Fucoidan Extracted from Fucus evanescens Prevents Endotoxin-Induced Damage in a Mouse Model of Endotoxemia
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of Fucoidan on Survival Times in Mouse Model of Endotoxemia
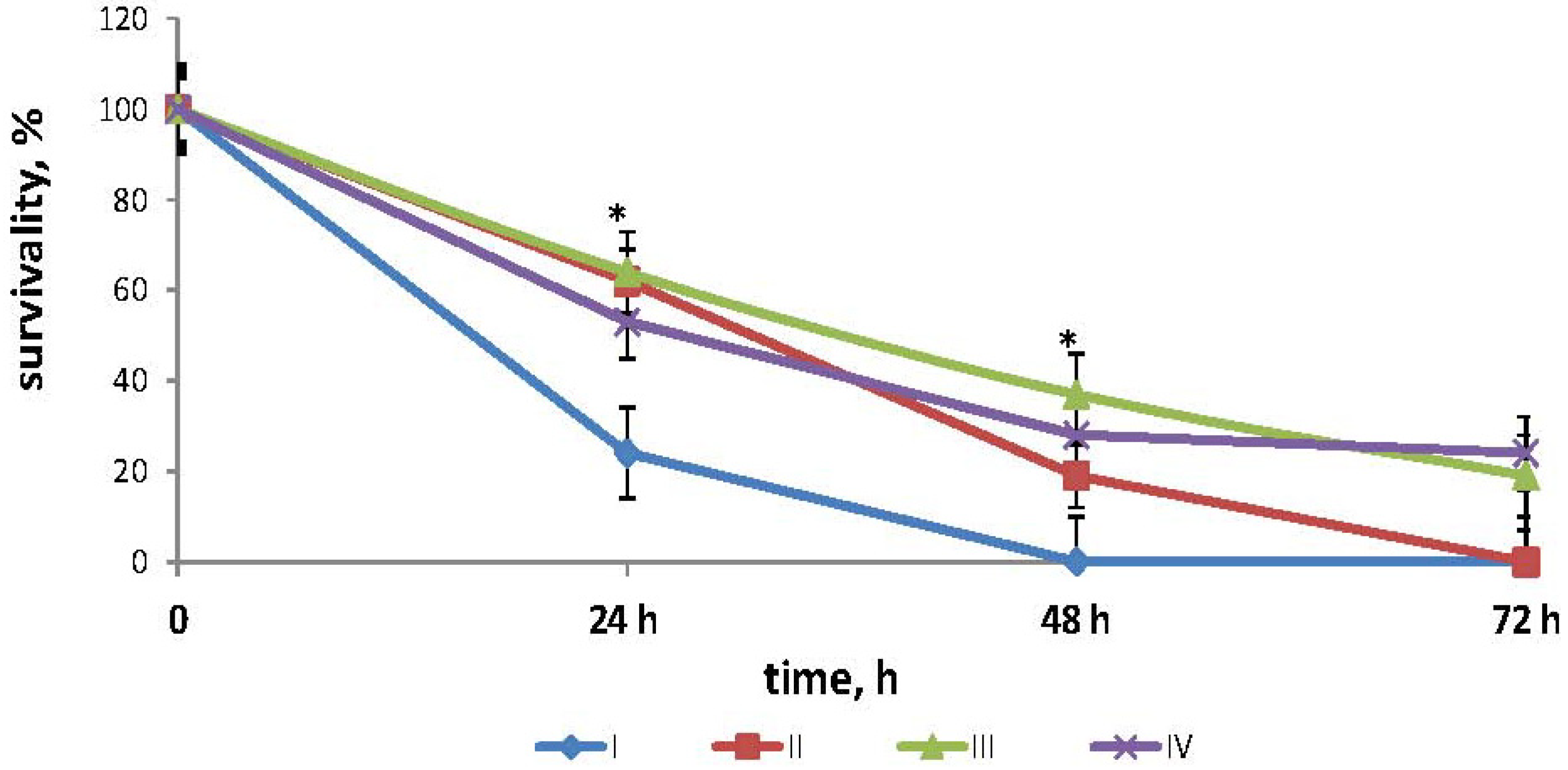
2.2. Effects of Fucoidan on Cytokines Production in Mouse Model of Endotoxemia
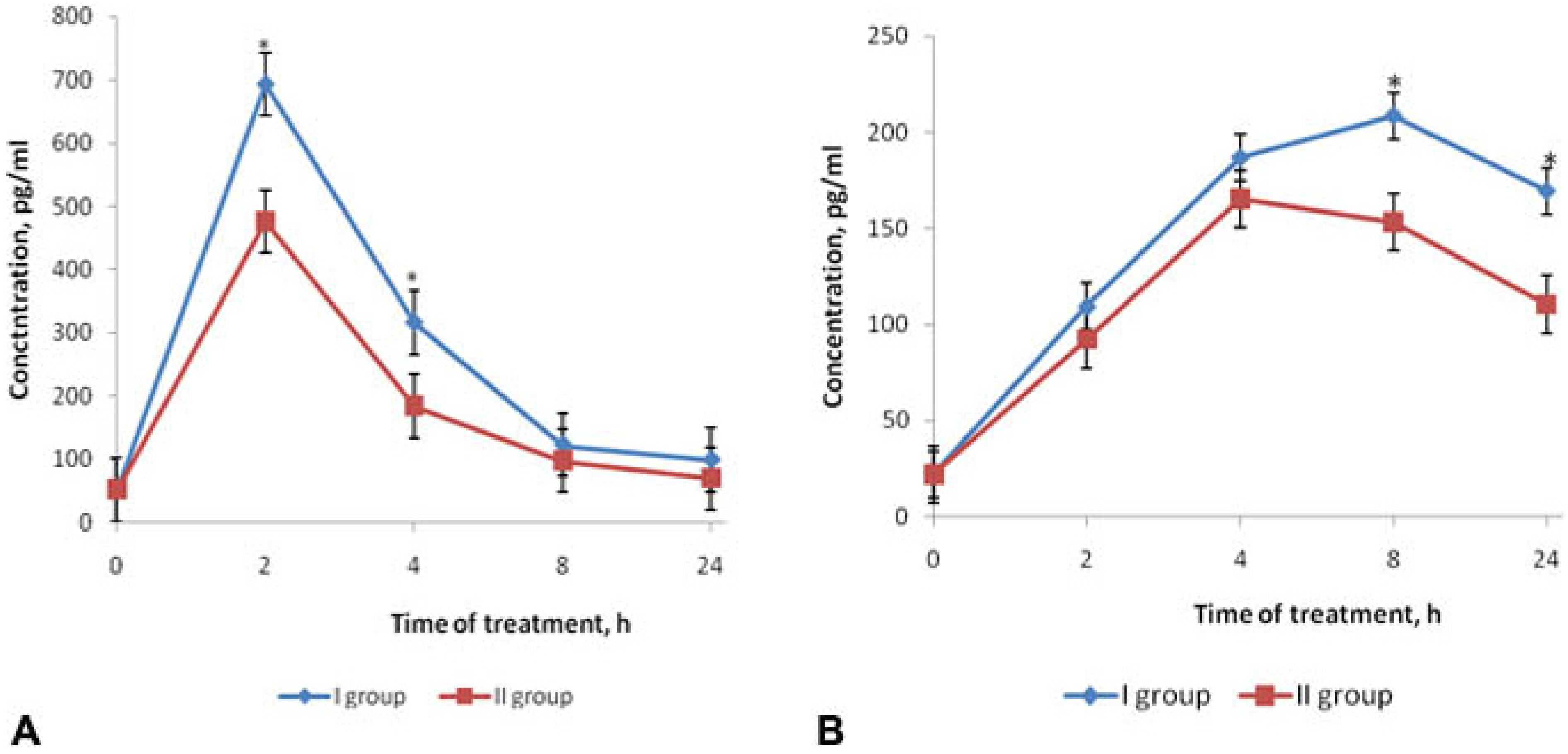
2.3. Effects of Fucoidan on Hemostasis Parameters in Mouse Model of Endotoxemia
| Hemostasis parameters | Group of mice | |||
|---|---|---|---|---|
| LPS (group I) | Fucoidan (group II) | Fucoidan (group III) | Control (0.85% NaCl) (group IV) | |
| APTT (s) | 25.0 ± 3.9 | 38.5 ± 3.9 | 75.6 ± 13.5 | 47.4 ± 5.7 p (IV–I) < 0.05 |
| p (II–I) < 0.05 | p (III–I) < 0.05 | |||
| p (II–IV) > 0.05 | p (III–IV) < 0.05 | |||
| PT (s) | 11.6 ± 1.8 | 14.0 ± 1.8 | 23.2 ± 4.8 | 16.8 ± 5.3 p (IV–I) > 0.05 |
| p (II–I) > 0.05 | p (III–I) < 0.05 | |||
| p (II–IV) > 0.05 | p (III–IV) >0.05 | |||
| TT (s) | 12.8 ± 0.8 | 15.7 ± 2.0 | 67.8 ± 9.8 | 18.6 ± 1.2 p (IV–I) < 0.05 |
| p (II–I) < 0.05 | p (III–I) < 0.05 | |||
| p (II–IV) < 0.05 | p (III–IV) < 0.05 | |||
| FA (min) | 533 ± 56 | 430 ± 58 | 350 ± 61 | 310 ± 65 p (IV–I) < 0.05 |
| p (II–I) < 0.05 | p (III–I) < 0.05 | |||
| p (II–IV) < 0.05 | p (III–IV) > 0.05 | |||
| FG (g/L) | 6.1 ± 0.9 | 4.8 ± 0.45 | 4.4 ± 0.42 | 4.1 ± 0.52 p (IV–I) < 0.05 |
| p (II–I) < 0.05 | p (III–I) < 0.05 | |||
