | 45.5 | 1.88 (ddd, 13.5, 11.2, 3.6) |
| 10 | 40.6 | - | - | 39.8 | - |
| 11α/eq | 21.8 | 1.47 (dq, 14.1, 3.8) | 9, 10, 12 | 21.7 | 1.48, m |
| 11β/ax | 1.37 a | 9, 12, 17 | 1.38 a | ||
| 12α/ax | 40.2 | 1.13, m | 11, 14 | 40.8 | 1.17 a |
| 12β/eq | 1.95 a | 9, 13, 14 | 1.94 (dt, 12.4, 3.4) | ||
| 13 | 42.9 | - | - | 43.4 | - |
| 14α | 56.2 | 1.05, m | 8, 13, 15, 16, 18 | 56.1 | 1.02, m |
| 15α | 24.4 | 1.57 a | 13, 14, 16, 17 | 24.4 | 1.55, m |
| 15β | 1.04, m | 8, 14, 16 | 1.07, m | ||
| 16α | 28.5 | 1.82 (ddd, 13.6, 9.5, 3.7) | 13, 15, 17 | 29.1 | 1.83 (ddd, 13.6, 9.4, 3.8) |
| 16β | 1.21 a | 13, 17, 20 | 1.23, m | ||
| 17 | 56.4 | 1.10, m | 13, 15, 16, 20, 22 | 56.9 | 1.11, m |
| 18 | 12.4 | 0.67, s | 12, 13, 14, 17 | 12.3 | 0.71, s |
| 19 | 18.7 | 1.60, s | 1, 5, 9, 10 | 18.5 | 1.47, s |
| 20 | 36 | 1.36, m | 17, 21, 22, 23 | 36.8 | 1.35, m |
| 21 | 19 | 0.96 (d, 6.5) | 17, 20, 22 | 19 | 0.96 (d, 6.5) |
| 22a | 36.5 | 1.38 a | 20, 21, 24 | 36.5 | 1.37 a |
| 22b | 1.03, m | 20, 21, 24 | 1.01, m | ||
| 23a | 24.2 | 1.38 a | 24 | 24.1 | 1.36 a |
