Abstract
Recently, the studies on the prevention and treatment of human papillomavirus (HPV) which is closely related to the cervical cancer and other genital diseases are attracting more and more attention all over the world. Marine-derived polysaccharides and other bioactive compounds have been shown to possess a variety of anti-HPV and related cancer activities. This paper will review the recent progress in research on the potential anti-HPV and related cancer agents from marine resources. In particular, it will provide an update on the anti-HPV actions of heparinoid polysaccharides and bioactive compounds present in marine organisms, as well as the therapeutic vaccines relating to marine organisms. In addition, the possible mechanisms of anti-HPV actions of marine bioactive compounds and their potential for therapeutic application will also be summarized in detail.
1. Introduction
Papillomavirus is a diverse group of non enveloped DNA viruses that infect the skin and mucosal tissues of a range of vertebrate species, including humans [1,2,3]. In recent years, human papillomavirus (HPV) was confirmed existing in reproductive tract and other organs, including respiratory, bladder, mouth and throat, and its pathogenesis was dependent on virus serotype, the site of infection, and the immunity [4,5]. Infection with genital HPV types is very common, with an estimated lifetime risk of infection of about 75% [6]. Although most genital HPV infections are subclinical and self-limiting, a subset of persistently infected individuals has lesions that progress to premalignancy or cancer [7]. Virtually 100% of cervical, ~43% of vulvar, and ~70% of vaginal tumors are attributable to human papillomavirus infection, which annually generating 530,000 cervical and 21,000 vulvar and vaginal cancers worldwide [6,8].
Although HPV infection is considered a sexually transmitted infection, HPVs can also be transmitted by on-sexual routes including casual physical contact and erinatal vertical transmission [9,10]. Some studies have suggested that condoms are, at best, only marginally effective for preventing the sexual transmission of HPV [11,12]. Recently, a highly effective group of prophylactic HPV vaccines are becoming publicly available in the developed countries [13]. However, there are two possible drawbacks for these vaccines that they are relatively expensive (at least initially) and are likely to be papillomavirus type-restricted in their protection [7]. Thus, the vaccines may not initially be available to women in all parts of the world and may not offer protection against all cancer associated HPV types. Therefore, the search for potential drug candidates with higher inhibitory activities against various HPV strains is increasing in the pharmaceutical industry nowadays. In this regard, marine derived natural bioactive compounds and their derivatives are great sources for the development of new generation anti-HPV therapeutics, which is more effective with fewer side effects.
This review presents an overview of recent progress in research on the prophylaxis and therapy for HPV and related cancer diseases. Moreover, this review will mainly focus on the heparinoid polysaccharides and bioactive compounds present in marine organisms. Recent developments in the possible mechanisms of anti-HPV actions of marine bioactive compounds and their potential for therapeutic application will also be discussed in detail.
2. Update on Pathogenesis and Therapy for HPV and its Related Cancer
2.1. The Pathogenesis of HPV and Its Related Cancer
Human papillomavirus (HPV) is a diverse group of non-enveloped DNA virus appearing icosahedra symmetry spherical particles with diameters of ~45 to 55 nm, and contains more than 130 serotypes [2,3]. HPV genome is a ~8 kb circular DNA, which codes for about 6 early (E1, E2, E4, E5, E6 and E7) and two late genes (L1 and L2) [14,15,16]. On the basis of their association with disease types, papillomaviruses are classified into high-risk (HR) and low-risk (LR) types [14]. HR-HPV types (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, 70) are often associated with high grade lesions and invasive cancer, whereas the LR-HPV types (HPV 6, 11, 12, 13, 15, 32, 34, 40, 42, 43, 44, 53, 54) are mainly found in low grade lesions, genital or skin warts and condyloma accuminata [14,17,18].
HPV infects squamous epithelial cells in the cervix, glans of the penis, penile shaft, scrotum and anal verge by interacting with putative host cell surface receptors such as heparan sulfate proteoglycans [19] and alpha 6 integrins [20]. The virus enters the host cell via clathrin-mediated or caveolin-mediated endocytosis, depending on the HPV type [21,22]. The viral entry is the first step in HPV pathogenesis followed by its persistence in epithelial cells. These two steps establish the viral genome into the host cell and have been the subjects of therapeutic interventions by vaccines [14]. The third step of HPV life cycle is its integration into the human genome, which is associated with higher lesions. The fourth key step of HPV life cycle is expression of viral oncogenes in differentiating epithelium. All these four steps provide means of interference with HPV life cycle and thus represent targets for the development of anti-HPV drugs [14,23].
Moreover, HPV DNA integration is closely tied to the development of cancer, as most cases of HPV-induced cervical cancer feature an integrated form of the HPV genome [3,4]. In the carcinogenic process of HPV, E2 gene normally integrates into the host cells, and result in the inactivation of E2 protein, which caused the over-expression of E6, E7 genes [24]. Meanwhile, E5 gene stimulates cell cycles via EGFR, which in turn raising the over-expression of E6, E7 genes [25]. The activities of p53 gene and pRb gene are inhibited by HPV E6 and E7 proteins, respectively [26,27], resulting in the activation of the transcription of human telomerase reverse transcriptase (hTERT) [28], and the imbalance of host cells, which eventually change the cells from the normal to the cancer. In summary, the carcinogenic process of HPV is a multi-factor and multi-step process, in which viral and host factors all play important roles.
2.2. HPV Vaccines
The viral entry is the first step in HPV pathogenesis, so some HPV vaccines targeting virus surface protein L1 can be used to prevention of HPV infection. There are two types of HPV vaccines including prophylactic and therapeutic vaccine [29,30,31,32,33], and recent studies show that virus like particles (VLP) could be the applied for preventive vaccine [13,34,35]. Vaccination with virus-like particles (VLP) has demonstrated efficacy in HPV prophylaxis but these vaccines lack therapeutic potential. Considering the vital role of HPV16 in carcinogenesis [3,36], most of the efforts for developing therapeutic HPV vaccines have been directed towards development of vaccines against HPV16 viral oncogenes E6 and E7 [37,38,39,40,41,42,43,44,45,46,47]. There are several groups of therapeutic vaccines including virus/bacterial vector vaccine, peptide antigens, recombinant protein vaccines and plasmid DNA vaccines [48]. The antigens of most therapeutic vaccines are the whole protein or peptides derived from HPV E6 and E7 proteins because of their oncogenic potential and they are invariably retained and expressed throughout HPV related disease progression and carcinogenesis [14]. Apart from therapeutic HPV vaccines, there have been attempts to pulse dendritic cells (DC) with tumor lysate expressing HPV16 antigens [49], which is currently being tested in clinic [43].
Moreover, it was reported that marine polysaccharide carrageenan was able to generate antigen-specific immune responses and anti-tumor effects in female (C57BL/6) mice vaccinated with HPV16 E7 peptide vaccine [50]. Furthermore, the enhancement was not restricted to E7 antigen but also applicable to other antigenic systems. Some other structurally similar compounds to carrageenan, such as dextran, can also generate similar immune enhancement [50]. Thus, carrageenan and its structurally related compounds may serve as adjuvants for enhancing peptide-based HPV vaccine potency. In summary, some marine derived polysaccharides could be used as adjutants to enhance the anti-HPV effects of HPV therapeutic vaccines.
2.3. Current Anti-HPV and Related Cancer Drugs
Even after establishment of causal relationship between HPV and cervical cancer, currently, presence or absence of HPV does not have any impact on deciding the treatment; the strategies are primarily anti-cancer rather than anti-viral [14], and there is still no FDA approved anti-HPV drug listed so far. Though scissor excision is the most preferred methods for genital warts, topical preparations of cytotoxic compounds like Podophyllin or Trichloroacetic acid are also utilized in USA and Europe [51]. The current anti-HPV drugs are mainly oral hormonal medicine such as acyclovir, ganciclovir, interferon and interleukin. Interferons (IFNs) are the only antiviral drugs approved for the therapy of benign HPV related lesions. However, the IFNα treatment has limited efficacy and not recommended for routine clinical practice in the treatment of high-grade HPV associated lesions [52].
