An Update on 2,5-Diketopiperazines from Marine Organisms
Abstract
:1. Introduction
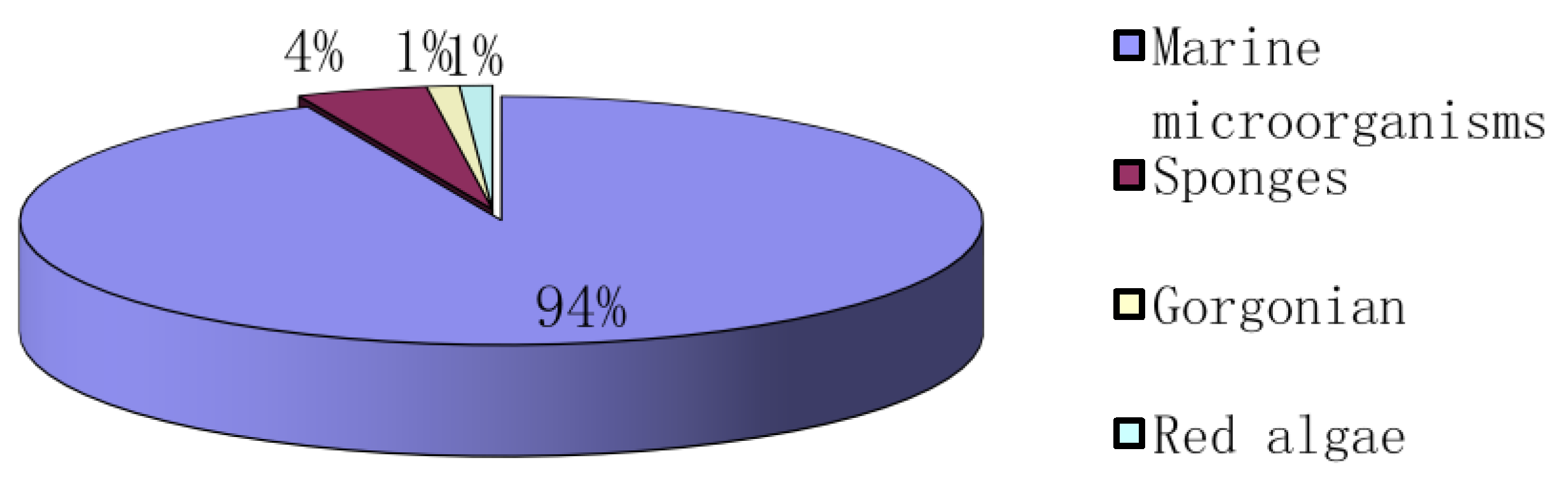
2. Marine Microorganisms
2.1. Actinomycetes
2.2. Bacteria
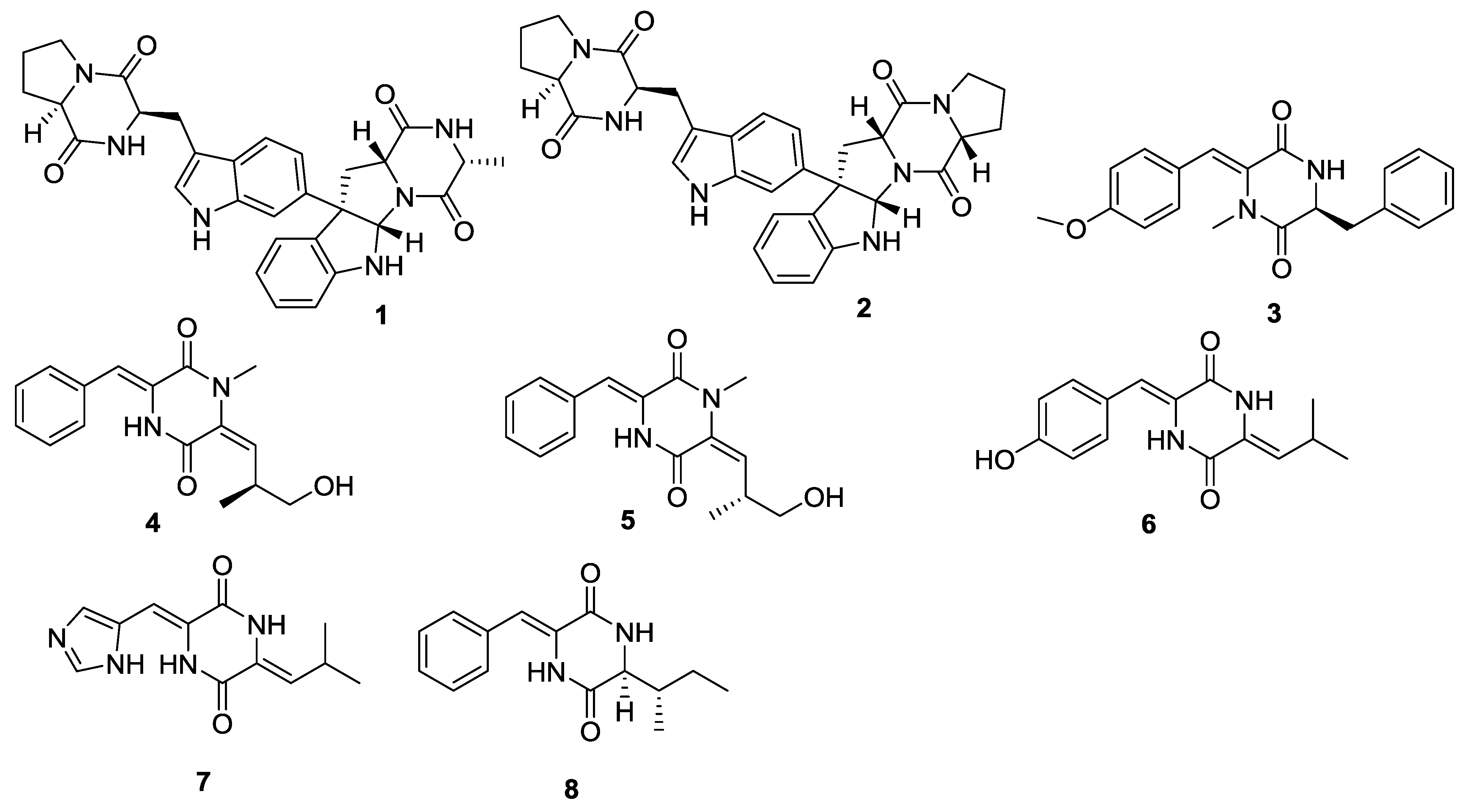
| Number | Name | Bioactivity | Source | Reference(s) |
|---|---|---|---|---|
| 1 | Naseseazine A | - | Streptomyces sp. | [4] |
| 2 | Naseseazine B | - | Streptomyces sp. | [4] |
| 3 | Nocazine C | - | Nocardiopsis dassonvillei | [5,6] |
| 4 | (3 Z,6E)-1-N-methyl-3-benzylidene-6-(2S-methyl-3-hydroxypropylidene)piperazine-2,5-dione | - | Streptomyces sp. | [7] |
| 5 | (3 Z,6E)-1-N-methyl-3-benzylidene-6-(2R-methyl-3-hydroxypropylidene)piperazine-2,5-dione | - | Streptomyces sp. | [7] |
| 6 | (3 Z,6Z)-3-(4-hydroxybenzylidene)-6-isobutylidenepiperazine-2,5-dione | Modest antivirus activity against influenza A (H1N1) virus | Streptomyces sp. | [7] |
| 7 | (3 Z,6Z)-3-((1H-imidazol-5-yl)-methylene)-6-isobutylidenepiperazine-2,5-dione (7) | - | Streptomyces sp. | [7] |
| 8 | (3 Z,6S)-3-benzylidene-6-(2S-but-2-yl)piperazine-2,5-dione | - | Streptomyces sp. | [7] |


| Number | Name | Bioactivity | Source | Reference |
|---|---|---|---|---|
| 9 | Bacillusamide A | Weak inhibition activity against A. niger | Bacillus sp. | [8] |
| 10 | Bacillusamide B | - | Bacillus sp. | [8] |
| 11 | Norcardioazine A | Inhibition of P-Glycoprotein | Nocardiopsis sp. | [9] |
| 12 | Norcardioazine B | - | Nocardiopsis sp. | [9] |
| 13 | Staphyloamide A | - | Staphylococcus sp. | [10] |
| 14 | Staphyloamide B | - | Staphylococcus sp. | [10] |
2.3. Fungi
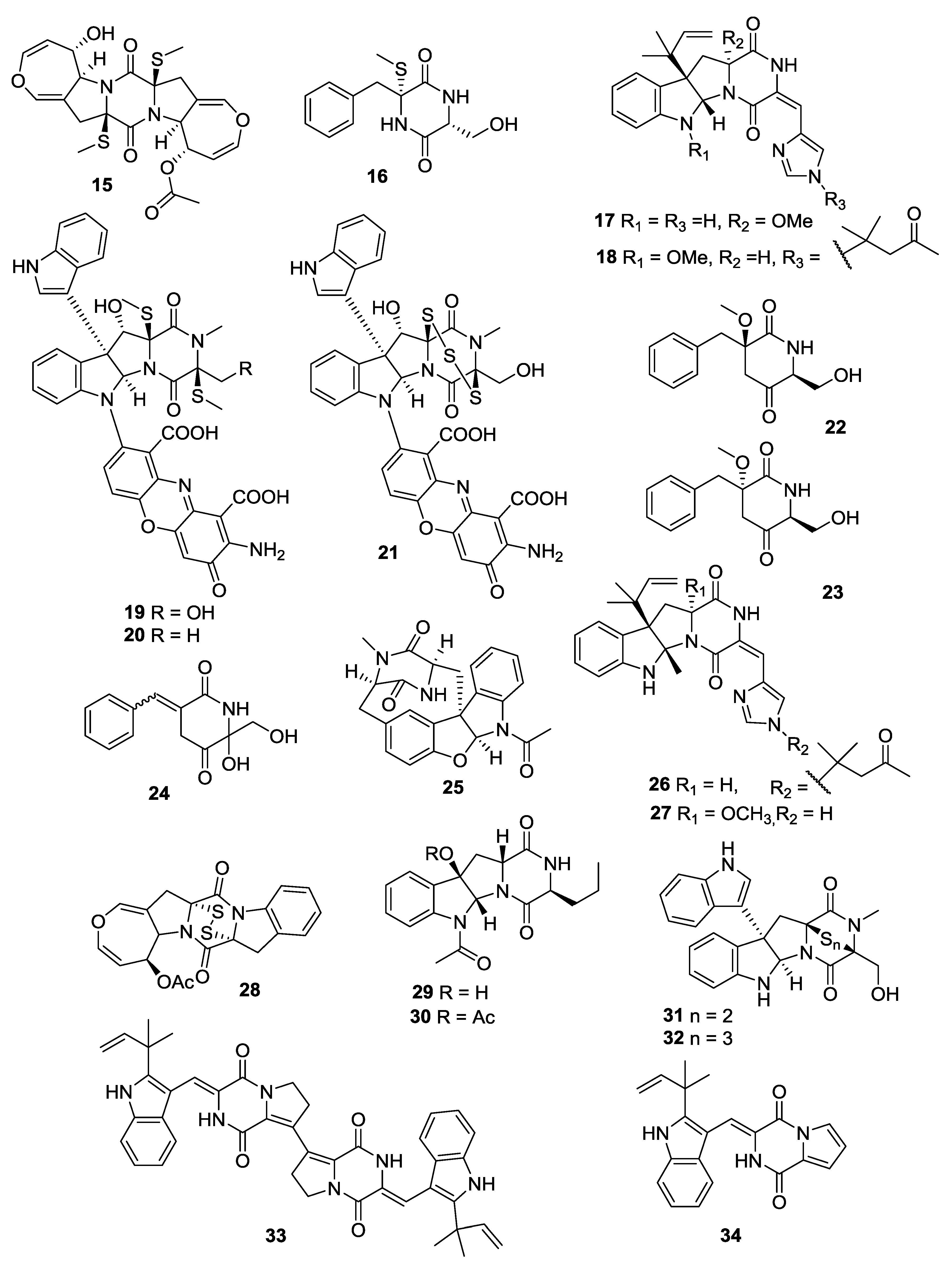

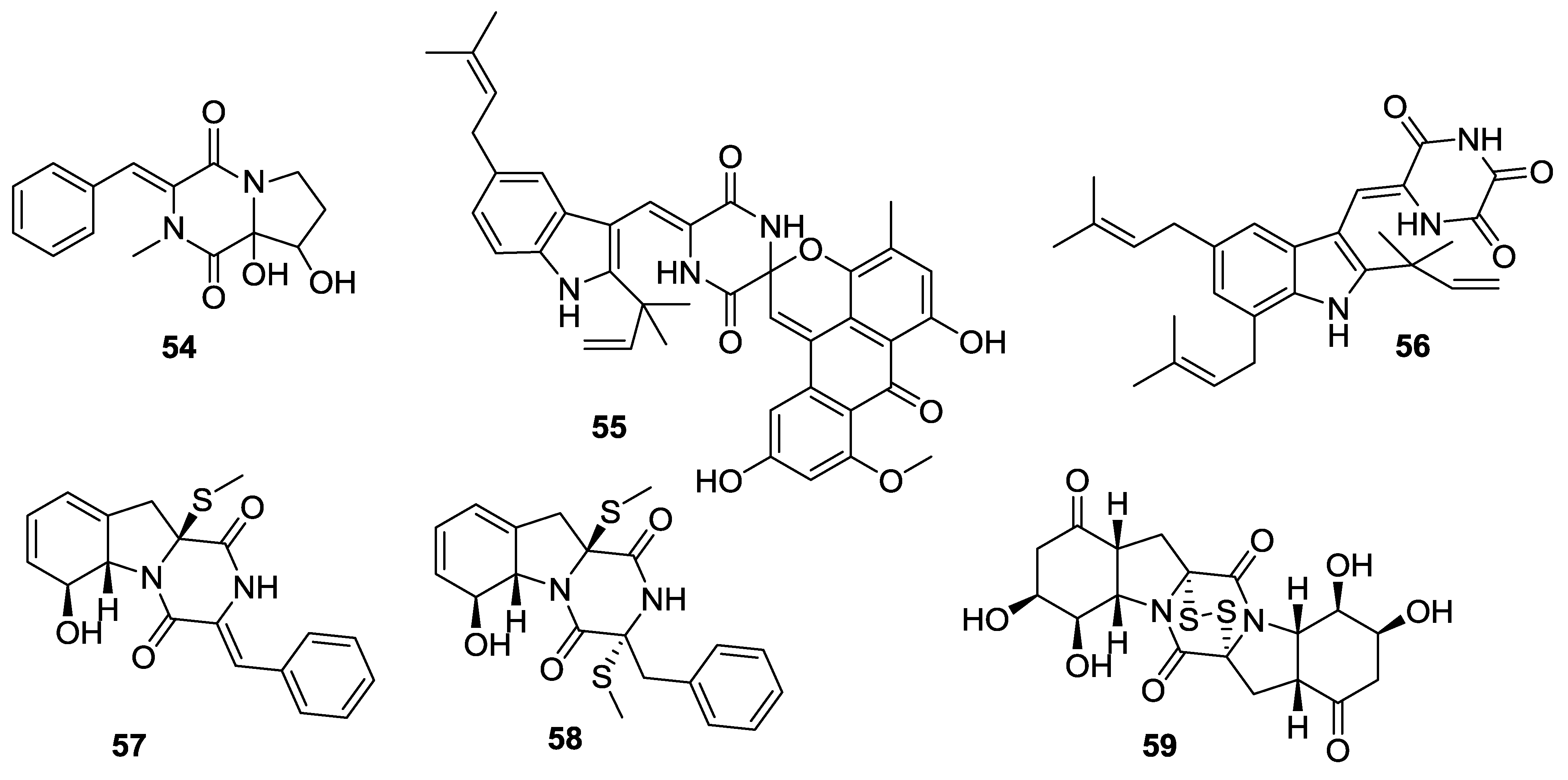

| Number | Name | Bioactivity | Source | Reference |
|---|---|---|---|---|
| 15 | Alternarosin A | - | Alternaria raphani | [11] |
| 16 | Not given | - | fumigatus | [12] |
| 17 | Roquefortine F | Cytotoxic activity | Penicillium sp. | [13] |
| 18 | Roquefortine G | Cytotoxic activity | Penicillium sp. | [13] |
| 19 | Plectoshpaeroic acid A | Inhibitor of indoleamine 2,3-dioxygenase (IDO) | Plectosphaerella cucumerina | [14] |
| 20 | Plectoshpaeroic acid B | Inhibitor of indoleamine 2,3-dioxygenase (IDO) | P. cucumerina | [14] |
| 21 | Plectoshpaeroic acid C | Inhibitor of indoleamine 2,3-dioxygenase (IDO) | P.cucumerina | [14] |
| 22 | Not given | - | A. fumigatus | [15] |
| 23 | Not given | - | A. fumigatus | [15] |
| 24 | Not given | - | A. fumigatus | [15] |
| 25 | Azonazine | Anti-inflammatory activity | A. insulicola | [16] |
| 26 | Roquefortine H | - | Penicillium sp. | [17] |
| 27 | Roquefortine I | - | Penicillium sp. | [17] |
| 28 | Deoxyapoaranotin | - | A. versicolor | [18] |
| 29 | Protuboxepin A | - | Aspergillus sp. | [19] |
| 30 | Protuboxepin B | - | Aspergillus sp. | [19] |
| 31 | Luteoalbusin A | Potent cytotoxins against several HTCLs | A. luteoaltus | [20] |
| 32 | Luteoalbusin B | Potent cytotoxins against several HTCLs | A. luteoaltus | [20] |