| 23b | 1.18 a | 24 | 1.17, m | ||
| 24a | 39.7 | 1.13, m | 23, 26, 27 | 39.7 | 1.14, m |
| 24b | 1.13, m | 23, 26, 27 | 1.14, m | ||
| 25 | 28.3 | 1.51, m | 23, 24, 26, 27 | 28.7 | 1.52 a |
| 26 | 22.7 | 0.88 (d, 6.6) | 24, 25 | 22.8 | 0.88 (d, 6.5) |
| 27 | 22.9 | 0.89 (d, 6.6) | 24, 25 | 22.9 | 0.88 (d, 6.5) |
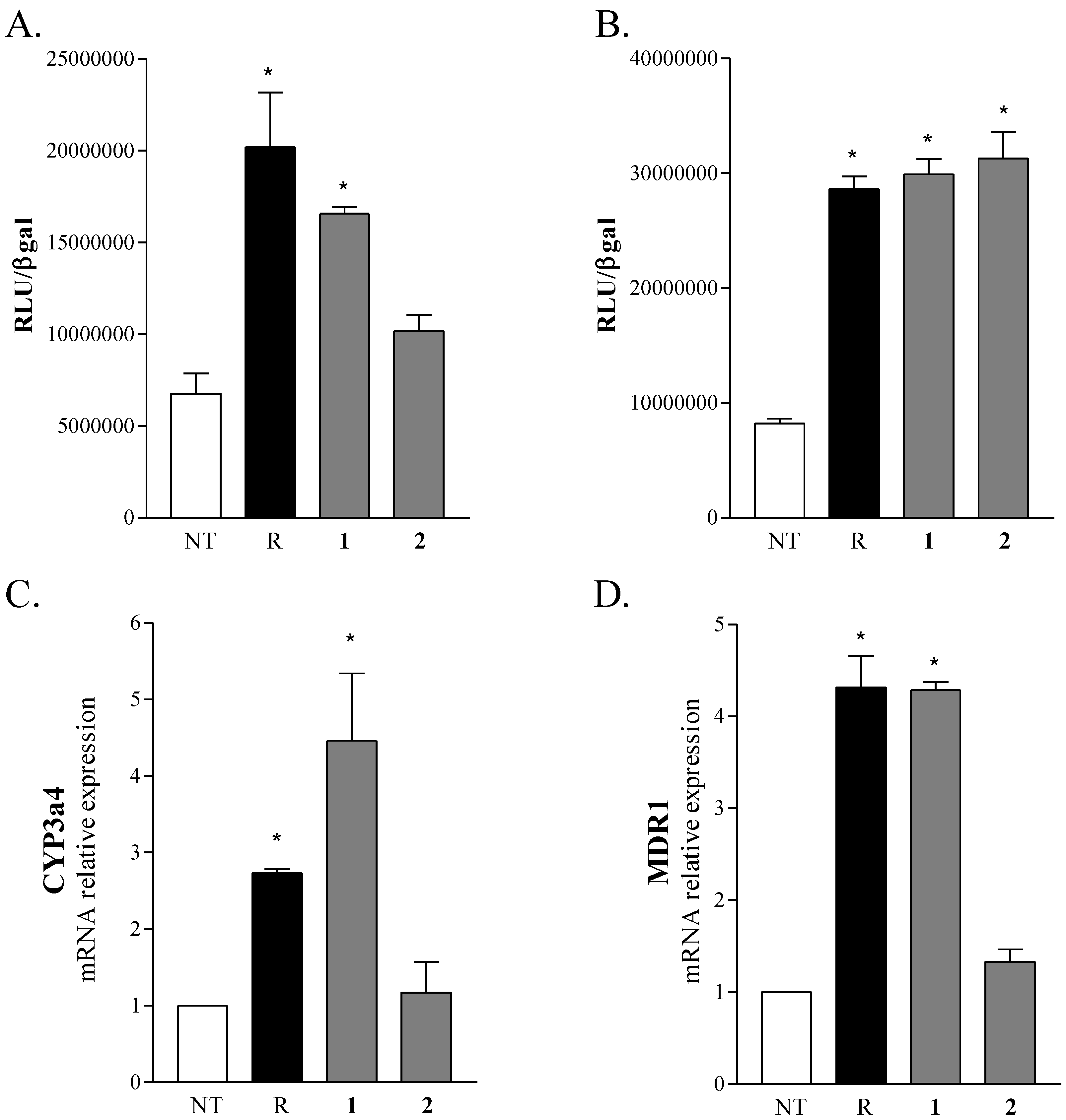
2.2. Biological Evaluation
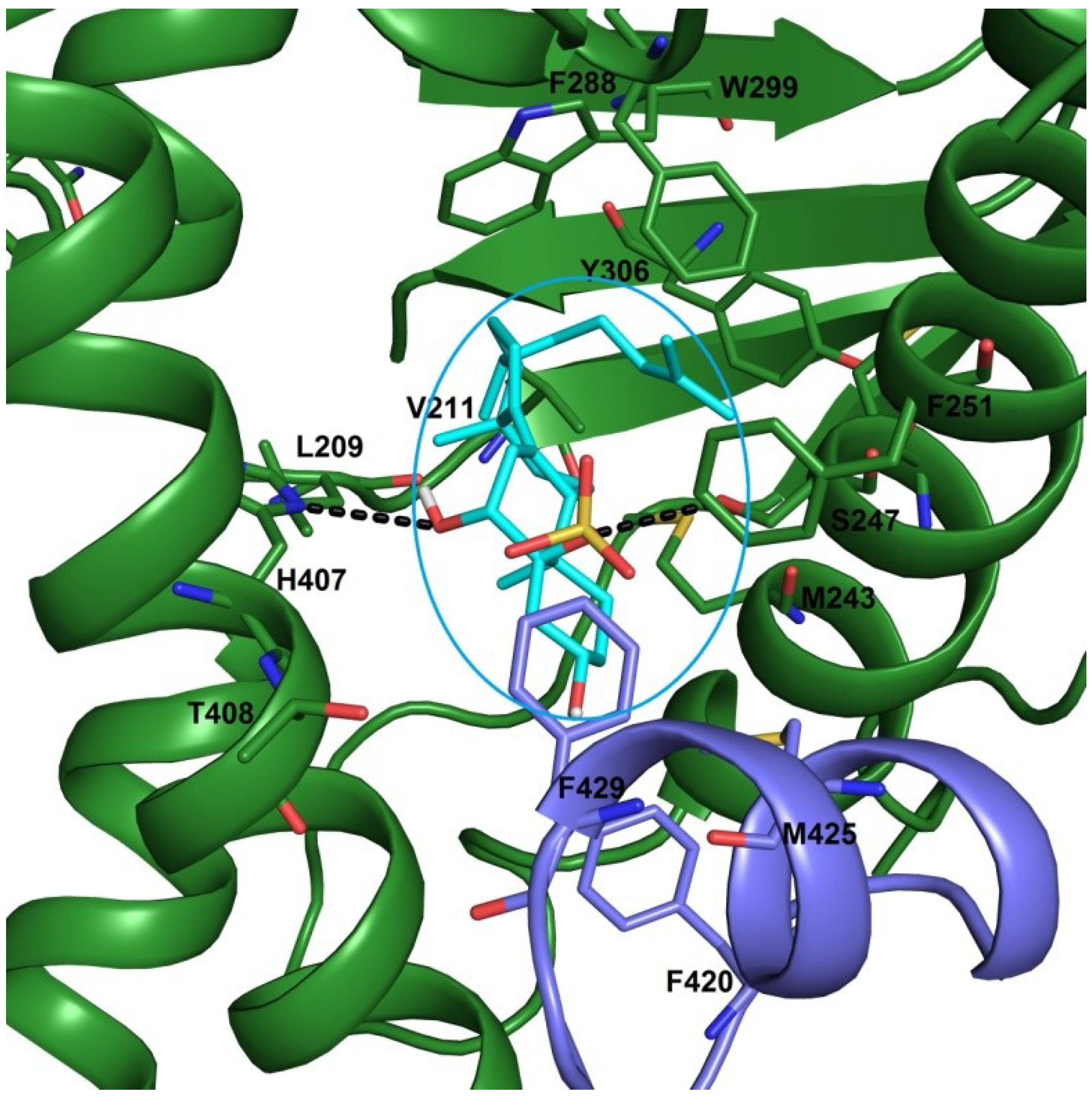
2.3. Docking Studies
3. Experimental Section
3.1. General Experimental Procedures
3.2. Collection, Extraction, and Isolation
3.3. Phallusiasterol A (1)
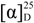 −3.5 (c 0.1, CHCl3); HRESIMS (positive ion mode, CH3OH) m/z 545.2858 ([M + Na]+, calcd. for C27H47SO6Na2+ 545.2889); 1H and 13C-NMR (C5D5N): see Table 1. 1H-NMR (CDCl3): δ 4.33 (1H, m, H-3), 3.96 (1H, br s, H-6), 2.34 (1H, dd, J = 13.1, 10.5 Hz, H-4β), 2.02 (1H, m, H-7β), 1.99 (1H, overlapping, H-4α), 1.98 (1H, overlapping, H-12β), 1.90 (1H, m, H-2α), 1.83 (1H, ddd, J = 13.6, 9.5, 3.7, H-16α), 1.74 (1H, qd, J = 12.0, 