Moreover, some traditional Chinese medicines possess good anti-HPV activities and have been used for prevention and treatment of HPV related cancer in China. Chinese medicine chaihu was reported to have good inhibition effects on HPV infection by interfering with the expression of HPV-DNA in genital warts [53]. Chinese medicine Youdujing can reverse the function of cervical lesions in high-risk HPV infected patients by inhibiting the expression of HPV-DNA [54]. Moreover, Chinese medicine Paiteling composed of folium, sophora, cnidium, gall, and javanica oil can eliminate or inhibit high risk HPV infection by destroying mitochondria and other membrane system selectively and then lead to cell degeneration and necrosis [55]. In addition, it was found that Xinfuning (recombinant human interferon α-2b vaginal effervescent capsule) could be applied to cure HPV infection in vagina by boosting NK cell activity, and Xinfuning combined with Baofukang suppository for treatment of patients with high-risk HPV infection was also effective [56].
However, these current anti-HPV drugs are usually expensive, easily lead to liver and kidney damage, and produce drug resistance after prolonged treatment. Therefore, the development of novel anti-HPV agents with low toxicity and high efficiency is of high importance.
3. Potential Anti-HPV and Related Cancer Agents from Marine Resources
3.1. Heparin and Marine Heparinoid Polysaccharides
Heparin is a member of the glycosaminoglycan (GAG) family of carbohydrates and consists of a variably sulfated repeating disaccharide unit. HPV have more than 100 kinds of serotypes, of which the type 5, type 11 [57,58] and the high risk sexually transmitted serotypes 16, 31, 33 and 39 [57,59] have been reported to be able to use heparin sulfate as a low affinity co-receptor on the cell surface. For example, HPV16 virus, which can cause cervical cancer, can bind to heparin sulfate through viral capsid protein L1. Moreover, some reports indicated that HPV-16/33 VLP could not infect the cell after heparanase acted on the COS-7 or HaCaT cell, and confirmed heparin sulfate is necessary for infection of HPV to cells [59,60,61,62]. Several heparan sulfate proteoglycans (HSPGs) can serve as HPV receptors and support a putative role for syndecan-1, rather than α6 integrin, as a primary receptor protein in natural HPV infection of keratinocytes [63]. A recent study showed that L1 binding to heparan sulfate makes the L2 protein and cyclophilin B together to promote the cyclophilin B-mediated conformational change of L2 protein, so that HPV can effectively invade host cells [64]. Furthermore, some studies have shown that the structure of heparan sulfate may also affect tissue tropism of HPV and other heparin sulfate-binding pathogens [57].
The marine heparinoid polysaccharides are similar to heparin in structure, and possess GAG-like biological properties, which contain alginates, ulvans, and their sulfated derivatives, as well as the dextran sulfate and chitosan sulfate (Figure 1) [65]. It was reported that sulfated polysaccharides, such as heparin, cellulose sulfate and dextran sulfate, can block the infectivity of papillomaviruses [22,66]. For many classes of virus, including papillomaviruses, initial attachment of the virion to host cells is thought to be mediated mainly by interactions between the virion and a type of cell surface glycosaminoglycan known as heparan sulfate [67]. Many previous studies have indicated that some marine heparinoid polysaccharides such as alginic acid and fucoidan could effectively block HPV pseudovirion infection just like heparin [7]. Moreover, it was reported that the sulfated derivatives of Escherichia coli K5 capsular polysaccharides such as K5-N,OS(H), K5-N,OS(L), and K5-OS(H) which have a backbone structure resembling the heparin/heparan biosynthetic precursor can significantly inhibit HPV-16, HPV-18, and HPV-6 pseudovirion infection (IC50 < 1.0 µg/mL) [68].
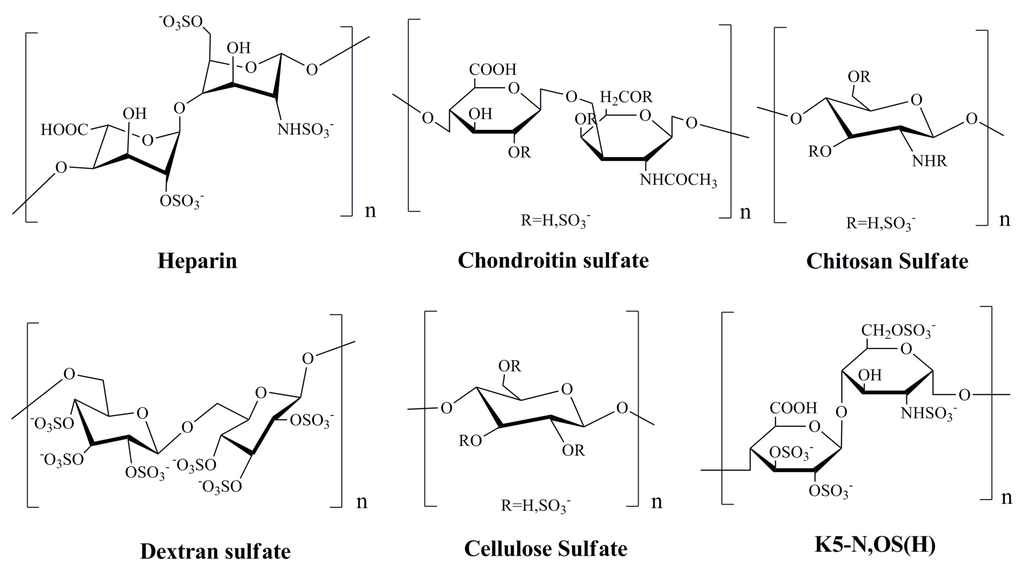
Figure 1.
Chemical structures of the repeat units of heparin and heparinoid polysaccharides with anti-Human papilloma virus (HPV) effects [65,66,68].
Figure 1.
Chemical structures of the repeat units of heparin and heparinoid polysaccharides with anti-Human papilloma virus (HPV) effects [65,66,68].
In summary, the sulfate polysaccharides such as heparin and heparinoid polysaccharides can inhibit the entry process of HPV by interfering with the initial attachment of the viral particle to the host cell, which suggests these polysaccharides can be developed into novel anti-HPV drugs in the future.
3.2. Polysaccharides Derived from Red Algae
Carrageenan, a sulfated polysaccharides of d-galactose and 3,6-anhydro-d-galactose extracted from red algae (Figure 2), has been widely used in use as a thickener in a variety of cosmetic and food products, ranging from sexual lubricants to infant feeding formulas [7]. Many researches indicated that carrageenans possess good inhibitory effects on different viruses such as HIV, HSV, and influenza virus [69,70,71,72,73], and they mainly interfere with virus adsorption or internalization into host cells [72]. Recently, Buck et al. [7], demonstrated that carrageenans particularly ι-carrageenans can inhibit HPV infection three orders magnitude more potent than heparin, and mainly block the initial infection process of HPV. Carrageenan acts primarily by preventing the binding of HPV virions to cells and blocks HPV infection through a second, post attachment heparin sulfate-independent effect. Moreover, some of milk-based products, which contain carrageenan, were also reported to be able to block HPV infection in vitro, even when diluted million-fold [7]. In addition, carrageenan has been reported to inhibit genital transmission of HPV in female mouse model of cervicovaginal [74,75].
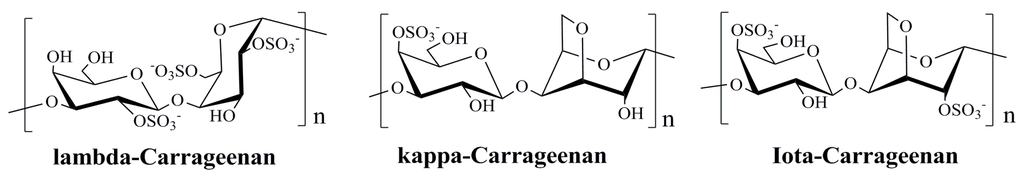
Figure 2.
Average structures of the repeat units of λ-, κ- and ι-carrageenans [76].
Figure 2.
Average structures of the repeat units of λ-, κ- and ι-carrageenans [76].