| 33 | Brevianamide S | Significant antibacterial activity against Bacille Calmette-Guerin (BCG) | A. versicolor | [21] |
| 34 | Brevianamide T | - | A. versicolor | [21] |
| 35 | Brevianamide U | - | A. versicolor | [21] |
| 36 | Brevianamide V | - | A. versicolor | [21] |
| 37 | Bis(dethio)-10a-methylthio-3a-deoxy-3,3a-didehydrogliotoxin | - | Penicillium sp. | [22] |
| 38 | 6-Deoxy-5a,6-didehydrogliotoxin | - | Penicillium sp. | [22] |
| 39 | 5-Chlorosclerotiamide | - | A. westerdijkiae | [23] |
| 40 | 10- Epi-sclerotiamide | - | A. westerdijkiae | [23] |
| 41 | Nocazine D | - | Nocardiopsis alba | [24] |
| 42 | Nocazine E | - | N. alba | [24] |
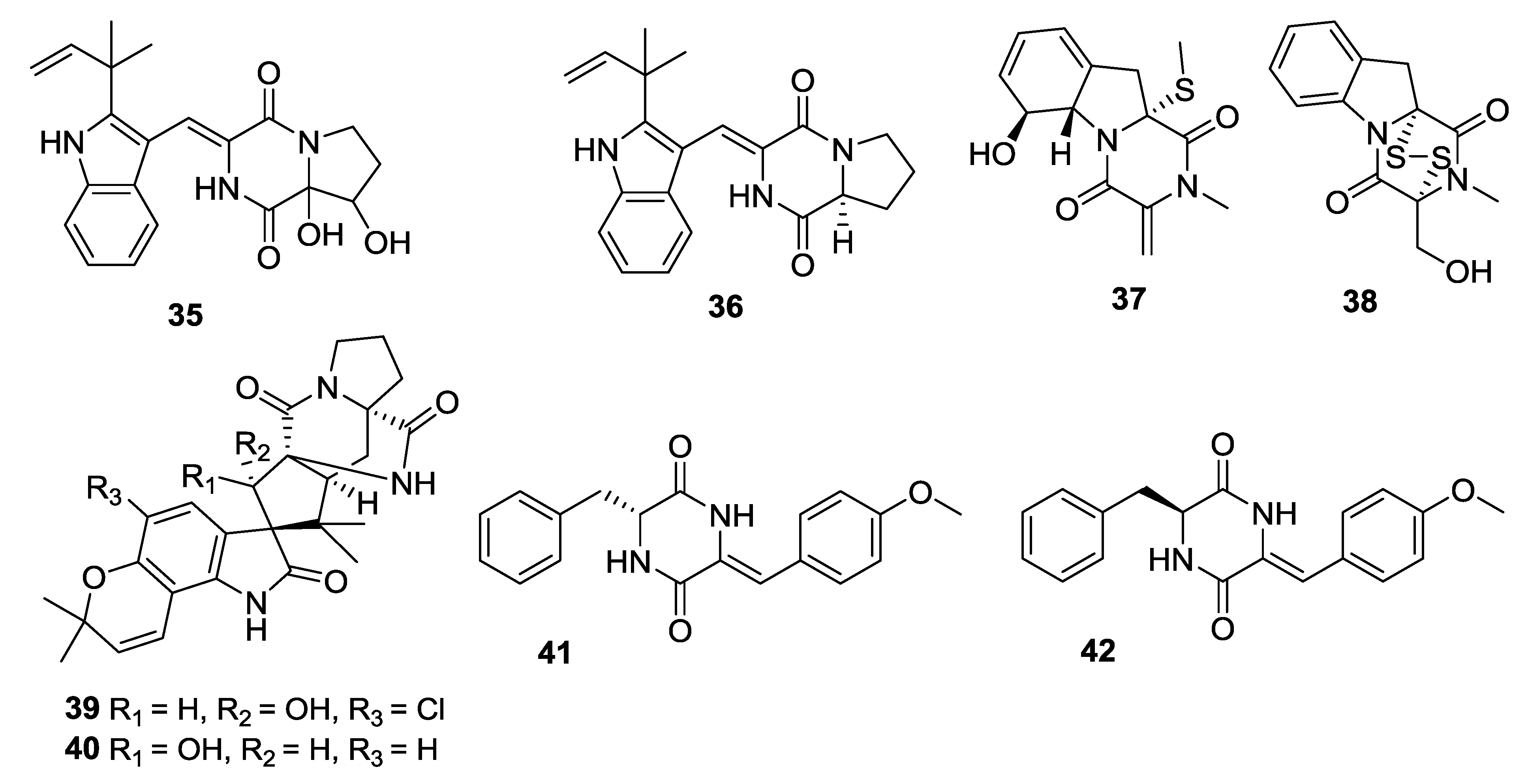
| Number | Name | Bioactivity | Source | Reference |
|---|---|---|---|---|
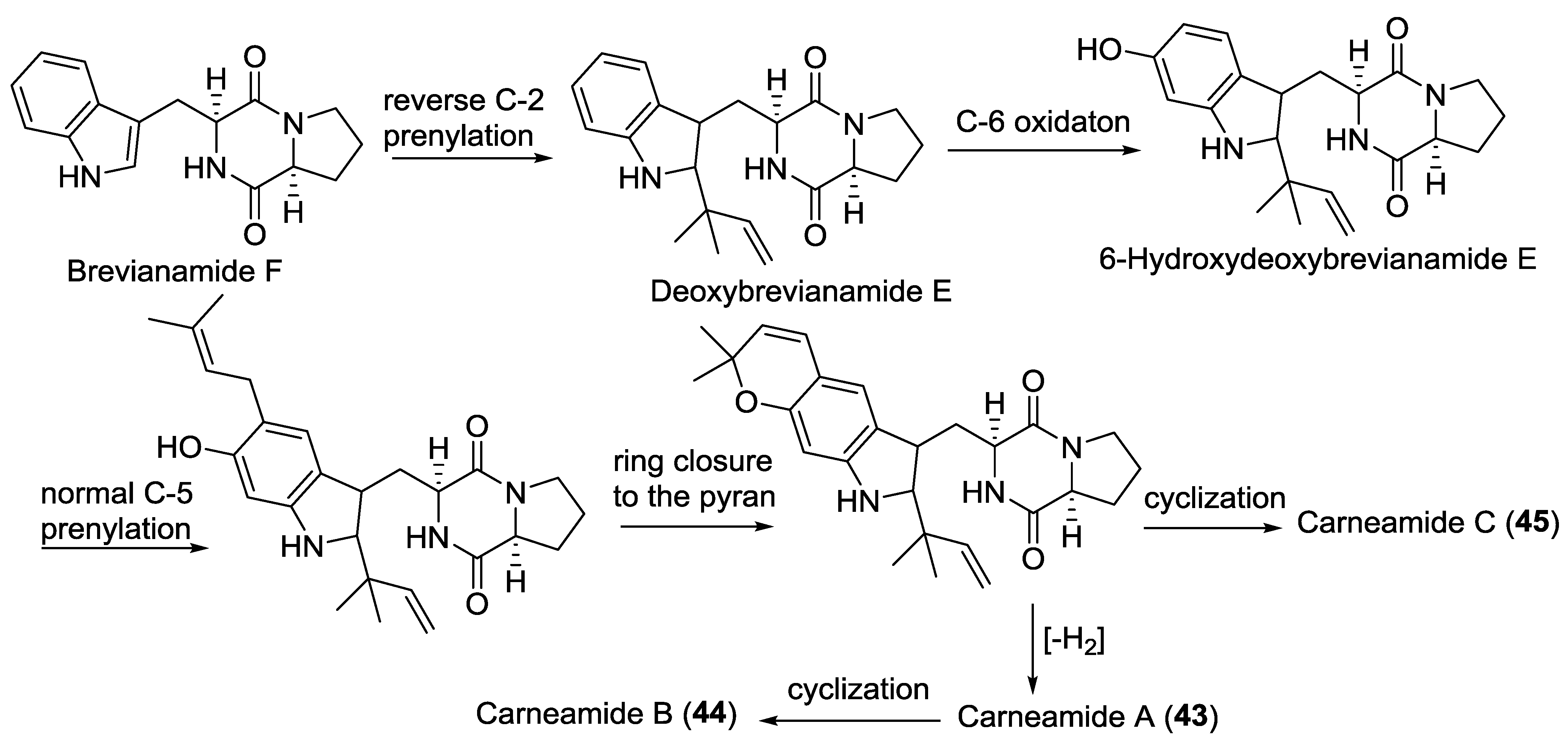
| 43 | Carneamide A | - | A. carneus | [25] |
| 44 | Carneamide B | - | A. carneus | [25] |
| 45 | Carneamide C | - | A. carneus | [25] |
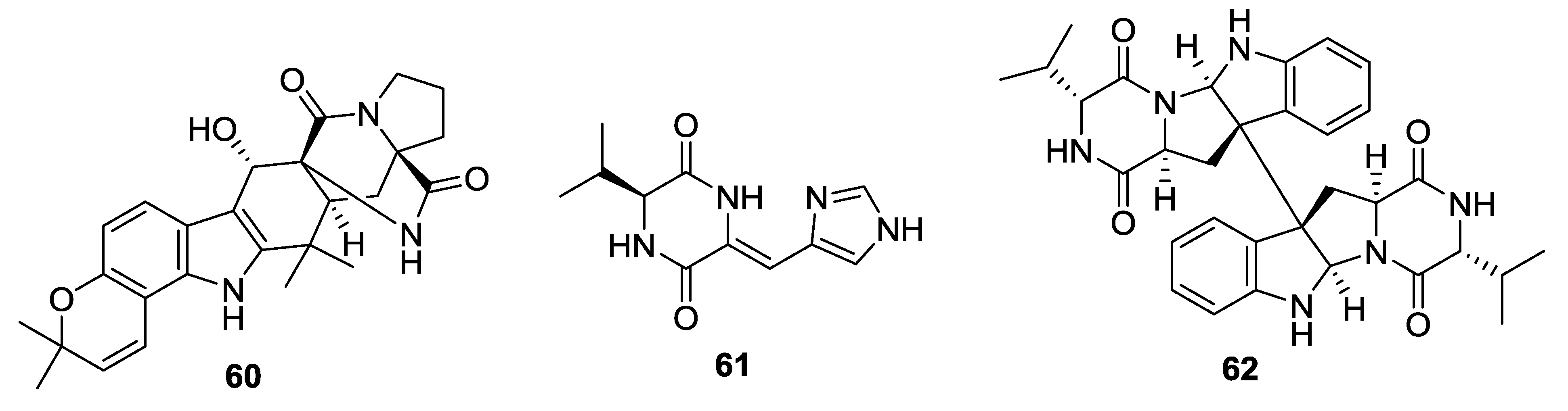
| 46 | Cristatumin A | Moderate activity against E. coli | E. cristatum | [26] |
| 47 | Cristatumin B | Moderate lethal activity against brine shrimp | E. cristatum | [26] |
| 48 | Cristatumin C | - | E.cristatum | [26] |
| 49 | 9Ɛ- O-2(2,3-dimethylbut-3-enyl)brevianamide Q | - | A. versicolor | [27] |
| Number | Name | Bioactivity | Source | Reference |
|---|---|---|---|---|
| 50 | Effusin A | - | A. effuses | [28] |
| 51 | Dihydrocryptoechinuline D | Potent activity on P388 cells with an IC50 value of 1.83 μM | A. effuses | [28] |
| 52 | Aspergilazine A | Weak activity against influenza A (H1N1) virus | A. taichungensis | [29] |
| 53 | Dihydroneochinulin B | Weak activity against BEL-7402 and A-549 cell lines | A. effuses | [30] |
| Number | Name | Bioactivity | Source | Reference |
|---|---|---|---|---|
| 54 | 3-Benzylidene-8,8a-dihydroxy-2-methyl-hexahydro-pyrrolo[1,2-a]pyrazine-1,4-dione | - | unidentified | [31] |
| 55 | 7- O-methylvariecoloride A | - | E. rubrum | [32] |
| 56 | 12-Demethyl-12-oxo-eurotechinulin B | Displayed cytotoxic activities | E. rubrum | [33] |
| 57 | Phomazine A | - | Phoma sp. | [34] |
| 58 | Phomazine B | Moderate cytotoxicities against the HL-60, HCT-116, K562, MGC-803 and A549 cell lines | Phoma sp. | [34] |
| 59 | Phomazine C | - | Phoma sp. | [34] |
| Number | Name | Bioactivity | Source | Reference |
|---|---|---|---|---|
| 60 | 21-Hydroxystephacidin | - | A. ostianus | [35] |
| 61 | Pre-aurantiamine | - | A. aculeatus | [36] |
| 62 | Eurocristatine | - | E. cristatum | [37] |
| Number | Name | Bioactivity | Source | Reference |
|---|---|---|---|---|
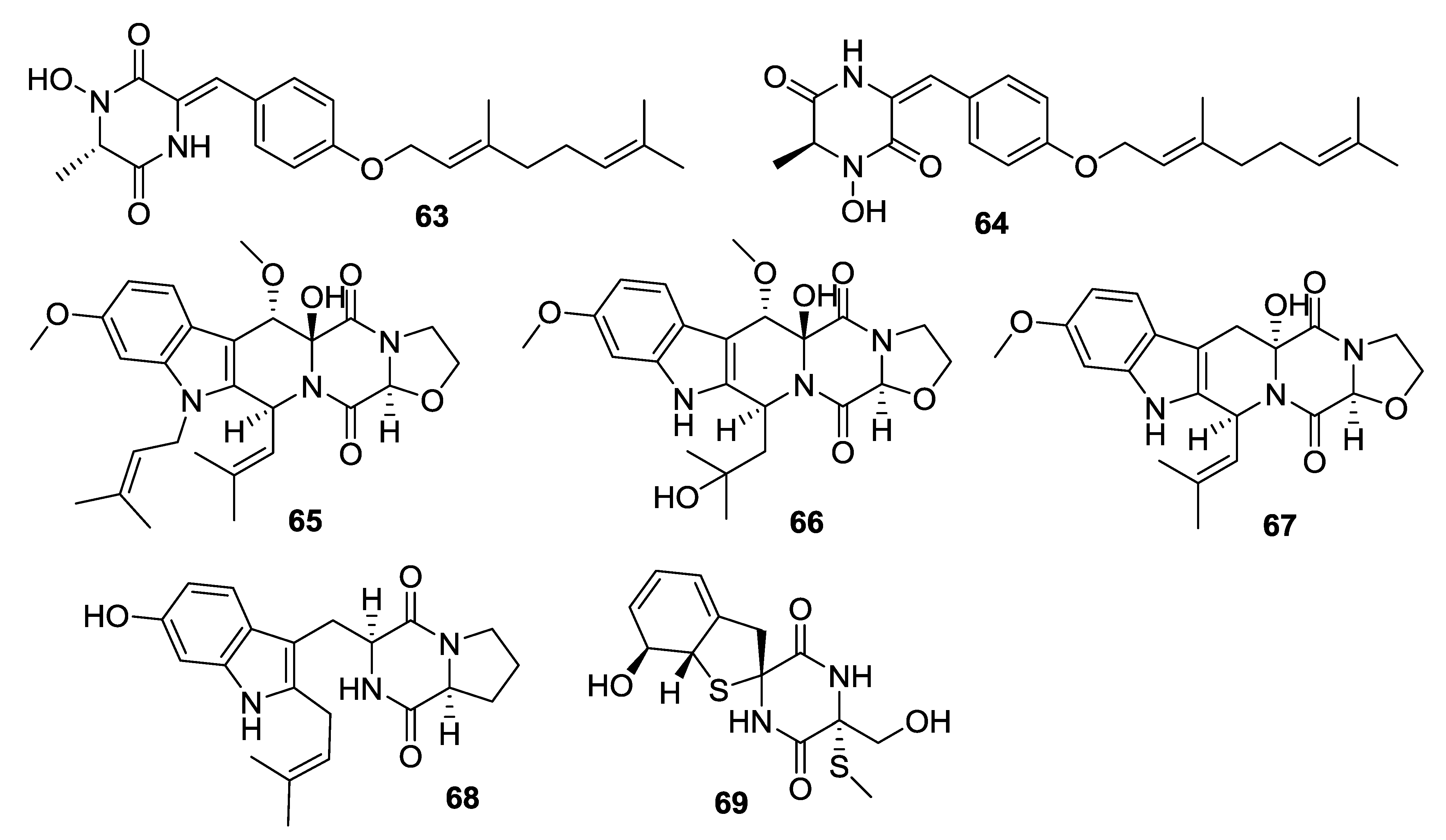
| 63 | Gliocladride A | Cytotoxic activity against HL-60, U937 and T47D with IC50 values form 12.80 μg/mL to 42.80 μg/mL | Gliocldium sp. | [38] |
| 64 | Gliocladride B | Cytotoxic activity against HL-60, U937 and T47D with IC50 values form 11.60 μg/mL to 52.83 μg/mL | Gliocldium sp. | [38] |