4.3 Hz, H-8β), 1.68 (1H, dt, J = 13.4, 4.2 Hz, H-1α), 1.60 (1H, overlapping, H-7α), 1.57 (1H, overlapping, H-2β), 1.57 (1H, overlapping, H-15α), 1.54 (1H, m, H-9α), 1.51 (1H, m, H-25), 1.48 (1H, ddd, J = 13.4, 4.5, 2.3, H-1β), 1.41 (1H, dq, J = 14.1, 3.8, H-11α), 1.37 (1H, m, H-20), 1.35–1.33 (2H, overlapping, H-22a and H-23a), 1.29 (1H, overlapping, H-11β), 1.28 (3H, s, Me-19), 1.26 (1H, overlapping, H-16β), 1.25 (1H, m, H-14α), 1.24 (1H, overlapping, H-23b), 1.18 (1H, m, H-12α), 1.15–1.10 (3H, m, H-17, H-24a, and H-24b), 1.07 (1H, m, H-15β), 1.00 (1H, m, H-22b), 0.91 (3H, d, J = 6.5 Hz, Me-21), 0.87 (3H, d, J = 6.6 Hz, Me-26), 0.86 (3H, d, J = 6.6 Hz, Me-27), 0.67 (3H, s, Me-18). 13C-NMR (CDCl3): δ 84.2 (C-5), 75.8 (C-6), 67.8 (C-3), 56.0 (C-17), 55.8 (C-14), 46.0 (C-9), 42.8 (C-13), 41.3 (C-4), 39.8 (C-10 and C-12), 39.6 (C-24), 36.2 (C-22), 35.8 (C-20), 34.0 (C-7), 33.6 (C-1), 30.5 (C-2), 30.4 (C-8), 28.2 (C-16), 28.0 (C-25), 24.0 (C-15), 23.8 (C-23), 21.3 (C-11), 22.7 (Me-26), 22.5 (Me-27), 18.8 (Me-21), 18.2 (Me-19), 12.1 (Me-18).