Based on these findings, carrageenan may be an alternative source of novel therapeutic candidate for HPV infection and some carrageenan-based sexual lubricant gels may be used to block the sexual transmission of HPV to some extend [7,74]. Moreover, there are some advantages of carrageenan over other classes of antiviral agents, such as low cytotoxicity, wide acceptability, and novel modes of action, which suggests that carrageenan merits further investigation as a promising anti-HPV agent in the future. However, most of the studies on anti-HPV effects of carrageenans have been observed in vitro or in mouse model systems, thus further studies with clinic trials are needed to determine whether carrageenan-based products are effective as topical microbicides against genital HPVs [7].
Furthermore, Buck et al. [7] reported that the sulfated polysaccharide agar derived from red algae could also effectively block HPV pseudovirion infection with the IC50 value of 0.27 µg/mL. In addition, carrageenan and its structurally related compounds such as dextran can also serve as adjuvants for enhancing peptide-based HPV vaccine potency [50]. Combined with the fact that iota carrageenans possess good anti-HPV activities in vitro and in vivo, we suppose that the sulfated galactose structure and the optimal sulfate content are very important for anti-HPV actions of carrageenans. In summary, the sulfated polysaccharides derived from red algae especially carrageenans merit further investigation as novel anti-HPV agents in the future.
3.3. Sulfated Polysaccharides from Brown Algae
It was reported that some sulfated polysaccharides from brown algae possess good antiviral and anti-tumor activities and some of them have been developed into novel antiviral agents [77,78,79,80,81]. Fucan is a term used to define a family of l-fucose-containing sulfated polysaccharides found in brown seaweed and the structures of these fucans vary among species and sometimes among different parts of the seaweed [82]. Some algal fucans exhibit important pharmacological activities such as anticoagulant [83], anti-inflammatory, antiviral [84], and antiproliferative [85]. It was found that the polysaccharide-rich extract from Sargassum filipendula C. Agardh showed significant anti-proliferative effect on HeLa cell (human uterine adenocarcinoma cell) proliferation [86]. In addition, a bioassay-guided fractionation of this extract led to the isolation of an antioxidant heterofucan denominated SF-1.5v, which exhibits good anti-proliferative activity against HeLa cells. However, the molecular mechanism underlying the heterofucan-induced anti-proliferative process remains unclear.
Moreover, Stevan et al. [87], reported that alginic acid isolated from the brown seaweed Laminaria brasiliensis (molar ratio M/G 1.2) and its M and G blocks (DP = 20) all could promote atypical mitoses in HeLa cells, besides the presence of acidophilic material in the cellular cytoplasm and occurrence of multinuclear cells present in the monolayer. In addition, the polysaccharide fraction SF isolated from the brown seaweed Sargassum stenophyllum containing mainly fucose could promote much accentuated morphologic modifications in HeLa cells at low concentrations (2.5 μg/mL) [87]. SF could cause significant alterations in the cellular morphology and reduction of cell growth in Hela cells in a dose dependent manner, which suggested this compound derived from brown algae could be used for treatment of HPV-related genital cancer in women in the future.
Furthermore, it was reported that fucoidan produced from brown algae could also inhibit HPV pesudovirus infection in vitro with the IC50 value of 1.1 µg/mL [7]. Although the anti-HPV effects of fucoidan are not as good as carrageenan (IC50 < 0.1 µg/mL), it also merits further investigation as novel anti-HPV or anti-cervical cancer candidate in the future. Taken together, brown algae-derived bioactive compounds, in particular the alginate polysaccharides and fucans, have good antiviral or anti-tumor activities, thus the sulfated polysaccharides from brown algae have the potential to become new resources for the development of anti-HPV and related cancer agents.
3.4. Agents from Marine Microbes
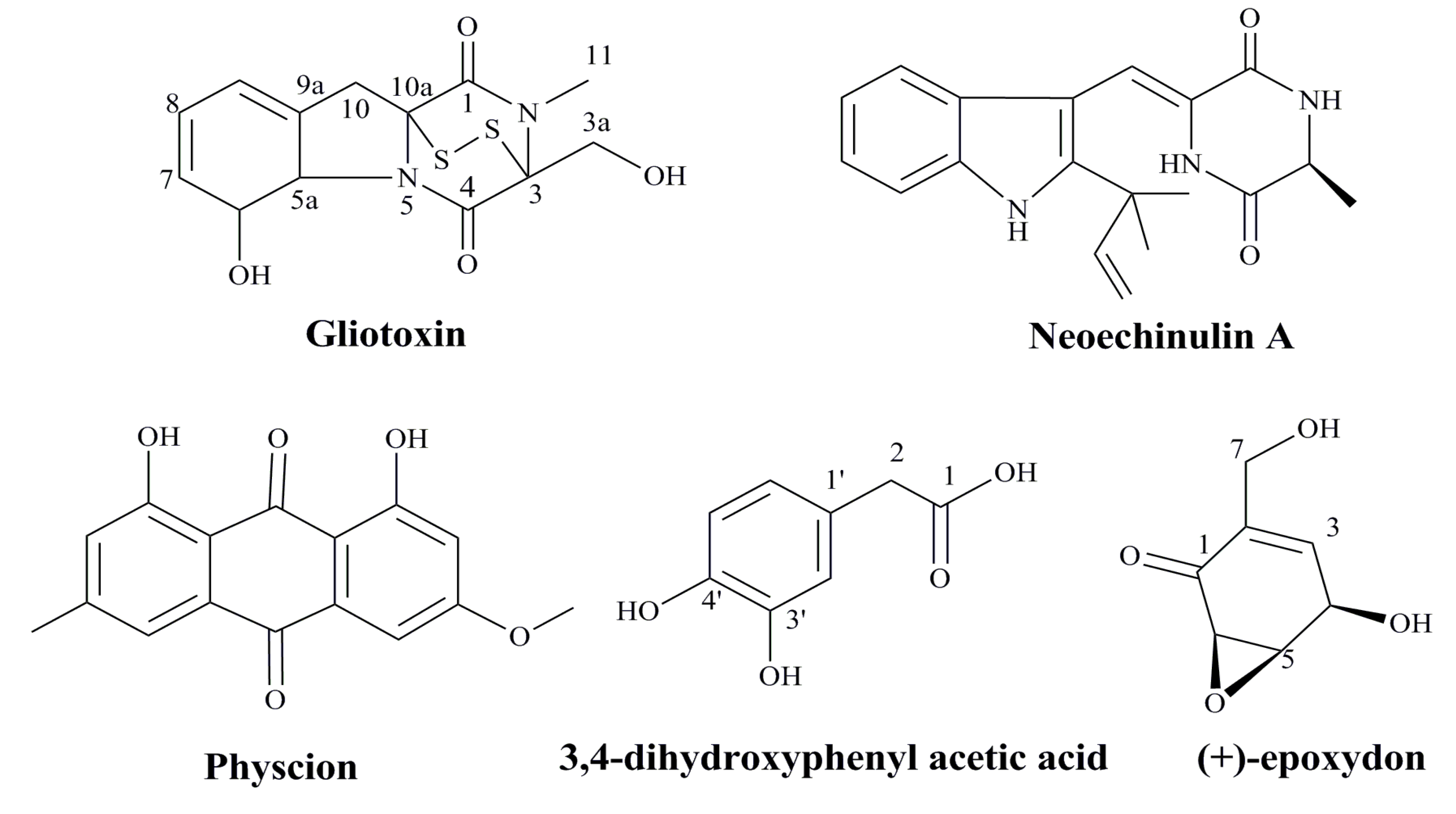
Marine-derived fungi have proven to be a promising source of bioactive metabolites and a growing number of marine fungi have been reported to produce bioactive secondary metabolites [88,89]. Aspergillus species are filamentous saprophytic fungi that can be found in almost all aerobic environments, which possessed antitumor, anti-inflammatory, antiviral and antibacterial activity [90]. Gliotoxin, one of the secondary metabolites produced by a number of Aspergillus, Gliocladium and Penicillium species, is a tricyclic alkaloid [91,92,93]. Gliotoxin effectively reduced the proliferation of HPV18 transformed Hela cells and could induce apoptotic cell death in association with the loss of mitochondrial membrane potential (MMP), and activation of Bax, caspase-3, caspase-8 and caspase-9, as well as suppression of Bcl-2 [94]. In a word, gliotoxin isolated from marine fungus Aspergillus sp. may induce apoptosis in HPV related cancer cells via the mitochondrial pathway followed by downstream events leading to apoptotic mode of cell death [94].