| 65 | Prenylcyclotryprostatin B | Most potent activities against both U937 and PC-3 cell lines | A. fumigatus | [39] |
| 66 | 20-Hydroxycyclotryprostatin B | Most potent activities against both U937 and PC-3 cell lines | fumigatus,A. sydowii | [39–41] |
| 67 | 9-Hydroxyfumitremorgin C | Most potent activities against both U937 and PC-3 cell lines | A. fumigatus | [39] |
| 68 | 6-Hydroxytryprostatin B | Most potent activities against both U937 and PC-3 cell lines | A. fumigatus | [39] |
| 69 | Spirogliotoxin | Most potent activities against both U937 and PC-3 cell lines | A. fumigatus | [39] |
| Number | Name | Bioactivity | Source | Reference |
|---|---|---|---|---|
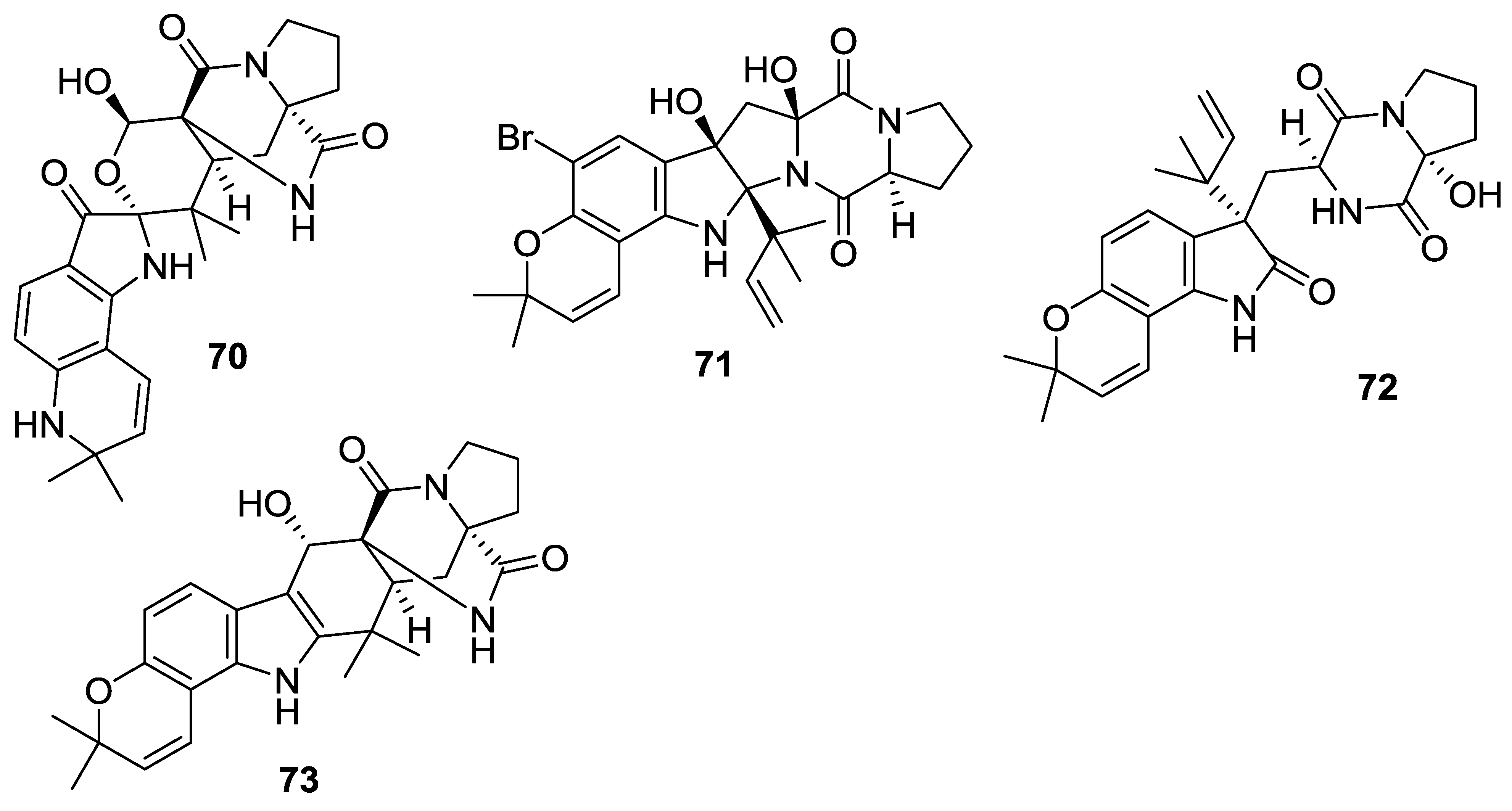
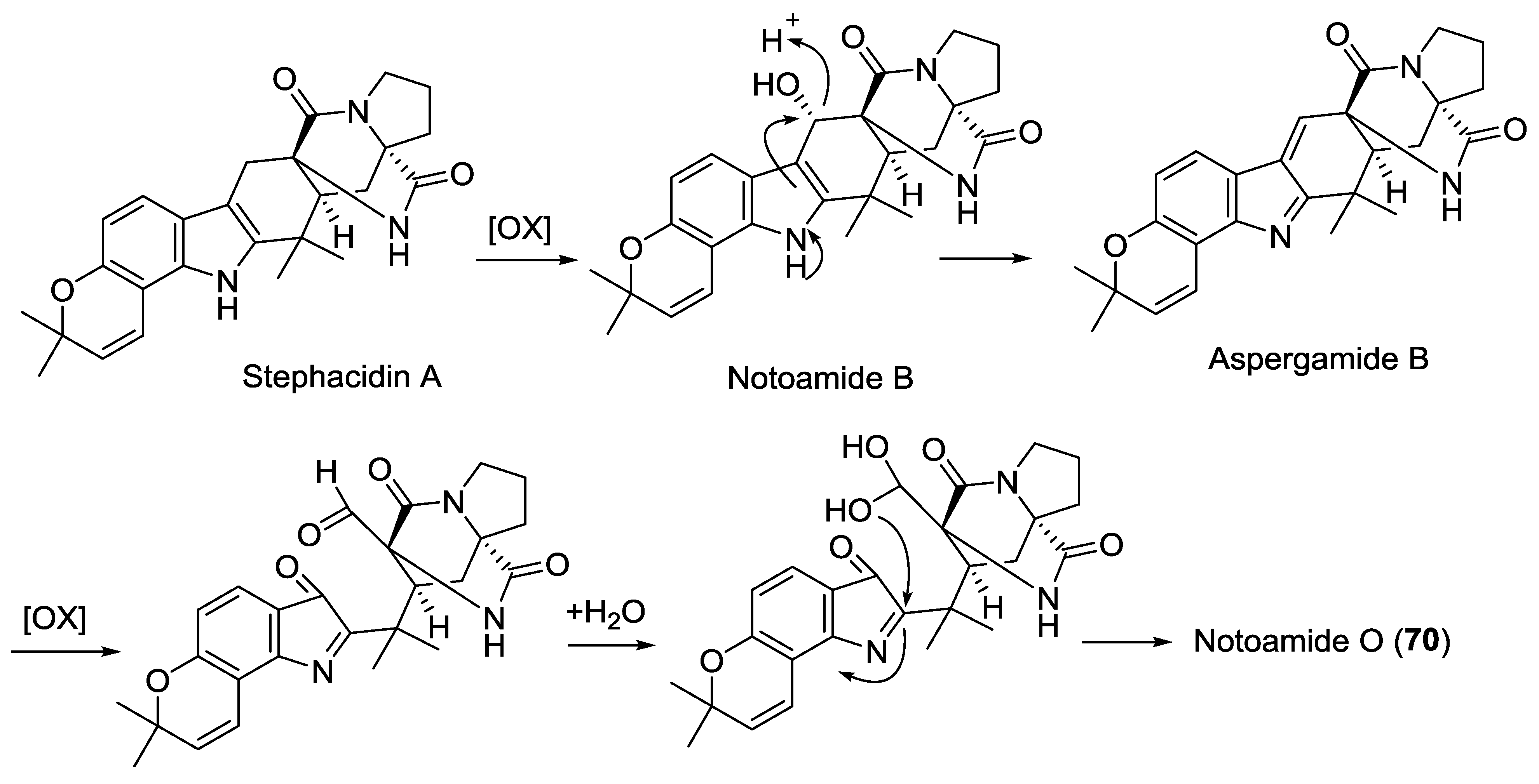
| 70 | Notoamide O | - | Aspergillus sp. | [42] |
| 71 | Notoamide P | - | Aspergillus sp. | [42] |
| 72 | Notoamide Q | - | Aspergillus sp. | [42] |
| 73 | Notoamide R | - | Aspergillus sp. | [42] |
| Number | Name | Bioactivity | Source | Reference |
|---|---|---|---|---|
| 74 | Notamide E | - | Aspergillus sp. | [43,44] |
| 75 | Notamide E2 | - | Aspergillus sp. | [45] |
| 76 | Notamide E3 | - | Aspergillus sp. | [45] |
| 77 | Notamide E4 | - | Aspergillus sp. | [45] |
| 78 | Notamide L | - | Aspergillus sp. | [46] |
| 79 | Notamide M | - | Aspergillus sp. | [46] |
| 80 | Notamide N | - | Aspergillus sp. | [46] |
| 81 | Cyclomarazine M | - | Salinispora arenicola | [47] |
| 82 | Cyclomarazine P | - | S. arenicola | [47] |
| 83 | Notoamide S | - | Aspergillus sp. | [48,49] |
| 84 | Spirotryprostatin F | Stimulatory phytoregulatory activity | A. fumigatus | [50] |
| 85 | Penilloid A | - | Penicillium sp. | [51] |
2.3.1. Fungi from Sediment Origin

2.3.2. Fungi from Algae Origin
2.3.3. Fungi from Mangrove Rhizosphere Soil Origin
2.3.4. Fungi from Mangrove Origin
2.3.5. Fungi from Sponge Origin
2.3.6. Fungi from Mud Origin
2.3.7. Fungi from Mollusk Origin
2.3.8. Fungi from Other Origins
3. Sponges

4. Gorgonian

5. Red Algae
6. Conclusions


Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huang, R.M.; Zhou, X.F.; Xu, T.H.; Yang, X.W.; Liu, Y.H. Diketopiperazines from marine organisms. Chem. Biodivers. 2010, 7, 2809–2829. [Google Scholar] [CrossRef] [PubMed]
- Borthwick, A.D. 2,5-Diketopiperazines: Synthesis, reactions, medicinal chemistry, and bioactive natural products. Chem. Rev. 2012, 112, 3641–3716. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.B.; Carvalho, I. Diketopiperazines: Biological activity and synthesis. Tetrahedron 2007, 63, 9923–9932. [Google Scholar] [CrossRef]
- Raju, R.; Piggott, A.M.; Conte, M.; Aalbersberg, W.G.L.; Feussner, K.; Capon, R.J. Naseseazines A and B: A new dimeric diketopiperazine framework from a marine-derived actinomycete, Streptomyces sp. Org. Lett. 2009, 11, 3862–3865. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Liu, P.P.; Qu, H.J.; Wang, Y.; Chen, D.F.; Wang, H.; Li, J.; Zhu, W.M. α-Pyrones and diketopiperazine derivatives from the marine-derived actinomycete Nocardiopsis dassonvillei HR10-5. J. Nat. Prod. 2011, 74, 2219–2223. [Google Scholar] [CrossRef] [PubMed]