−3.5 (c 0.1, CHCl3); HRESIMS (positive ion mode, CH3OH) m/z 545.2858 ([M + Na]+, calcd. for C27H47SO6Na2+ 545.2889); 1H and 13C-NMR (C5D5N): see Table 1. 1H-NMR (CDCl3): δ 4.33 (1H, m, H-3), 3.96 (1H, br s, H-6), 2.34 (1H, dd, J = 13.1, 10.5 Hz, H-4β), 2.02 (1H, m, H-7β), 1.99 (1H, overlapping, H-4α), 1.98 (1H, overlapping, H-12β), 1.90 (1H, m, H-2α), 1.83 (1H, ddd, J = 13.6, 9.5, 3.7, H-16α), 1.74 (1H, qd, J = 12.0, 4.3 Hz, H-8β), 1.68 (1H, dt, J = 13.4, 4.2 Hz, H-1α), 1.60 (1H, overlapping, H-7α), 1.57 (1H, overlapping, H-2β), 1.57 (1H, overlapping, H-15α), 1.54 (1H, m, H-9α), 1.51 (1H, m, H-25), 1.48 (1H, ddd, J = 13.4, 4.5, 2.3, H-1β), 1.41 (1H, dq, J = 14.1, 3.8, H-11α), 1.37 (1H, m, H-20), 1.35–1.33 (2H, overlapping, H-22a and H-23a), 1.29 (1H, overlapping, H-11β), 1.28 (3H, s, Me-19), 1.26 (1H, overlapping, H-16β), 1.25 (1H, m, H-14α), 1.24 (1H, overlapping, H-23b), 1.18 (1H, m, H-12α), 1.15–1.10 (3H, m, H-17, H-24a, and H-24b), 1.07 (1H, m, H-15β), 1.00 (1H, m, H-22b), 0.91 (3H, d, J = 6.5 Hz, Me-21), 0.87 (3H, d, J = 6.6 Hz, Me-26), 0.86 (3H, d, J = 6.6 Hz, Me-27), 0.67 (3H, s, Me-18). 13C-NMR (CDCl3): δ 84.2 (C-5), 75.8 (C-6), 67.8 (C-3), 56.0 (C-17), 55.8 (C-14), 46.0 (C-9), 42.8 (C-13), 41.3 (C-4), 39.8 (C-10 and C-12), 39.6 (C-24), 36.2 (C-22), 35.8 (C-20), 34.0 (C-7), 33.6 (C-1), 30.5 (C-2), 30.4 (C-8), 28.2 (C-16), 28.0 (C-25), 24.0 (C-15), 23.8 (C-23), 21.3 (C-11), 22.7 (Me-26), 22.5 (Me-27), 18.8 (Me-21), 18.2 (Me-19), 12.1 (Me-18).3.4. Phallusiasterol B (2)
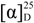 +7.9 (c 0.1, CHCl3); HRESIMS (positive ion mode, CH3OH) m/z 545.2870 ([M + Na]+, calcd. for C27H47SO6Na2+ 545.2889); 1H and 13C-NMR (C5D5N): see Table 1. 1H-NMR (CDCl3): δ 4.05 (1H, m, H-3), 3.84 (1H, d, J = 5.4, H-6), 2.25 (1H, t, J = 13.2, H-4β), 1.99 (1H, dt, J = 12.4, 3.4, H-12β), 1.96 (1H, m, H-7β), 1.67 (1H, dd, J = 13.2, 2.1, H-4α), 1.87–1.84 (3H, overlapping, H-2α, H-7α, H-8β H-16α), 1.68 (1H, dd, J = 13.1, 4.7, H-9α), 1.56-1.54 (3H, overlapping, H-1α, H-2β, and H-15α), 1.51 (1H, m, H-25), 1.42–1.41 (2H, m, H-1β and H-11α), 1.37 (1H, m, H-20), 1.33 (2H, overlapping, H-22a and H-23a), 1.30 (1H, overlapping, H-11β), 1.28 (3H, s, Me-19), 1.27-1.24 (3H, overlapping, H-14α, H-16β, and H-23b), 1.12 (1H, m, H-12α), 1.20-1.11 (3H, m, H-17, H-24a, and H-24b), 1.07 (1H, m, H-15β), 1.00 (1H, m, H-22b), 0.91 (3H, d, J = 6.5 Hz, Me-21), 0.87 (3H, d, J = 6.6 Hz, Me-26), 0.86 (3H, d, J = 6.6 Hz, Me-27), 0.70 (3H, s, Me-18). 