Marine microbes are valuable sources of structurally diverse bioactive compounds with anticancer activity. It was reported that a prenylated indole alkaloid, neoechinulin A, could be isolated from the culture broth extract of a marine-derived fungus, Microsporum sp. [95]. Neoechinulin A had good cytotoxic effect on human cervical carcinoma HeLa cells and it could induce cell apoptosis through down-regulating of Bcl-2 expression, up-regulating of Bax expression, and activating the caspase-3 pathway. Thus, neoechinulin A from marine-derived fungus merits further investigation as a potential candidate in the field of anticancer drug discovery against human cervical cancer [95]. In summary, some agents from marine microbes especially the bioactive metabolites have good inhibitory effects on the proliferation of HPV related cancer cells (Figure 3), which suggest these natural compounds can be developed into novel anti-HPV related cancer agents in the future.
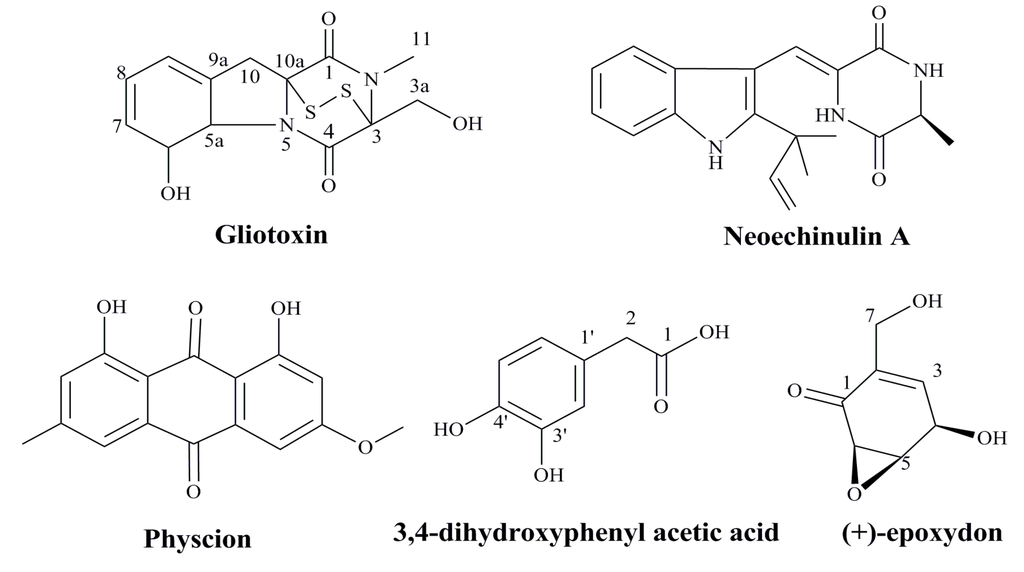
Figure 3.
The structures of anti-HPV related cancer agents from marine microbes [94,95,96,97].
Figure 3.
The structures of anti-HPV related cancer agents from marine microbes [94,95,96,97].
3.5. Bioactive Compounds from Marine Animals
Marine life is a treasure trove of natural medicine, and the polysaccharides isolated from a variety of marine animals have been shown to possess good pharmacological activities such as antitumor, anti-inflammatory, and antiviral activity. Marine japonicus polysaccharide isolated from Stichopus Japonicus is a class of natural polysaccharides, which is rich in marine coelenterata. Some studies reported that marine japonicus polysaccharide SJAMP could significantly inhibit the proliferation of human cervical carcinoma Hela cell in vitro [98]. SJAMP could reduce the abnormal expression of proliferating cell nuclear antigen (PCNA) and cell cycle inhibitor protein Mdm2 protein, and induce cell cycle arrest in G1 phase, thus plays a role in the inhibition of HeLa cell proliferation. In a word, marine japonicus polysaccharide can inhibit HPV related cancer in vitro through interfering with the cell cycle of cervical cancer cell [98].
Moreover, Clam (Meretrix meretrix Linnaeus) is one of the main beach cultured shellfishes in China. Clams possess many biological activities, such as anti-tumor, antiviral, and antioxidant effects [99]. There are a variety of active natural products in Clams, such as proteins, peptides, polysaccharides, nucleic acids and sterols, and some of them can significantly inhibit tumor growth in vitro and in vivo. Zhang et al. [100] reported that a low molecular weight polypeptide Mer2 isolated from the body of Clam could effectively inhibit the growth of Hela cells in a dose-and time-dependent manner. Mer2 could induce obvious cell morphology change and the apoptosis phenomenon, but without a significant cell cycle arrest, which suggest that Mer2 probably inhibits the proliferation of cervical cancer cells by induction of cell apoptosis [100].
Furthermore, chitosan, a partially deacetylated polymer of N-acetylglucosamine, is produced by deacetylation of chitin derived from the shells of crabs and shrimps, and has been reported to have good pharmacological properties such as antiviral activities [65]. Recently, some researchers reported that a chitosan cervical antimicrobial film which contains mainly chitosan and gelatin possessed good inhibitory effects on chronic cervicitis combined HPV infection, and the HPV negative conversion rates in chitosan treated group were superior to that in interferon α-2b gel treated group after three courses of treatment [101,102]. Moreover, the diethylaminoethyl chitosan was found to be able to induce apoptosis in human cervix cancer Hela cells via up-regulation of caspases, p53, and Bax expression, and down-regulation of Bcl-2 expression [103]. In summary, some bioactive compounds from marine animals such as polysaccharides and polypeptides possess good inhibitory effects on HPV and its related cancer, which suggest these natural compounds merit further investigation as novel anti-HPV and related cancer agents in the future. In order to summarize the data available in the literature, marine derived anti-HPV and related cancer agents described in this paper were all shown in Table 1.

Table 1.
Anti-HPV and related cancer agents from marine resources.
| Marine Organisms | Specific Compounds | Mechanisms of Action | References |
|---|---|---|---|
| Red Algae | λ-carrageenan | Blocking HPV infection | [7,104] |
| κ-carrageenan | Blocking HPV infection | [7,104] | |
| ι-carrageenan | Blocking HPV infection | [7,74,104] | |
| Agar | Blocking HPV infection | [7] | |
| Brown Algae | Alginic acid | Inhibiting HPV and cancer cell proliferation | [7,87] |
| Fucoidan | Blocking HPV infection | [7] | |
| Marine Fungus | Gliotoxin | Inducing apoptosis in cancer cells | [94] |
| Neoechinulin A | Inducing apoptosis in cancer cells | [95] | |
| Physcion | Inducing apoptosis in cancer cells | [96] | |
| (+)-epoxydon | Inducing apoptosis in cancer cells | [97] | |
| Echinoderm | japonicus polysaccharide | Inducing apoptosis in cancer cells | [98] |
| Shellfish | Clam polypeptide | Inducing apoptosis in cancer cells | [100] |
| Crustacean | Chitosan | Inhibiting HPV and cancer cell proliferation | [101,102,103] |
4. Prospects of Marine Derived Anti-HPV and Related Cancer Agents
HPV-caused cancer is a major health problem worldwide, especially in developing countries. The discovery of medicinal agents specifically capable of inhibiting HPV and related cancer is urgently required to prevent HPV infection. Marine derived bioactive compounds especially the heparinoid polysaccharides have similar pharmacological activities to the natural heparin, which can effectively block the entry process of HPV. Carrageenan-containing sexual lubricant gels were reported to be able to inhibit the infectivity of HPV16 pseudovirus in vitro, which suggests use of such carrageenan based gels may be able to block the sexual transmission of HPV [7]. Moreover, the anti-HPV related cancer agents produced by natural compounds often can not only target against the viral oncogenes but also inhibit the dysregulated gene expression of the host cells [14]. So natural products derived from marine organisms are excellent sources for the effective discovery of anti-HPV and related cancer agents.
However, the possibility that some genital HPVs might exhibit natural resistance to inhibition by carrageenan or other marine derived natural compounds would be an important factor to consider in the design of clinical efficacy trials [7]. In addition, despite having good anti-HPV activities, marine polysaccharides are structurally diverse and heterogeneous, which makes studies of their structures challenging, and may also have hindered their development as therapeutic agents to date [105]. Moreover, most of studies on anti-HPV activity of marine-derived HPV inhibitors have been observed in vitro or in mouse model systems until now. Therefore, further studies are needed to explore their activities in clinic in the future.