- Bryans, J.; Charlton, P.; ChicarelliRobinson, I.; Collins, M.; Faint, R.; Latham, C.; Shaw, I.; Trew, S. Inhibition of plasminogen activator inhibitor-1 activity by two diketopiperazines, XR330 and XR334 produced by Streptomyces sp. J. Antibiot. 1996, 49, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xi, L.J.; Liu, P.P.; Wang, Y.; Wang, W.; Huang, Y.; Zhu, W.M. Diketopiperazine derivatives from the marine-derived actinomycete Streptomyces sp. FXJ7.328. Mar. Drugs 2013, 11, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, K.; Yamada, K.; Kouno, I. New diketopiperazine derivatives isolated from sea urchin-derived Bacillus sp. Chem. Pharm. Bull. 2011, 59, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Raju, R.; Piggott, A.M.; Huang, X.C.; Capon, R.J. Nocardioazines: A novel bridged diketopiperazine scaffold from a marine-derived bacterium inhibits P-glycoprotein. Org. Lett. 2011, 13, 2770–2773. [Google Scholar] [CrossRef] [PubMed]
- Khedr, A.I.M.; Kouno, I.; Tanaka, T.; Yamada, K. New diketopiperazine derivatives from culture broth of Staphylococcus sp. isolated from Corallina officinalis Lineaus. Heterocycles 2013, 87, 1029–1037. [Google Scholar] [CrossRef]
- Wang, W.L.; Wang, Y.; Tao, H.W.; Peng, X.P.; Liu, P.P.; Zhu, W.M. Cerebrosides of the halotolerant fungus Alternaria raphani isolated from a sea salt field. J. Nat. Prod. 2009, 72, 1695–1698. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.Y.; Zhu, T.J.; Han, X.X.; Fan, G.T.; Liu, H.B.; Zhu, W.M.; Gu, Q.Q. A new gliotoxin analogue from a marine-derived fungus Aspergillus fumigatus Fres. Nat. Prod. Res. 2009, 23, 203–207, Correction 1457. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Li, D.H.; Zhu, T.J.; Cai, S.X.; Wang, F.P.; Xiao, X.; Gu, Q.Q. New alkaloids and diterpenes from a deep ocean sediment derived fungus Penicillium sp. Tetrahedron 2009, 65, 1033–1039. [Google Scholar] [CrossRef]
- Carr, G.; Tay, W.; Bottriell, H.; Andersen, S.K.; Mauk, A.G.; Andersen, R.J. Plectosphaeroic acids A, B, and C, indoleamine 2,3-dioxygenase inhibitors produced in culture by a marine isolate of the fungus Plectosphaerella cucumerina. Org. Lett. 2009, 11, 2996–2999. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.Y.; Zhu, T.J.; Fan, G.T.; Liu, H.B.; Fang, Y.C.; Gu, Q.Q.; Zhu, W.M. Three new dioxopiperazine metabolites from a marine-derived fungus Aspergillus fumigatus Fres. Nat. Prod. Res. 2010, 24, 953–957. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.X.; Crews, M.S.; Draskovic, M.; Sohn, J.; Johnson, T.A.; Tenney, K.; Valeriote, F.A.; Yao, X.J.; Bjeldanes, L.F.; Crews, P. Azonazine, a novel dipeptide from a Hawaiian marine sediment-derived fungus, Aspergillus insulicola. Org. Lett. 2010, 12, 4458–4461. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Feng, T.; Zhao, B.Y.; Li, D.H.; Cai, S.X.; Zhu, T.J.; Wang, F.P.; Xiao, X.; Gu, Q.Q. Alkaloids from a deep ocean sediment-derived fungus Penicillium sp. and their antitumor activities. J. Antibiot. 2010, 63, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Park, J.S.; Kim, Y.J.; Jung, J.H.; Lee, J.K.; Kwon, H.C.; Yang, H.O. Apoptosis-inducing effect of diketopiperazine disulfides produced by Aspergillus sp. KMD 901 isolated from marine sediment on HCT116 colon cancer cell lines. J. Appl. Microbiol. 2011, 110, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.U.; Asami, Y.; Lee, D.; Jang, J.H.; Ahn, J.S.; Oh, H. Protuboxepins A and B and protubonines A and B from the marine-derived fungus Aspergillus sp. SF-5044. J. Nat. Prod. 2011, 74, 1284–1287. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.Z.; Huang, Z.; Shi, X.F.; Chen, Y.C.; Zhang, W.M.; Tian, X.P.; Li, J.; Zhang, S. Cytotoxic indole diketopiperazines from the deep sea-derived fungus Acrostalagmus luteoalbus SCSIO F457. Bioorg. Med. Chem. Lett. 2012, 22, 7265–7267. [Google Scholar] [CrossRef] [PubMed]
- Song, F.H.; Liu, X.R.; Guo, H.; Ren, B.; Chen, C.X.; Piggott, A.M.; Yu, K.; Gao, H.; Wang, Q.; Liu, M.; et al. Brevianamides with antitubercular potential from a marine-derived isolate of Aspergillus versicolor. Org. Lett. 2012, 14, 4770–4773. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Takada, K.; Takemoto, Y.; Yoshida, M.; Nogi, Y.; Okada, S.; Matsunaga, S. Gliotoxin analogues from a marine-derived fungus, Penicillium sp., and their cytotoxic and histone methyltransferase inhibitory activities. J. Nat. Prod. 2012, 75, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zhang, X.Y.; Tu, Z.C.; Xu, X.Y.; Qi, S.H. Alkaloids from the deep-sea-derived fungus Aspergillus westerdijkiae DFFSCS013. J. Nat. Prod. 2013, 76, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.B.; Li, S.M.; Chen, Y.C.; Tian, X.P.; Zhang, H.B.; Zhang, G.T.; Zhu, Y.G.; Zhang, S.; Zhang, W.M.; Zhang, C.S. New diketopiperazine derivatives from a deep-sea-derived Nocardiopsis alba SCSIO 03039. J. Antibiot. 2013, 66, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Zhuravleva, O.I.; Afiyatullov, S.S.; Denisenko, V.A.; Ermakova, S.P.; Slinkina, N.N.; Dmitrenok, P.S.; Kim, N.Y. Secondary metabolites from a marine-derived fungus Aspergillus carneus Blochwitz. Phytochemistry 2012, 80, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Du, F.Y.; Li, X.M.; Li, C.S.; Shang, Z.; Wang, B.G. Cristatumins A–D, new indole alkaloids from the marine-derived endophytic fungus Eurotium cristatum EN-220. Bioorg. Med. Chem. Lett. 2012, 22, 4650–4653. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.P.; Li, X.D.; Liu, X.H.; Cichewicz, R.H.; Ji, N.Y. Secondary metabolites from an algicolous Aspergillus versicolor strain. Mar. Drugs 2012, 10, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.Q.; Liu, W.Z.; Zhu, T.J.; Mo, X.M.; Mandi, A.; Kurtan, T.; Li, J.; Ai, J.; Gu, Q.Q.; Li, D.H. Diketopiperazine alkaloids from a mangrove rhizosphere soil derived fungus Aspergillus effuses H1-1. Org. Biomol. Chem. 2012, 10, 9501–9506. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.X.; Kong, X.L.; Wang, W.; Zhou, H.N.; Zhu, T.J.; Li, D.H.; Gu, Q.Q. Aspergilazine A, a diketopiperazine dimer with a rare N-1 to C-6 linkage, from a marine-derived fungus Aspergillus taichungensis. Tetrahedron Lett. 2012, 53, 2615–2617. [Google Scholar] [CrossRef]
- Gao, H.Q.; Zhu, T.J.; Li, D.H.; Gu, Q.Q.; Liu, W.Z. Prenylated indole diketopiperazine alkaloids from a mangrove rhizosphere soil derived fungus Aspergillus effuses H1-1. Arch. Pharm. Res. 2013, 36, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.G.; Shao, C.L.; Huang, Z.J.; Zhang, Y.; Cai, X.L.; She, Z.G.; Zhou, S.N.; Lin, Y.C. Structure elucidation and NMR assignments for two amide alkaloids from a mangrove endophytic fungus (No. ZZF-22). Magn. Reson. Chem. 2009, 47, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Li, D.L.; Li, X.M.; Proksch, P.; Wang, B.G. 7-O-Methylvariecolortide A, a new spirocyclic diketopiperazine alkaloid from a marine mangrove derived endophytic fungus, Eurotium rubrum. Nat. Prod. Commun. 2010, 5, 1583–1586. [Google Scholar] [PubMed]
- Yan, H.J.; Li, X.M.; Li, C.S.; Wang, B.G. Alkaloid and anthraquinone derivatives produced by the marine-derived endophytic fungus Eurotium rubrum. Helv. Chim. Acta 2012, 95, 163–168. [Google Scholar] [CrossRef]
- Kong, F.D.; Wang, Y.; Liu, P.P.; Dong, T.H.; Zhu, W.M. Thiodiketopiperazines from the marine-derived fungus Phoma sp. OUCMDZ-1847. J. Nat. Prod. 2014, 77, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Kito, K.; Ookura, R.; Kusumi, T.; Namikoshi, M.; Ooi, T. X-ray structures of two stephacidins, heptacyclic alkaloids from the marine-derived fungus Aspergillus ostianus. Heterocycles 2009, 78, 2101–2106. [Google Scholar] [CrossRef]
- Antia, B.S.; Aree, T.; Kasettrathat, C.; Wiyakrutta, S.; Ekpa, O.D.; Ekpe, U.J.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Itaconic acid derivatives and diketopiperazine from the marine-derived fungus Aspergillus aculeatus CRI322-03. Phytochemistry 2011, 72, 816–820. [Google Scholar] [CrossRef] [PubMed]
- Gomes, N.M.; Dethoup, T.; Singburaudom, N.; Gales, L.; Silva, A.M.S.; Kijjoa, A. Eurocristatine, a new diketopiperazine dimer from the marine sponge-associated fungus Eurotium cristatum. Phytochem. Lett. 2012, 5, 717–720. [Google Scholar] [CrossRef]
- Yao, Y.; Tian, L.; Li, J.; Cao, J.Q.; Pei, Y.H. Cytotoxic piperazine-2,5-dione derivatives from marine fungus Gliocladium sp. Pharmazie 2009, 64, 616–618. [Google Scholar] [PubMed]