| p (II–IV) > 0.05 | p (III–IV) > 0.05 | |||
2.4. Effects of Fucoidan on Histopathological Changes in Target Organs of Mice during Endotoxemia
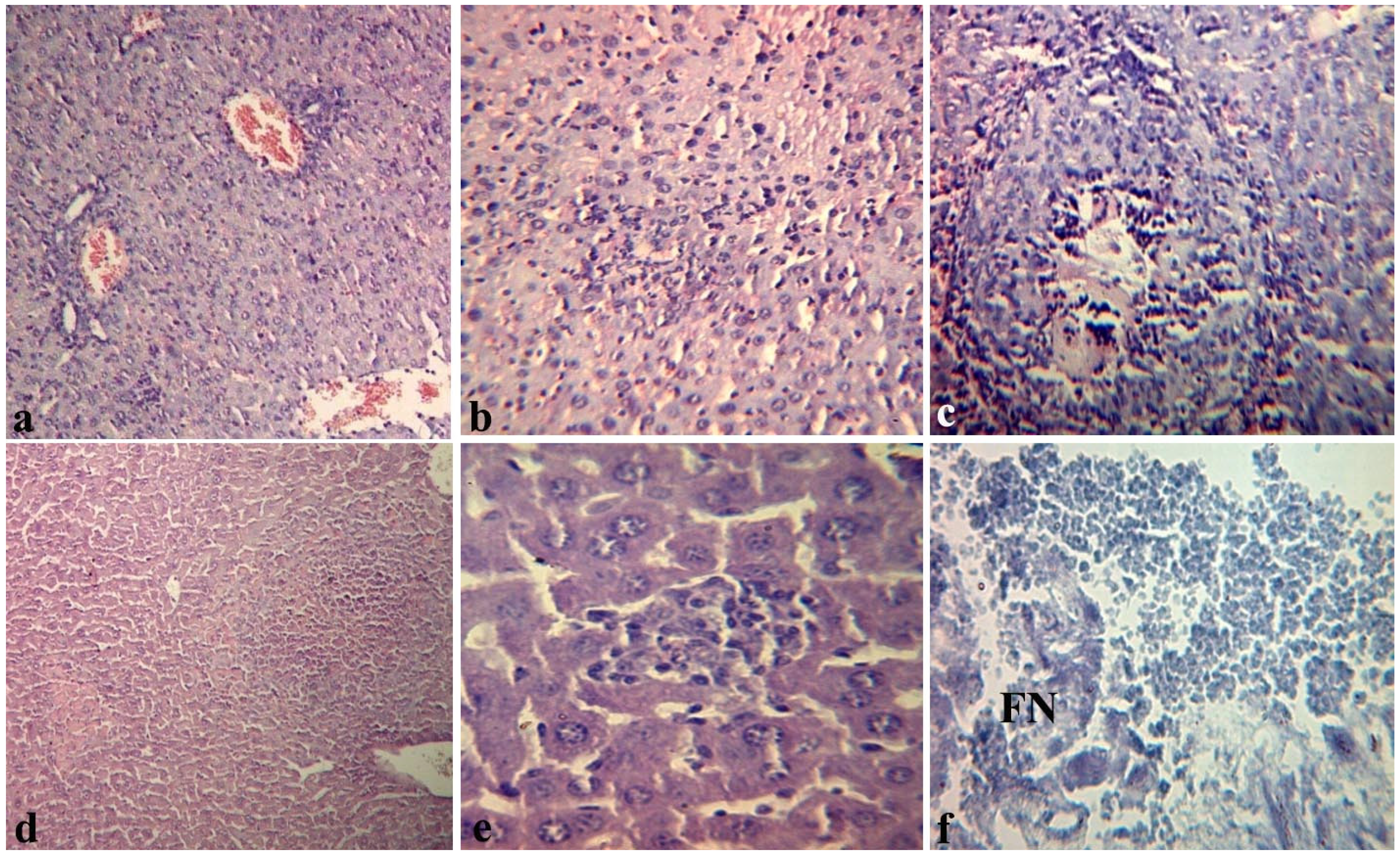
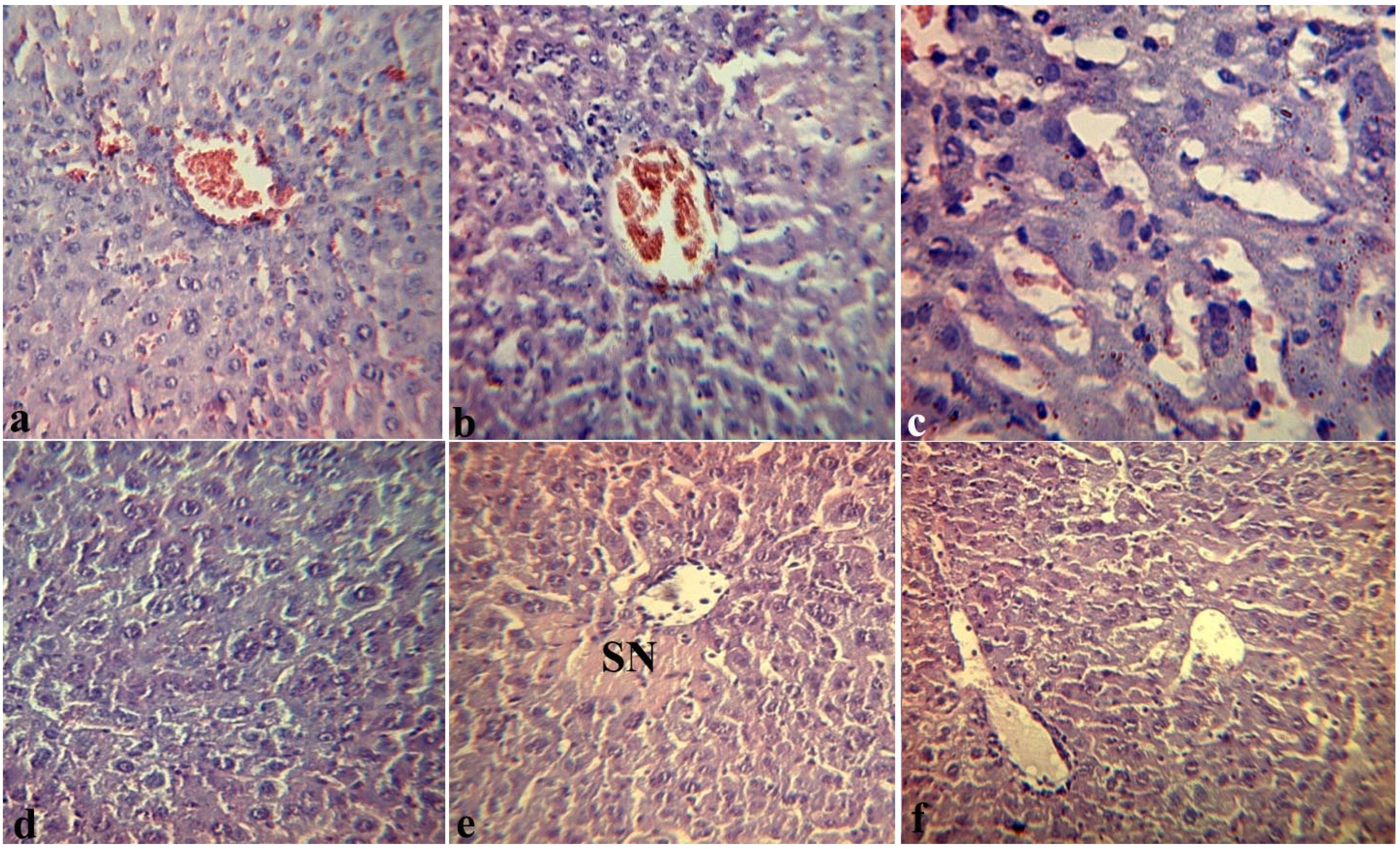
3. Experimental Section
3.1. Animals
3.2. Reagents
3.3. Model of Endotoxemia
3.4. The Survival Time of Mice
3.5. Determining the Level of Cytokines in the Serum of Mice
3.6. Determination of Hemostasis Parameters
3.7. Histopathological Studies
3.8. Statistical Analysis
4. Conclusions
Acknowledgements
References
- Berteau, O.; Mulloy, B. Sulfated fucans, fresh perspectives: Structures, functions, and biological priperties of sulfated fucans and overview of enzymes active toward this class of polysaccharide. Glycobiology 2003, 13, 29–40. [Google Scholar] [CrossRef]
- Cumashi, A.; Ushakova, N.; Preobrazhenskaya, M.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and Bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef]
- Wijesekara, I.; Pangestuti, R.; Kim, S. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011, 84, 14–21. [Google Scholar] [CrossRef]
- Jeong, B.E.; Ko, E.J.; Joo, H.G. Cytoprotective effects of fucoidan, an algae-derived polysaccharide on 5-fluorouracil-treated dendritic cells. Food Chem. Toxicol. 2012, 50, 1480–1484. [Google Scholar] [CrossRef]