5. Conclusions
In summary, the anti-HPV and related cancer agents derived from marine organisms have the potential to treat both unapparent HPV infection as well as visible clinical diseases. Besides that, marine natural products especially polysaccharides and polypeptides have many advantages, such as relatively low cytotoxicity, low production costs, and wide acceptability, which suggest these compounds merit further investigation as novel drug candidates to reduce or regulate HPV infection related cancer [106,107]. Furthermore, the underlying molecular mechanisms of antiviral actions of marine-derived HPV inhibitors need to be elucidated clearly by intensive studies in the future [108].
Acknowledgments
This work was supported in part by the National Natural Science Foundation of China (81302811), Shandong Provincial Natural Science Foundation (ZR2011HQ012), the Special Fund for Marine Scientific Research in the Public Interest (201005024), and Qingdao science and technology development project (12-1-4-1-(20)-jch).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mammas, I.; Sourvinos, G.; Giannoudis, A.; Spandidos, D.A. Human papilloma virus (HPV) and host cellular interactions. Pathol. Oncol. Res. 2008, 14, 345–354. [Google Scholar] [CrossRef]
- Sanclemente, G.; Gill, D.K. Human papillomavirus molecular biology and pathogenesis. J. Eur. Acad. Dermatol. Venereol. 2002, 16, 231–240. [Google Scholar] [CrossRef]
- Zur, H.H. Papillomaviruses and cancer: From basic studies to clinical application. Nat. Rev. Cancer 2002, 2, 342–350. [Google Scholar] [CrossRef]
- Bosch, F.X.; Munoz, N. The viral etiology of cervical cancer. Virus Res. 2002, 89, 183–190. [Google Scholar] [CrossRef]
- Steben, M.; Duarte, F.E. Human papillomavirus infection: Epidemiology and pathophysiology. Gynecol. Oncol. 2007, 107, S2–S5. [Google Scholar] [CrossRef]
- Forman, D.; Martel, C.; Lacey, C.J.; Soerjomataram, I.; Lortet, T.J.; Bruni, L.; Vignat, J.; Ferlay, J.; Bray, F.; Plummer, M.; et al. Global burden of human papillomavirus and related diseases. Vaccine 2012, 30, F12–F23. [Google Scholar] [CrossRef]
- Buck, C.B.; Thompson, C.D.; Roberts, J.N.; Muller, M.; Lowy, D.R.; Schiller, J.T. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathog. 2006, 2, 69. [Google Scholar] [CrossRef]
- Lowy, D.R.; Schiller, J.T. Reducing HPV-associated cancer globally. Cancer Prev. Res. 2012, 5, 18–23. [Google Scholar] [CrossRef]
- Sidbury, R. What’s new in pediatric dermatology: Update for the pediatrician. Curr. Opin. Pediatr. 2004, 16, 410–414. [Google Scholar] [CrossRef]
- Syrjanen, S.; Puranen, M. Human papillomavirus infections in children: The potential role of maternal transmission. Crit. Rev. Oral. Biol. Med. 2002, 11, 259–274. [Google Scholar] [CrossRef]
- Manhart, L.E.; Koutsky, L.A. Do condoms prevent genital HPV infection, external genital warts, or cervical neoplasia: A meta-analysis. Sex Transm. Dis. 2002, 29, 725–735. [Google Scholar] [CrossRef]
- Holmes, K.K.; Levine, R.; Weaver, M. Effectiveness of condoms in preventing sexually transmitted infections. Bull. World Health Organ. 2004, 82, 454–461. [Google Scholar]
- Mao, C.; Koutsky, L.A.; Ault, K.A.; Wheeler, C.M.; Brown, D.R.; Wiley, D.J.; Alvarez, F.B.; Bautista, O.M.; Jansen, K.U.; Barr, E. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: A randomized controlled trial. Obstet. Gynecol. 2006, 107, 18–27. [Google Scholar] [CrossRef]
- Bharti, A.C.; Shirish, S.; Sutapa, M.; Suresh, H.; Bhudev, C.D. Anti-human papillomavirus therapeutics: Facts & future. Indian. J. Med. Res. 2009, 130, 296–310. [Google Scholar]
- Lauri, E.M.; Eileen, F.D.; Mona, S.; Herschel, W.L.; Harrell, C.; Elizabeth, R.U. Quadrivalent human Papillomavirus vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2007, 56, 1–24. [Google Scholar]
- Wang, Q.; Griffin, N.; Southern, S.; Jackson, D.; Martin, A. Functional analysis of the human Papillomavirus type 16 E1–E4 protein provides a mechanism for in vivo and in vitro keratin filament reorganization. J. Virol. 2004, 78, 821–833. [Google Scholar] [CrossRef]
- Munõz, N.; Bosch, F.X.; de Sanjosé, S.; Herrero, R.; Castellsagué, X.; Shah, K.V.; Snijders, P.J.; Meijer, C.J. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 2003, 348, 518–527. [Google Scholar] [CrossRef]
- Smith, J.S.; Lindsay, L.; Hoots, B.; Keys, J.; Franceschi, S.; Winer, R.; Clifford, G.M. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: A meta-analysis update. Int. J. Cancer 2007, 121, 621–632. [Google Scholar] [CrossRef]
- Patterson, N.A.; Smith, J.L.; Ozbun, M.A. Human papillomavirus type 31b infection of human keratinocytes does not require heparan sulfate. J. Virol. 2005, 79, 6838–6847. [Google Scholar] [CrossRef]
- Doorbar, J. Molecular biology of human papillomavirus infection and cervical cancer. Clin. Sci. 2006, 110, 525–541. [Google Scholar] [CrossRef]
- Day, P.M.; Lowy, D.R.; Schiller, J.T. Papillomaviruses infect cells via a clathrin-dependent pathway. Virology 2003, 307, 1–11. [Google Scholar] [CrossRef]
- Bousarghin, L.; Touzé, A.; Sizaret, P.-Y.; Coursaget, P. Human papillomavirus types 16, 31, and 58 use different endocytosis pathways to enter cells. J. Virol. 2003, 77, 3846–3850. [Google Scholar] [CrossRef]
- Thierry, F. Transcriptional regulation of the papillomavirus oncogenes by cellular and viral transcription factors in cervical carcinoma. Virology 2009, 384, 375–379. [Google Scholar] [CrossRef]
- Faridi, R.; Zahra, A.; Khan, K.; Idrees, M. Oncogenic potential of Human Papillomavirus (HPV) and its relation with cervical cancer. Virol. J. 2011, 8, 269–277. [Google Scholar] [CrossRef]
- Disbrow, G.L.; Hanover, J.A.; Schlegel, R. Endoplasmic reticulum-localized human Papillomavirus type 16 E5 protein alters endosomal pH but not trans-Golgi pH. J. Virol. 2005, 79, 5839–5846. [Google Scholar] [CrossRef]
- Shai, A.; Brake, T.; Somoza, C.; Lambert, P.F. The human Papillomavirus E6 oncogene dysregulates the cell cycle and contributes to cervical carcinogenesis through two independent activities. Cancer Res. 2007, 67, 1626–1635. [Google Scholar] [CrossRef]
- Snijders, P.J.; van Duin, M.; Walboomers, J.M.; Steenbergen, R.D.; Risse, E.K.; Helmerhorst, T.J.; Verheijen, R.H.; Meijer, C.J. Telomerase activity exclusively in cervical carcinomas and a subset of cervical intraepithelial neoplasia grade III lesions: Strong association with elevated messenger RNA levels of its catalytic subunit and high-risk human Papillomavirus DNA. Cancer Res. 1998, 58, 3812–3818. [Google Scholar]