- Wang, Y.; Li, Z.L.; Bai, J.; Zhang, L.M.; Wu, X.; Zhang, L.; Pei, Y.H.; Jing, Y.K.; Hua, H.M. 2,5-Diketopiperazines from the marine-derived fungus Aspergillus fumigatus YK-7. Chem. Biodivers. 2012, 9, 385–393. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Sun, Y.L.; Liu, K.S.; Zhang, X.Y.; Qian, P.Y.; Wang, Y.F.; Qi, S.H. Indole alkaloids from marine-derived fungus Aspergillus sydowii SCSIO 00305. J. Antibiot. 2012, 65, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Zhang, Q.; Zhang, A.L.; Gao, J.M. Metabolites from Aspergillus fumigatus, an endophytic fungus associated with melia azedarach, and their antifungal, antifeedant, and toxic activities. J. Agr. Food Chem. 2012, 60, 3424–3431. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Umaoka, H.; Yoshikawa, K.; Ikeda, T.; Hirota, H. Notoamide O, a structurally unprecedented prenylated indole alkaloid, and notoamides P–R from a marine-derived fungus, Aspergillus sp. J. Nat. Prod. 2010, 73, 1438–1440, Correction 2013, 76, 1232. [Google Scholar]
- Kato, H.; Yoshida, T.; Tokue, T.; Nojiri, Y.; Hirota, H.; Ohta, T.; Williams, R.M.; Tsukamoto, S. Notoamides A–D: Prenylated indole alkaloids isolated from a marine-derived fungus, Aspergillus sp. Angew. Chem. Int. Edit. 2007, 46, 2254–2256. [Google Scholar] [CrossRef]
- Grubbs, A.W.; Artman, G.D.; Tsukamoto, S.; Williams, R.M. A concise total synthesis of the notoamides C and D. Angew. Chem. Int. Edit. 2007, 46, 2257–2261. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Kato, H.; Greshock, T.J.; Hirota, H.; Ohta, T.; Williams, R.M. Isolation of notoamide E, a key precursor in the biosynthesis of prenylated indole alkaloids in a marine-derived fungus, Aspergillus sp. J. Am. Chem. Soc. 2009, 131, 3834–3835. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Kawabata, T.; Kato, H.; Greshock, T.J.; Hirota, H.; Ohta, T.; Williams, R.M. Isolation of antipodal (−)-versicolamide B and notoamides L–N from a marine-derived Aspergillus sp. Org. Lett. 2009, 11, 1297–1300. [Google Scholar] [CrossRef] [PubMed]
- Schultz, A.W.; Lewis, C.A.; Luzung, M.R.; Baran, P.S.; Moore, B.S. Functional characterization of the cyclomarin/cyclomarazine prenyltransferase CymD directs the biosynthesis of unnatural cyclic peptides. J. Nat. Prod. 2010, 73, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.S.; de Wet, J.R.; Cavalcoli, J.; Li, S.Y.; Greshock, T.J.; Miller, K.A.; Finefield, J.M.; Sunderhaus, J.D.; McAfoos, T.J.; Tsukamoto, S.; et al. Genome-based characterization of two prenylation steps in the assembly of the stephacidin and notoamide anticancer agents in a marine-derived Aspergillus sp. J. Am. Chem. Soc. 2010, 132, 12733–12740. [Google Scholar] [CrossRef] [PubMed]
- McAfoos, T.J.; Li, S.Y.; Tsukamoto, S.; Sherman, D.H.; Williams, R.M. Studies on the biosynthesis of the stephacidins and notoamides. Total synthesis of notoamide S. Heterocycles 2010, 82, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Afiyatullov, S.S.; Zhuravleva, O.I.; Chaikina, E.L.; Anisimov, M.M. A new spirotryprostatin from the marine isolate of the fungus Aspergillus fumigatus. Chem. Nat. Compd. 2012, 48, 95–98. [Google Scholar] [CrossRef]