- Vishchuk, O.S.; Ermakova, S.P.; Zvyagintseva, T.N. The Effect of Sulfated (1→3)-α-l-Fucan from the Brown Alga Saccharina cichorioides Miyabe on Resveratrol-Induced Apoptosis in Colon Carcinoma Cells. Mar. Drugs 2013, 11, 194–212. [Google Scholar] [CrossRef]
- Costa, L.S.; Fidelis, G.P.; Telles, C.B. Antioxidant and Antiproliferative Activities of Heterofucans from the Seaweed Sargassum filipendula. Mar. Drugs 2011, 9, 952–966. [Google Scholar] [CrossRef]
- Opal, S.M. Endotoxins and Other Sepsis Triggers. Contrib. Nephrol. 2010, 167, 14–24. [Google Scholar] [CrossRef]
- Enukidze, G.; Anikhovskaya, I.; Marachev, A.; Yakovlev, M. Endotoxin aggression in the pathogenesis of chronic inflammatory diseases of small pelvis organs and infertility, or an antiendotoxin approach to their treatment. Hum. Physiol. 2006, 32, 351–356. [Google Scholar] [CrossRef]
- Yakovlev, M. Endotoxin aggression as premorbidity or universal factor of the pathogenesis of human and animal. Biology Bulletin Reviews 2003, 123, 31–40. [Google Scholar]
- Meshkov, M.V.; Anikhovskaia, I.A.; Urazaev, R.A.; Iakovlev, M.I. Endotoxin aggression in the development of hemostatic disorders in children with surgical diseases. Khirurgiia 2006, 3, 32–37. [Google Scholar]
- Eun-Ju, K.; Hong-Gu, J. Fucoidan Enhances the Survival and Sustains the Number of Splenic Dendritic Cells in Mouse Endotoxemia. Korean J. Physiol. Pharmacol. 2011, 15, 89–94. [Google Scholar] [CrossRef]