13C-NMR (CDCl3): δ 77.2 (C-5), 67.3 (C-3), 63.5 (C-6), 56.4 (C-17), 55.6 (C-14), 46.1 (C-9), 42.9 (C-13), 41.6 (C-4), 40.2 (C-12), 39.6 (C-24), 39.4 (C-10), 36.1 (C-22), 35.8 (C-20), 35.2 (C-7), 32.6 (C-1), 30.6 (C-2), 29.7 (C-8), 29.1 (C-16), 27.9 (C-25), 24.0 (C-15), 23.8 (C-23), 21.1 (C-11), 22.7 (Me-26), 22.5 (Me-27), 18.5 (Me-21), 18.2 (Me-19), 12.0 (Me-18).
+7.9 (c 0.1, CHCl3); HRESIMS (positive ion mode, CH3OH) m/z 545.2870 ([M + Na]+, calcd. for C27H47SO6Na2+ 545.2889); 1H and 13C-NMR (C5D5N): see Table 1. 1H-NMR (CDCl3): δ 4.05 (1H, m, H-3), 3.84 (1H, d, J = 5.4, H-6), 2.25 (1H, t, J = 13.2, H-4β), 1.99 (1H, dt, J = 12.4, 3.4, H-12β), 1.96 (1H, m, H-7β), 1.67 (1H, dd, J = 13.2, 2.1, H-4α), 1.87–1.84 (3H, overlapping, H-2α, H-7α, H-8β H-16α), 1.68 (1H, dd, J = 13.1, 4.7, H-9α), 1.56-1.54 (3H, overlapping, H-1α, H-2β, and H-15α), 1.51 (1H, m, H-25), 1.42–1.41 (2H, m, H-1β and H-11α), 1.37 (1H, m, H-20), 1.33 (2H, overlapping, H-22a and H-23a), 1.30 (1H, overlapping, H-11β), 1.28 (3H, s, Me-19), 1.27-1.24 (3H, overlapping, H-14α, H-16β, and H-23b), 1.12 (1H, m, H-12α), 1.20-1.11 (3H, m, H-17, H-24a, and H-24b), 1.07 (1H, m, H-15β), 1.00 (1H, m, H-22b), 0.91 (3H, d, J = 6.5 Hz, Me-21), 0.87 (3H, d, J = 6.6 Hz, Me-26), 0.86 (3H, d, J = 6.6 Hz, Me-27), 0.70 (3H, s, Me-18). 13C-NMR (CDCl3): δ 77.2 (C-5), 67.3 (C-3), 63.5 (C-6), 56.4 (C-17), 55.6 (C-14), 46.1 (C-9), 42.9 (C-13), 41.6 (C-4), 40.2 (C-12), 39.6 (C-24), 39.4 (C-10), 36.1 (C-22), 35.8 (C-20), 35.2 (C-7), 32.6 (C-1), 30.6 (C-2), 29.7 (C-8), 29.1 (C-16), 27.9 (C-25), 24.0 (C-15), 23.8 (C-23), 21.1 (C-11), 22.7 (Me-26), 22.5 (Me-27), 18.5 (Me-21), 18.2 (Me-19), 12.0 (Me-18).3.5. 3β,6β-Diacetate-5α-cholestan-5α-yl Sodium Sulfate (3)
3.6. Transactivation Experiments
3.7. Cells Culture, RNA Extraction and Real-Time PCR
- hGAPDH: GAAGGTGAAGGTCGGAGT and CATGGGTGGAATCATATTGGAA;
- hCYP3A4: CAAGACCCCTTTGTGGAAAA and CGAGGCGACTTTCTTTCATC;
- hMDR1: GTGGGGCAAGTCAGTTCATT and TCTTCACCTCCAGGCTCAGT.