- Horikawa, I.; Barrett, J.C. Transcriptional regulation of the telomerase hTERT gene as a target for cellular and viral oncogenic mechanisms. Carcinogenesis 2003, 24, 1167–1178. [Google Scholar] [CrossRef]
- Fausch, S.C.; Da, S.D.M.; Kast, W.M. Heterologous papillomavirus virus-like particles and human papillomavirus virus-like particle immune complexes activate human Langerhans cells. Vaccine 2005, 23, 1720–1729. [Google Scholar] [CrossRef]
- Pagliusi, S.R.; Teresa, A.M. Efficacy and other milestones for human papillomavirus vaccine introduction. Vaccine 2004, 23, 569–578. [Google Scholar] [CrossRef]
- Rose, R.C.; White, W.I.; Li, M.; Suzich, J.A.; Lane, C.; Garcea, R.L. Human papillomavirus type 11 recombinant capsomeres induce virus-neutralizing antibodies. J. Virol. 1998, 72, 6151–6154. [Google Scholar]
- White, W.I.; Wilson, S.D.; Frances, J.P.H.; Robert, M.W.; Ghim, S.J.; Lisa, A.H.; Daniel, M.G.; Steven, J.B.; A. Bennett, J.; Scott, K.; et al. Characterization of a major neutralizing epitope on human papillomavirus type 16 L1. J. Virol. 1999, 73, 4882–4889. [Google Scholar]
- Huh, W.K.; Roden, R.B.S. The future of vaccines for cervical cancer. Gynecol. Oncol. 2008, 109, S48–S56. [Google Scholar] [CrossRef]
- Paavonen, J.; Naud, P.; Salmerón, J.; Wheeler, C.M.; Chow, S.N.; Apter, D.K.H.; Castellsague, X.; Teixeira, J.C.; Skinner, S.R. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): Final analysis of a double-blind, randomised study in young women. Lancet 2009, 374, 301–314. [Google Scholar] [CrossRef]
- Munõz, N.; Kjaer, S.K.; Sigurdsson, K.; Iversen, O.E.; Hernandez, A.; MWheeler, C.M.; Perez, G.; Brown, D.R.; Koutsky, L.A.; Tay, E.H. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-assocated genital diseases in young women. J. Natl. Cancer Inst. 2010, 102, 325–339. [Google Scholar] [CrossRef]
- Steenbergen, R.D.; De, W.J.; Wilting, S.M.; Brink, A.A.; Snijders, P.J.; Meijer, C.J. HPV-mediated transformation of the anogenital tract. J. Clin. Virol. 2005, 32, 25–33. [Google Scholar] [CrossRef]
- Borysiewicz, L.K.; Fiander, A.; Nimako, M.; Man, S.; Wilkinson, G.W.; Westmoreland, D. A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet 1996, 347, 1523–1527. [Google Scholar] [CrossRef]
- Greenstone, H.L.; Nieland, J.D.; Visser, K.E.; Bruijn, M.L.; Kirnbauer, R.; Roden, R.B. Chimeric papillomavirus virus-like particles elicit antitumor immunity against the E7 oncoprotein in an HPV16 tumor model. Proc. Natl. Acad. Sci. USA 1998, 95, 1800–1805. [Google Scholar]
- Muderspach, L.; Wilczynski, S.; Roman, L.; Bade, L.; Felix, J.; Small, L.A. A phase I trial of a human papillomavirus (HPV) peptide vaccine for women with high-grade cervical and vulvar intraepithelial neoplasia who are HPV 16 positive. Clin. Cancer Res. 2000, 6, 3406–3416. [Google Scholar]
- De Jong, A.; O’Neill, T.; Khan, A.Y.; Kwappenberg, K.M.C.; Chisholm, S.E.; Whittle, N.R.; Dobson, J.A.; Jack, L.C.; Roberts, J.A.S.C.; Offringa, R.; et al. Enhancement of human papillomavirus (HPV) type 16 E6 and E7-specific T-cell immunity in healthy volunteers through vaccination with TA-CIN, an HPV16 L2E7E6 fusion protein vaccine. Vaccine 2002, 20, 3456–3464. [Google Scholar] [CrossRef]
- Frazer, I.H.; Quinn, M.; Nicklin, J.L.; Tan, J.; Perrin, L.C.; Ng, P. Phase 1 study of HPV16-specific immunotherapy with E6E7 fusion protein and ISCOMATRIX adjuvant in women with cervical intraepithelial neoplasia. Vaccine 2004, 23, 172–181. [Google Scholar] [CrossRef]
- Kaufmann, A.M.; Nieland, J.D.; Jochmus, I.; Baur, S.; Friese, K.; Gabelsberger, J. Vaccination trial with HPV16 L1E7 chimeric virus-like particles in women suffering from high grade cervical intraepithelial neoplasia (CIN 2/3). Int. J. Cancer 2007, 121, 2794–2800. [Google Scholar] [CrossRef]
- Santin, A.D.; Bellone, S.; Palmieri, M.; Zanolini, A.; Ravaggi, A.; Siegel, E.R. Human papillomavirus type 16 and 18 E7-pulsed dendritic cell vaccination of stage IB or IIA cervical cancer patients: A phase I escalating-dose trial. J. Virol. 2008, 8, 1968–1979. [Google Scholar]
- Welters, M.J.; Kenter, G.G.; Piersma, S.J.; Vloon, A.P.; Lowik, M.J.; Berends-van der, M.D.M.; Drijfhout, J.W.; Valentijn, A.R.; Wafelman, A.R.; Oostendorp, J. Induction of tumor-specific CD4+ and CD8+ T-cell immunity in cervical cancer patients by a human papillomavirus type 16 E6 and E7 long peptides vaccine. Clin. Cancer Res. 2008, 14, 178–187. [Google Scholar] [CrossRef]
- Klencke, B.; Matijevic, M.; Urban, R.G.; Lathey, J.L.; Hedley, M.L.; Berry, M. Encapsulated plasmid DNA treatment for human papillomavirus 16-associated anal dysplasia: A Phase I study of ZYC101. Clin. Cancer Res. 2002, 8, 1028–1037. [Google Scholar]
- Sheets, E.E.; Urban, R.G.; Crum, C.P.; Hedley, M.L.; Politch, J.A.; Gold, M.A. Immunotherapy of human cervical high-grade cervical intraepithelial neoplasia with microparticle-delivered human papillomavirus 16 E7 plasmid DNA. Am. J. Obstet. Gynecol. 2003, 188, 916–926. [Google Scholar] [CrossRef]
- Peng, S.; Trimble, C.; Alvarez, R.D.; Huh, W.K.; Lin, Z.; Monie, A. Cluster intradermal DNA vaccination rapidly induces E7-specific CD8+ T-cell immune responses leading to therapeutic antitumor effects. Gene Ther. 2008, 15, 1156–1166. [Google Scholar] [CrossRef]
- Stern, P.L. Immune control of human papillomavirus (HPV) associated anogenital disease and potential for vaccination. J. Clin. Virol. 2005, 32, 72–81. [Google Scholar] [CrossRef]
- Adams, M.; Navabi, H.; Jasani, B.; Man, S.; Fiander, A.; Evans, A.S. Dendritic cell (DC) based therapy for cervical cancer: use of DC pulsed with tumour lysate and matured with a novel synthetic clinically non-toxic double stranded RNA analogue poly [I]:poly[C(12)U] (Ampligen R). Vaccine 2003, 21, 787–790. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Tsai, Y.C.; Monie, A.; Hung, C.F.; Wu, T.C. Carrageenan as an adjuvant to enhance peptide-based vaccine potency. Vaccine 2010, 28, 5212–5219. [Google Scholar] [CrossRef]
- Greenfield, I.; Cuthill, S. Antivirals. Clinical and Scientific Advances. In Human Papillomaviruses; Sterling, J.C., Tyring, S.K., Eds.; Arnorld: London, UK, 2001; pp. 120–130. [Google Scholar]
- Cirelli, R.; Tyring, S.K. Interferons in human papillomavirus infections. Antivir. Res. 1994, 24, 191–204. [Google Scholar] [CrossRef]
- Li, J.; Luo, K.Z.; Lin, Y.; Chen, W.N.; Lai, S.P. Laboratory study on anti-human papillomavirus activity of Bupleurum chinese. Chin. J. Dermato. Venerol. Integ. Trad. W. Med. 2005, 4, 171–173. (In Chinese) [Google Scholar]