- Kuznetsova, T.A.; Besednova, N.N.; Mamaev, A.N.; Momot, A.P.; Shevchenko, N.M.; Zvyagintseva, T.N. Anticoagulant activity of fucoidan from brown algae of the Okhotsk Sea Fucus evanescens. Bull. Exp. Biol. Med. 2003, 136, 53–59. [Google Scholar]
- Zaporozhets, T.S.; Kuznetsova, T.A.; Smolina, T.P.; Shevchenko, N.M.; Zvyagintseva, T.N.; Besednova, N.N. Immunotropic and anticoagulant properties of fucoidan from brown algae Fucus evanescens: Prospects of application in medicine. J. Microbiol. 2006, 3, 54–58. [Google Scholar]
- Khil’chenko, S.R.; Zaporozhets, T.S.; Shevchenko, N.M.; Zvyagintseva, T.N.; Vogel, U.; Seeberger, P.; Lepenies, B. Immunostimulatory Activity of Fucoidan from the Brown Alga Fucus evanescens: Role of Sulfates and Acetates. J. Carbohydr. Chem. 2011, 30, 291–305. [Google Scholar] [CrossRef]
- Makarenkova, I.D.; Akhmatova, N.K.; Semenova, I.B.; Zvyagintseva, T.N.; Besednova, N.N. Effect of fucoidan from brown algae on the maturation of dendritic cells generated from peripheral blood monocytes in vitro. Russ. J. Immunol. 2012, 6, 105–106. [Google Scholar]
- Hack, C.E.; Poll, T.; Thijs, L.G. Sepsis and coagulation. Sepsis 1999, 3, 85. [Google Scholar] [CrossRef]
- Levi, M.D.; Cate, H. Disseminated Intravascular Coagulation. N. Engl. J. Med. 1999, 34, 586–592. [Google Scholar] [CrossRef]
- Cavaillon, J.M.; Adib-Conquy, M.; Fitting, C.; Adrie, C.; Payen, D. Cytokine cascade in sepsis. Scand. J. Infect. Dis. 2003, 35, 535–544. [Google Scholar]