3.8. Statistical Analysis
3.9. Computational Details
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kerr, R.G.; Baker, B.J. Marine sterols. Nat. Prod. Rep. 1991, 8, 465–497. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Hu, W.P.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2007, 24, 31–86. [Google Scholar] [CrossRef]
- Stonik, V.A. Marine polar steroids. Russ. Chem. Rev. 2001, 70, 673–715. [Google Scholar] [CrossRef]
- D’Auria, M.V. Polyoxygenated steroids of marine origin. Chem. Rev. 1993, 93, 1839–1895. [Google Scholar] [CrossRef]
- Sarma, N.S.; Krishna, M.S.R.; Rao, S.R. Sterol ring system oxidation pattern in marine sponges. Mar. Drugs 2005, 3, 84–111. [Google Scholar] [CrossRef]
- Sica, D.; Musumeci, D. Secosteroids of marine origin. Steroids 2004, 69, 743–756. [Google Scholar] [CrossRef]
- Guyot, M.; Durgeat, M. Occurrence of 9(11)-unsaturated peroxides in Tunicates. Tetrahedron Lett. 1981, 22, 1391–1392. [Google Scholar] [CrossRef]
- Guyot, M.; Davoust, D. Hydroperoxy-24-vinyl-24-cholésterol, nouvel hydroperoxide naturel isolé de deux tuniciers: Phallusia mamillata et Ciona intestinalis. Tetrahedron Lett. 1982, 23, 1905–1906. [Google Scholar] [CrossRef]
- Gunatilaka, A.L.; Gopichand, Y.; Schmitz, F.J.; Djerassi, F.J. Minor and trace sterols in marine invertebrates 26. Isolation and structure elucidation of nine new 5α,8α-epidioxy sterols from marine organisms. J. Org. Chem. 1981, 46, 3860–3866. [Google Scholar] [CrossRef]
- Tam Ha, T.B.; Kokke, W.C.; Djerassi, C. Minor sterols of marine invertebrates 37. Isolation of novel coprostanols and 4α-H3thyl sterols from the tunicate Ascidia nigra. Steroids 1982, 40, 433–453. [Google Scholar] [CrossRef]
- Palermo, J.A.; Rodriguez Brasco, M.F.; Hughes, E.A.; Seldes, A.M.; Balzaretti, V.T.; Cabezas, E. Short side chain sterols from the tunicate Polizoa opuntia. Steroids 1996, 61, 2–6. [Google Scholar] [CrossRef]
- Aiello, A.; Esposito, G.; Fattorusso, E.; Iuvone, T.; Luciano, P.; Menna, M. Aplidiasterols A and B, two new cytotoxic 9,11-secosterols from the Mediterranean ascidian Aplidium conicum. Steroids 2003, 68, 719–723. [Google Scholar] [CrossRef]
- Fiorucci, S.; Distrutti, E.; Bifulco, G.; D’Auria, M.V.; Zampella, A. Marine sponge steroids as nuclear receptor ligands. Trends Pharm. Sci. 2012, 33, 591–600. [Google Scholar] [CrossRef]
- Demarco, P.V.; Farkas, E.; Doddrell, D.; Mylari, B.V.; Wenkert, E. Pyridine-Induced Solvent shifts in the Nuclear Magnetic Resonance Spectra of Hydroxylic Compounds. J. Am. Chem. Soc. 1968, 90, 5480–5486. [Google Scholar]
- Migliuolo, A.; Notaro, G.; Piccialli, V.; Sica, D. New tetrahydroxylated sterots from the marine sponge Spongia officinalis. J. Nat. Prod. 1990, 53, 1414–1429. [Google Scholar] [CrossRef]
- Aiello, A.; Fattorusso, E.; Menna, M.; Carnuccio, R.; Iuvone, T. New cytotoxic steroids from the marine sponge Dysidea fragilis coming from the lagoon of Venice. Steroids 1995, 60, 660–673. [Google Scholar]