- Xiao, J.; Wu, J.; Yu, B. Therapeutic efficacy of Youdujing preparation in treating cervical high-risk papilloma virus infection patients. Zhongguo Zhong Xi Yi Jie He Za Zhi 2012, 32, 1212–1215. (In Chinese) [Google Scholar]
- Huang, G.Q. Clinical observation of paiteling on treatment of cervicitis combined with high-risk HPV infection. Chin. J. Woman Child Health Res. 2012, 23, 675–677. (In Chinese) [Google Scholar]
- Song, X.X.; Liu, Y.L.; Hao, Q.Y. Xinfuning combined with Baofukang suppository for 53 cases with cervical high risk human papillomavirus infection. J. Oncol. 2011, 17, 825–827. [Google Scholar]
- Johnson, K.M.; Kines, R.C.; Roberts, J.N.; Lowy, D.R.; Schiller, J.T.; Day, P.M. Role of heparan sulfate in attachment to and infection of the murine female genital tract by human papillomavirus. J. Virol. 2009, 83, 2067–2074. [Google Scholar] [CrossRef]
- Joyce, J.G.; Tung, J.S.; Przysiecki, C.T.; Cook, J.C.; Lehman, E.D.; Sands, J.A.; Jansen, K.U.; Keller, P.M. The L1 major capsid protein of human papillomavirus type 11 recombinant virus-like particles interacts with heparin and cell-surface glycosaminoglycans on human keratinocytes. J. Biol. Chem. 1999, 274, 5810–5822. [Google Scholar] [CrossRef]
- Giroglou, T.; Florin, L.; Schafer, F.; Streeck, R.E.; Sapp, M. Human papillomavirus infection requires cell surface heparan sulfate. J. Virol. 2001, 75, 1565–1570. [Google Scholar] [CrossRef]
- Drobni, P.; Mistry, N.N.; Millan, M. Carboxy-fluorescein diacetate, succinimidyl ester labeled papillomavirus virus like particles fluoresce after internalization and interact with heparin sulfate for binding and entry. Virology 2003, 310, 163–172. [Google Scholar] [CrossRef]
- Hans, C.S.; Luise, F.; Hetal, D. Inhibition of transfer to secondary receptors by heparan sulfate-binding drug or antibody induces noninfectious uptake of human papillomavirus. J. Virol. 2007, 81, 10970–10980. [Google Scholar] [CrossRef]
- Rommel, O.; Dillner, J.; Fligge, C. Heparan sulfate proteoglycans interact exclusively with conformationally intact HPV L1 assemblies: Basis for a virus-like particle ELISA. J. Med. Virol. 2005, 75, 114–121. [Google Scholar] [CrossRef]
- Saeed, S.K.; Alessandra, H.; Ernst, K.; Guerrino, M.; Katharina, S.; Reinhard, K. Different heparin sulfate proteoglycans serve as cellular receptors for human papillomaviruses. J. Virol. 2003, 77, 13125–13135. [Google Scholar] [CrossRef]
- Malgorzata, B.H.; Patel, H.D.; Sapp, M. Target cell cyclophilins facilitate human papilloma-virus type 16 infection. PLoS Pathog. 2009, 5, e1000524. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.X.; Guan, H.S. The antiviral activities and mechanisms of marine polysaccharides: An overview. Mar. Drugs 2012, 10, 2795–2816. [Google Scholar] [CrossRef]
- Christensen, N.D.; Reed, C.A.; Culp, T.D.; Hermonat, P.L.; Howett, M.K. Papillomavirus microbicidal activities of high-molecular-weight cellulose sulfate, dextran sulfate, and polystyrene sulfonate. Antimicrob. Agents Chemother. 2001, 45, 3427–3432. [Google Scholar] [CrossRef]
- Esko, J.D.; Selleck, S.B. Order out of chaos: Assembly of ligand binding sites in heparan sulfate. Annu. Rev. Biochem. 2002, 71, 435–471. [Google Scholar] [CrossRef]
- Lembo, D.; Donalisio, M.; Rusnati, M.; Bugatti, A.; Cornaglia, M.; Cappello, P.; Giovarelli, M.; Oreste, P.; Landolfo, S. Sulfated K5 Escherichia coli polysaccharide derivatives as wide-range inhibitors of genital types of human papillomavirus. Antimicrob. Agents Chemother. 2008, 52, 1374–1381. [Google Scholar] [CrossRef]
- Carlucci, M.J.; Scolaro, L.A.; Noseda, M.D.; Cerezo, A.S.; Damonte, E.B. Protective effect of a natural carrageenan on genital herpes simplex virus infection in mice. Antivir. Res. 2004, 64, 137–141. [Google Scholar] [CrossRef]
- Andreas, L.; Christiane, M.; Marielle, K.S.; Regina, W.; Donata, K.; Bettina, P.; Philipp, G.; Britta, F.G.; Martin, B.; Tamas, F.; et al. Iota-Carrageenan is a potent inhibitor of rhinovirus infection. Virology 2008, 5, 107. [Google Scholar] [CrossRef]
- Pujol, C.A.; Scolaro, L.A.; Ciancia, M.; Matulewicz, M.C.; Cerezo, A.S.; Damonte, E.B. Antiviral activity of a carrageenan from Gigartinatina skottsbergii against intraperitoneal murine Herpes Simplex virus infection. Planta Med. 2006, 72, 121–125. [Google Scholar] [CrossRef]
- Talarico, L.B.; Damonte, E.B. Interference in dengue virus adsorption and uncoating by carrageenans. Virology 2007, 363, 473–485. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, P.; Hao, C.; Zhang, X.E.; Cui, Z.Q.; Guan, H.S. In vitro inhibitory effect of carrageenan oligosaccharide on influenza A H1N1 virus. Antivir. Res. 2011, 92, 237–246. [Google Scholar] [CrossRef]
- Roberts, J.N.; Buck, C.B.; Thompson, C.D.; Kines, R.; Bernardo, M.; Choyke, P.L.; Lowy, D.R.; Schiller, J.T. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat. Med. 2007, 13, 857–861. [Google Scholar] [CrossRef]
- Schiller, T.S.; Davies, P. Delivering on the promise: HPV vaccines and cervical cancer. Nat. Rev. Microbiol. 2004, 2, 343–347. [Google Scholar] [CrossRef]
- Van de Velde, F.; Knutsen, S.H.; Usov, A.I.; Rollema, H.S.; Cerezo, A.S. 1H and 13C high resolution NMR spectroscopy of carrageenans: Application in research and industry. Trends Food Sci. Technol. 2002, 13, 73–92. [Google Scholar] [CrossRef]
- Xin, X.L.; Geng, M.Y.; Guan, H.S.; Li, Z.L. Study on the mechanism of inhibitory action of 911 on replication of HIV-1 in vitro. Chin. J. Mar. Drugs 2000, 19, 15–18. (In Chinese) [Google Scholar]
- Xin, X.L.; Ding, H.; Geng, M.Y.; Liang, P.F.; Li, Y.X.; Guan, H.S. Studies of the anti-AIDS effects of marine polysaccharide drug 911 and its related mechanisms of action. Chin. J. Mar. Drugs 2000, 6, 4–8. (In Chinese) [Google Scholar]
- Geng, M.Y.; Li, F.C.; Xin, X.L.; Li, J.; Yan, Z.W.; Guan, H.S. The potential molecular targets of marine sulfated polymannuroguluronate interfering with HIV-1 entry Interaction between SPMG and HIV-1 gp120 and CD4 molecule. Antivir. Res. 2003, 59, 127–135. [Google Scholar] [CrossRef]
- Miao, B.C.; Geng, M.Y.; Li, J.; Li, F.; Chen, H.; Guan, H.S.; Ding, J. Sulfated polymannuroguluronate, a novel anti-acquired immune deficiency syndrome (AIDS) drug candidate, targeting CD4 in lymphocytes. Biochem. Pharmacol. 2004, 68, 641–649. [Google Scholar] [CrossRef]
- Liu, H.Y.; Geng, M.Y.; Xin, X.L.; Li, F.C.; Chen, H.X.; Guan, H.S.; Ding, J. Multiple and multivalent interactions of novel anti-AIDS drug candidates, sulfated polymannuronate (SPMG)-derived oligosaccharides, with gp120 and their anti-HIV activities. Glycobiology 2005, 15, 501–510. [Google Scholar]