- Igonin, A.A.; Kukes, V.G.; Paltsev, M.A. Sepsis: Molecular mechanisms of systemic inflammation as a model for the study of promising therapeutic targets. Mol. Med. 2004, 2, 3–12. [Google Scholar]
- Pernerstorfer, T.; Hollenstein, U.; Hansen, J.; Stohlawetz, P.; Eichler, H.-G.; Handler, S.; Speiser, W.; Jilma, B. Lepirudin blunts endotoxin-induced coagulation activation. Blood 2000, 95, 1729–1734. [Google Scholar]
- Levi, M.; Dorffler-Melly, J.; Reitsma, P.; Buller, H.; Florquin, S.; van der Poll, T.; Carmeliet, P. Aggravation of endotoxin-induced disseminated intravascular coagulation and cytokine activation in heterozygous protein-C-deficient mice. Blood 2003, 101, 4823–4827. [Google Scholar] [CrossRef]
- Pawlinski, R.; Pedersen, B.; Schabbauer, G.; Tencati, M.; Holscher, T.; Boisvert, W.; Andrade-Gordon, P.; Frank, R.D.; Mackman, N. Role of tissue factor and protease-activated receptors in a mouse model of endotoxemia. Blood 2004, 103, 1342–1347. [Google Scholar]
- Levi, M.; Poll, T.; Cate, H.; Deventer, S.J. The cytokine mediated imbalance between coagulant and anticoagulant mechanisms in sepsis and endotoxaemia. Eur. J. Clin. Investig. 1997, 27, 3–9. [Google Scholar]
- Kerr, R.; Stirling, D.; Ludlam, C.A. Interleukin-6 and haemostasis. Br. J. Haematol. 2001, 115, 3–12. [Google Scholar] [CrossRef]
- Schiffer, E.R.; Reber, G.; De Moerloose, P.; Morel, D.R. Evaluation of unfractionated heparin and recombinant hirudin on survival in a sustained ovine endotoxin shock model. Crit. Care Med. 2002, 30, 2689–2699. [Google Scholar] [CrossRef]