- Fujimoto, Y.; Yamada, T.; Ikekawa, N. Pyridine-induced deshielding of 4-methylene protons for the determination of C-6 stereochemistry of sterols having a 5et,6-diol moiety. Revision of the C-6 stereochemistry of marine sterol isolated from a sponge, Dysidea sp. Chem. Pharm. Bull. (Tokyo) 1985, 33, 3129–3133. [Google Scholar] [CrossRef]
- Notaro, G.; Piccialli, V.; Sica, D.; Corriero, G. 3β,5α,6β-Trihydroxylated sterols with a saturated nucleus from two populations of the marine sponge Cliona copiosa. J. Nat. Prod. 1991, 54, 1570–1575. [Google Scholar] [CrossRef]
- Das, B.; Srinivas, N.S. Studies on marine chemicals, part IV. Isolation of cholesterol derivatives from the marine sponge Spirastrella incostans. J. Nat. Prod. 1992, 55, 1310–1312. [Google Scholar] [CrossRef]
- Das, B.; Padma Rao, S.; Srinivas, N.S. Studies on marine chemicals, part VI. A new clionasterol derivative from the marine sponge Spirastrella incostans. J. Nat. Prod. 1993, 56, 2210–2211. [Google Scholar] [CrossRef]
- Festa, C.; de Marino, S.; D’Auria, M.V.; Bifulco, G.; Renga, B.; Fiorucci, S.; Petek, S.; Zampella, A. Solomonsterols A and B from Thenoella swinhoei. The first example of C-24 and C-23 sulfated sterols from a marine source endowed with a PXR agnostic activity. J. Med. Chem. 2011, 54, 401–405. [Google Scholar] [CrossRef]
- Sepe, V.; Ummarino, R.; D’Auria, M.V.; Mencarelli, A.; D’Amore, C.; Renga, B.; Zampella, A.; Fiorucci, S. Total synthesis and pharmacological characterization of solomonsterol A, a potent marine pregnane-X-receptor agonist endowed with anti-inflammatory activity. J. Med. Chem. 2011, 54, 4590–4599. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Imperatore, C.; D'Aniello, F.; Aiello, A.; Fiorucci, S.; D'Amore, C.; Sepe, V.; Menna, M. Phallusiasterols A and B: Two New Sulfated Sterols from the Mediterranean Tunicate Phallusia fumigata and Their Effects as Modulators of the PXR Receptor. Mar. Drugs 2014, 12, 2066-2078. https://doi.org/10.3390/md12042066
Imperatore C, D'Aniello F, Aiello A, Fiorucci S, D'Amore C, Sepe V, Menna M. Phallusiasterols A and B: Two New Sulfated Sterols from the Mediterranean Tunicate Phallusia fumigata and Their Effects as Modulators of the PXR Receptor. Marine Drugs. 2014; 12(4):2066-2078. https://doi.org/10.3390/md12042066
Chicago/Turabian StyleImperatore, Concetta, Filomena D'Aniello, Anna Aiello, Stefano Fiorucci, Claudio D'Amore, Valentina Sepe, and Marialuisa Menna. 2014. "Phallusiasterols A and B: Two New Sulfated Sterols from the Mediterranean Tunicate Phallusia fumigata and Their Effects as Modulators of the PXR Receptor" Marine Drugs 12, no. 4: 2066-2078. https://doi.org/10.3390/md12042066
APA StyleImperatore, C., D'Aniello, F., Aiello, A., Fiorucci, S., D'Amore, C., Sepe, V., & Menna, M. (2014). Phallusiasterols A and B: Two New Sulfated Sterols from the Mediterranean Tunicate Phallusia fumigata and Their Effects as Modulators of the PXR Receptor. Marine Drugs, 12(4), 2066-2078. https://doi.org/10.3390/md12042066