- He, F.; Han, Z.; Peng, J.; Qian, P.Y.; Qi, S.H. Antifouling indole alkaloids from two marine derived fungi. Nat. Prod. Commun. 2013, 8, 329–332. [Google Scholar] [PubMed]
- Tsukamoto, S.; Kato, H.; Samizo, M.; Nojiri, Y.; Onuki, H.; Hirota, H.; Ohta, T. Notoamides F–K, prenylated indole alkaloids isolated from a marine-derived Aspergillus sp. J. Nat. Prod. 2008, 71, 2064–2067. [Google Scholar] [CrossRef] [PubMed]
- Qian-Cutrone, J.F.; Huang, S.; Shu, Y.Z.; Vyas, D.; Fairchild, C.; Menendez, A.; Krampitz, K.; Dalterio, R.; Klohr, S.E.; Gao, Q. Stephacidin A and B: Two structurally novel, selective inhibitors of the testosterone-dependent prostate LNCaP cells. J. Am. Chem. Soc. 2002, 124, 14556–14557. [Google Scholar] [CrossRef] [PubMed]
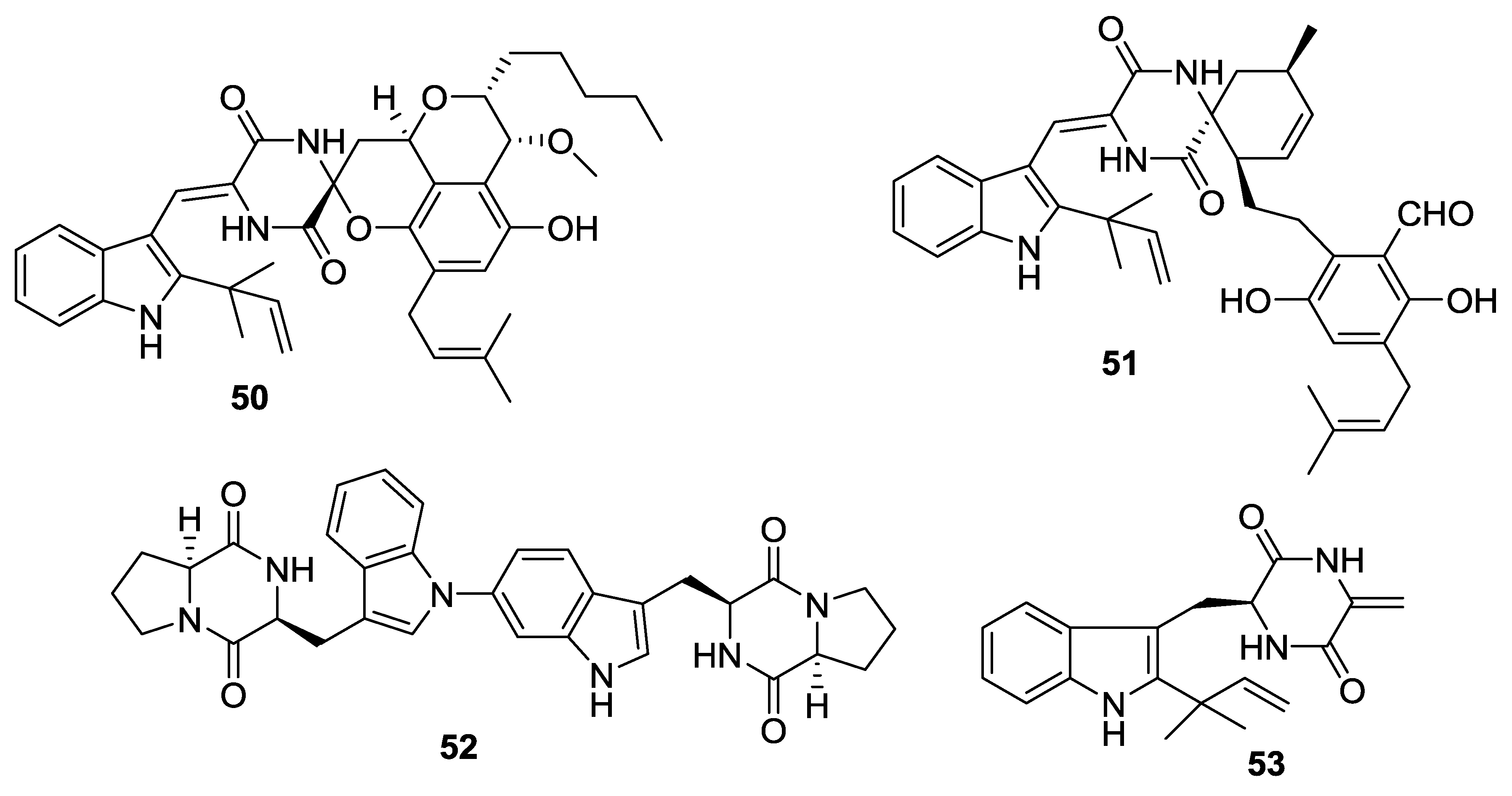
- Yang, B.; Dong, J.D.; Zhou, X.F.; Yang, X.W.; Lee, K.J.; Wang, L.S.; Zhang, S.; Liu, Y.H. Proline-containing dipeptides from a marine sponge of a Callyspongia species. Helv. Chim. Acta 2009, 92, 1112–1117. [Google Scholar] [CrossRef]
- Ovenden, S.P.B.; Nielson, J.L.; Liptrot, C.H.; Willis, R.H.; Tapiolas, D.M.; Wright, A.D.; Motti, C.A. A new diketopiperazine, cyclo-(4-S-hydroxy-R-proline-R-isoleucine), from an Australian specimen of the sponge Stelletta sp. Mar. Drugs 2011, 9, 2469–2478. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.N.; Peng, Y.; Gao, C.H.; Huang, R.M. A new diketopiperazine from South China Sea marine sponge Callyspongia sp. Nat. Prod. Res. 2014, 28, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.H.; Lin, L.; Long, B.; Chen, Y.N.; He, B.J.; Sun, H.Y.; Huang, R.M. A new diketopiperazine from the gorgonian coral Menella kanisa. Nat. Prod. Res. 2014, 28, 473–476. [Google Scholar] [CrossRef] [PubMed]
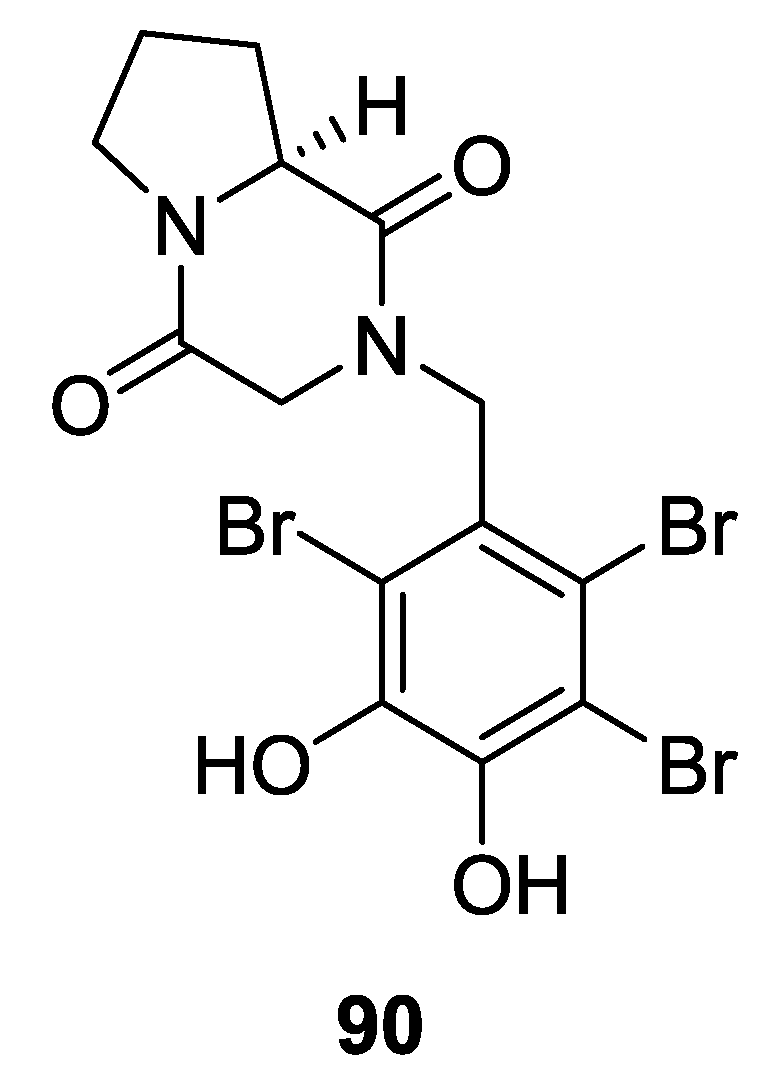
- Xu, X.L.; Yin, L.Y.; Fang, N.Q.; Fan, X.; Song, F.H. Bromophenol coupled with diketopiperazine from marine red alga Symphyocladia latiuscula. Chem. Nat. Compd. 2012, 48, 622–624. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2014, 31, 160–258. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2013, 30, 237–323. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, R.-M.; Yi, X.-X.; Zhou, Y.; Su, X.; Peng, Y.; Gao, C.-H. An Update on 2,5-Diketopiperazines from Marine Organisms. Mar. Drugs 2014, 12, 6213-6235. https://doi.org/10.3390/md12126213
Huang R-M, Yi X-X, Zhou Y, Su X, Peng Y, Gao C-H. An Update on 2,5-Diketopiperazines from Marine Organisms. Marine Drugs. 2014; 12(12):6213-6235. https://doi.org/10.3390/md12126213
Chicago/Turabian StyleHuang, Ri-Ming, Xiang-Xi Yi, Yuying Zhou, Xiangdong Su, Yan Peng, and Cheng-Hai Gao. 2014. "An Update on 2,5-Diketopiperazines from Marine Organisms" Marine Drugs 12, no. 12: 6213-6235. https://doi.org/10.3390/md12126213
APA StyleHuang, R.-M., Yi, X.-X., Zhou, Y., Su, X., Peng, Y., & Gao, C.-H. (2014). An Update on 2,5-Diketopiperazines from Marine Organisms. Marine Drugs, 12(12), 6213-6235. https://doi.org/10.3390/md12126213