- Li, Y.; Sun, J.-F.; Cui, X.; Mani, H.; Danner, R. L.; Li, X.; Su, J.-W.; Fitz, Y.; Eichacker, P.Q. The effect of heparin administration in animal models of sepsis: A prospective study in Escherichia coli-challenged mice and a systematic review and metaregression analysis of published studies. Crit. Care Med. 2011, 39, 1104–1112. [Google Scholar] [CrossRef]
- Thomas, W.S. Coagulation Activation by Lipopolysaccharides. Clin. Appl. Thromb Hemost. 2009, 15, 209–219. [Google Scholar] [CrossRef]
- Doğanyiğit, Z.; Küp, F.Ö.; Silici, S.; Deniz, K.; Yakan, B.; Atayoglu, T. Protective effects of propolis on female rats histopathological, biochemical and genotoxic changes during LPS induced endotoxemia. Phytomedicine 2013, 20, 632–639. [Google Scholar] [CrossRef]
- Gouda, K.H. Thymoquinone supplementation ameliorates acute endotoxemia-induced liver dysfunction in rats. Pak. J. Pharm. Sci. 2010, 23, 131–137. [Google Scholar]
- Zager, R.A.; Johnson, A.C.; Lund, S.; Hanson, S.Y.; Abrass, C.K. Levosimendan protects against experimental endotoxemic acute renal failure. Am. J. Physiol. Renal Physiol. 2006, 290, 1453–1462. [Google Scholar] [CrossRef]
- Zvyagintseva, T.N.; Shevchenko, N.M.; Popivnich, I.B.; Svetasheva, T.G.; Isakov, V.V.; Skobun, A.S.; Elyakova, L.A. Procedure for the separation of water-soluble polysaccharides from brown seaweeds. Russian Patent 2135518, 27 08 1999. [Google Scholar]