- Dietrich, C.P.; Farias, G.G.M.; Abreu, L.R.; Leite, E.L.; Silva, L.F.; Nader, H.B. A new approach for the characterization of polysaccharides from algae: Presence of four main acidic polysaccharides in three species of the class Phaeophycea. Plant Sci. 1995, 108, 143–153. [Google Scholar] [CrossRef]
- Albuquerque, I.R.L.; Queiroz, K.C.S.; Alves, L.G.; Santos, E.A.; Leite, E.L.; Rocha, H.A.O. Heterofucans from Dictyota menstrualis have anticoagulant activity. Braz. J. Med. Biol. Res. 2004, 37, 167–171. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and Bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef]
- Synytsya, A.; Kim, W.J.; Kim, S.M.; Pohl, R.; Synytsya, A.; Kvasnička, F.; Čopíková, J.; Park, Y.L. Structure and antitumour activity of fucoidan isolated from sporophyll of Korean brown seaweed Undaria pinnatifida. Carbohydr. Polym. 2010, 81, 41–48. [Google Scholar] [CrossRef]
- Costa, L.S.; Fidelis, G.P.; Cordeiro, S.L.; Oliveira, R.M.; Sabry, D.A; Câmara, R.B.G.; Nobre, L.T.D.B.; Costa, M.S.S.P.; Jailma, A.L.; Farias, E.H.C.; et al. Biological activities of sulfated polysaccharides from tropical seaweeds. Biomed. Pharmacother. 2010, 64, 21–28. [Google Scholar] [CrossRef]
- Stevan, F.R.; Oliveira, M.B.; Bucchi, D.F.; Noseda; Iacomini, M.; Duarte, M.E. Cytotoxic effects against HeLa cells of polysaccharides from seaweeds. J. Submicrosc. Cytol. Pathol. 2001, 33, 477–484. [Google Scholar]
- Shao, C.L.; Wang, C.Y.; Wei, M.Y.; Gu, Y.C.; She, Z.G.; Qian, P.Y.; Lin, Y.C. Aspergilones A and B, two benzylazaphilones with an unprecedented carbon skeleton from the gorgonian-derived fungus Aspergillus sp. Bioorg. Med. Chem. Lett. 2011, 21, 690–693. [Google Scholar] [CrossRef]
- Wei, M.Y.; Wang, C.Y.; Liu, Q.A.; Shao, C.L.; She, Z.G.; Lin, Y.C. Five sesquiterpenoids from a marine-derived fungus Aspergillus sp. isolated from a Gorgonian Dichotella gemmacea. Mar. Drugs 2010, 8, 941–949. [Google Scholar] [CrossRef]
- Lee, D.S.; Jeong, G.S.; Li, B.; Lee, S.U.; Oh, H.C.; Kim, Y.C. Asperlin from the marine-derived fungus Aspergillus sp. SF-5044 exerts antiinflammatory effects through heme oxygenase-1 expression in murine macrophages. J. Pharmacol. Sci. 2011, 116, 283–295. [Google Scholar] [CrossRef]
- Axelsson, V.; Holback, S.; Sjogren, M.; Gustafsson, H.; Forsby, A. Gliotoxin induces caspase-dependent neurite degeneration and calpain-mediated general cytotoxicity in differentiated human neuroblastoma SH-SY5Y cells. Biochem. Biophys. Res. Commun. 2006, 345, 1068–1074. [Google Scholar] [CrossRef]
- Comera, C.; Andre, K.; Laffitte, J.; Collet, X.; Galtier, P.; Isabelle, M.P. Gliotoxin from Aspergillus fumigatus affects phagocytosis and the organization of the actin cytoskeleton by distinct signalling pathways in human neutrophils. Microbes. Infect. 2007, 94, 47–54. [Google Scholar]
- Nieminen, S.M.; Jorm, M.P.; Hirvonen, M.R.; Roponen, M.; Wright, A.V. Genotoxicity of gliotoxin, a secondary metabolite of Aspergillus fumigatus, in a battery of short-term test systems. Mutat. Res. 2002, 520, 161–170. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Lee, J.S.; Qian, Z.J.; Li, Y.X.; Kim, K.N.; Heo, S.J.; Jeon, Y.J.; Park, W.S.; Choi, I.W.; Je, J.Y.; et al. Gliotoxin isolated from marine fungus Aspergillus sp. induces apoptosis of human cervical cancer and chondrosarcoma cells. Mar. Drugs 2014, 12, 69–87. [Google Scholar]
- Isuru, W.; Li, Y.X.; Vo, T.S.; Quang, V.T.; Ngo, D.H.; Kim, S.K. Induction of apoptosis in human cervical carcinoma HeLa cells by neoechinulin A from marine-derived fungus Microsporum sp. Process Biochem. 2013, 48, 68–72. [Google Scholar] [CrossRef]
- Isuru, W.; Zhang, C.; Van Ta, Q.; Vo, T.S.; Li, Y.X.; Kim, S.K. Physcion from marine-derived fungus Microsporum sp. induces apoptosis in human cervical carcinoma HeLa cells. Microbiol. Res. 2014, 169, 255–261. [Google Scholar] [CrossRef]
- Jo, M.J.; Bae, S.J.; Son, B.W.; Kim, C.Y.; Kim, G.D. 3,4-dihydroxyphenyl acetic acid and (+) epoxydon isolated from marine algae-derived microorganisms induce down regulation of epidermal growth factor activated mitogenic signaling cascade in Hela cells. Cancer Cell Int. 2013, 13, 49. [Google Scholar] [CrossRef]
- Niu, J.J.; Song, Y. Effect of stichopus Japonicus acidic mucopolysaccharide on cell cycle of Hela cells and its mechanism. Med. J. Qilu 2010, 25, 386–388. (In Chinese) [Google Scholar]
- Wei, N.; Lin, X.K.; Niu, R.L.; Li, H.Y. Overview on anticancer agents from Meretrix meretrix. Food Drug 2007, 9, 63–65. (In Chinese) [Google Scholar]
- Zhang, J.; Kang, J.H.; Liu, F.J.; Fan, C.C.; Li, H.L.; Chen, Q.X. Effect of the polypeptides from Meretrix meretrix Linnaeus on proliferation of cervical cancer Hela cells. J. Xiamen Univ. 2009, 48, 729–732. (In Chinese) [Google Scholar]
- Huang, X.Y.; He, W.M.; Liao, Q.X.; Ruan, G.Y. Clinical nursing observation of the treatment of chronic cervicitis combined HPV infection using chitosan antimicrobial film. China Pract. Med. 2013, 8, 196–198. (In Chinese) [Google Scholar]
- Tong, Y.P.; Shen, S.H.; Zheng, W. Clinical application of chitosan nano-iodine for treatment of cervical CIN and HPV infection. J. Chin. Phys. 2013, 15, 1411–1413. (In Chinese) [Google Scholar]
- Lee, S.H.; Ryu, B.; Je, J.-Y.; Kim, S.K. Diethylaminoethyl chitosan induces apoptosis in HeLa cells via activation of caspase-3 and p53 expression. Carbohydr. Polym. 2011, 84, 571–578. [Google Scholar] [CrossRef]
- Roberts, J.N.; Kines, R.C.; Katki, H.A.; Lowy, D.R.; Schiller, J.T. Effect of Pap smear collection and carrageenan on cervicovaginal human papillomavirus-16 infection in a rhesus macaque model. J. Natl. Cancer Inst. 2011, 103, 737–743. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, S.H. Chemical structures and bioactivities of sulphated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef]
- Ghosh, T.; Chattopadhyay, K.; Marschall, M.; Karmakar, P.; Mandal, P.; Ray, B. Focus on antivirally active sulfated polysaccharides: From structure-activity analysis to clinical evaluation. Glycobiology 2009, 19, 2–15. [Google Scholar]
- Vo, T.S.; Kim, S.K. Potential anti-HIV agents from marine resources: An Overview. Mar. Drugs 2010, 8, 2871–2892. [Google Scholar] [CrossRef]
- Patel, S. Therapeutic importance of sulfated polysaccharides from seaweeds: Updating the recent findings. 3 Biotech 2012, 2, 171–185. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