- Westphal, O.; Jann, K. Bacterial Lipopolysaccharides. Extraction with Phenol-Water and Further Applications of the Procedures. In Methods in Carbohydrate Chemistry; Whistler, B.L., Wolfrom, M.L., Eds.; Academic Press: New York, NY, USA, 1965; Volume 9, pp. 83–91. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kuznetsova, T.A.; Besednova, N.N.; Somova, L.M.; Plekhova, N.G. Fucoidan Extracted from Fucus evanescens Prevents Endotoxin-Induced Damage in a Mouse Model of Endotoxemia. Mar. Drugs 2014, 12, 886-898. https://doi.org/10.3390/md12020886
Kuznetsova TA, Besednova NN, Somova LM, Plekhova NG. Fucoidan Extracted from Fucus evanescens Prevents Endotoxin-Induced Damage in a Mouse Model of Endotoxemia. Marine Drugs. 2014; 12(2):886-898. https://doi.org/10.3390/md12020886
Chicago/Turabian StyleKuznetsova, Tatyana A., Natalya N. Besednova, Larisa M. Somova, and Natalya G. Plekhova. 2014. "Fucoidan Extracted from Fucus evanescens Prevents Endotoxin-Induced Damage in a Mouse Model of Endotoxemia" Marine Drugs 12, no. 2: 886-898. https://doi.org/10.3390/md12020886
APA StyleKuznetsova, T. A., Besednova, N. N., Somova, L. M., & Plekhova, N. G. (2014). Fucoidan Extracted from Fucus evanescens Prevents Endotoxin-Induced Damage in a Mouse Model of Endotoxemia. Marine Drugs, 12(2), 886-898. https://doi.org/10.3390/md12020886





