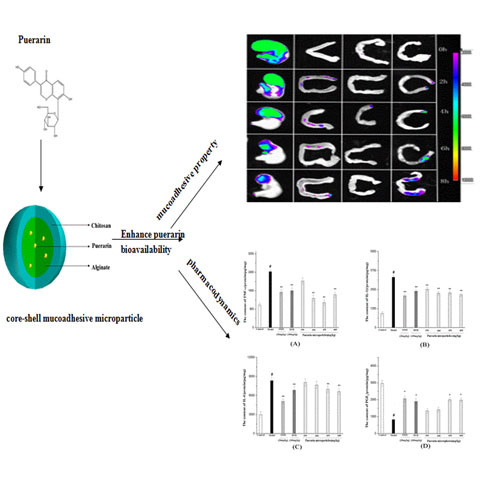
Mucoadhesive Microparticles for Gastroretentive Delivery: Preparation, Biodistribution and Targeting Evaluation
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Animals
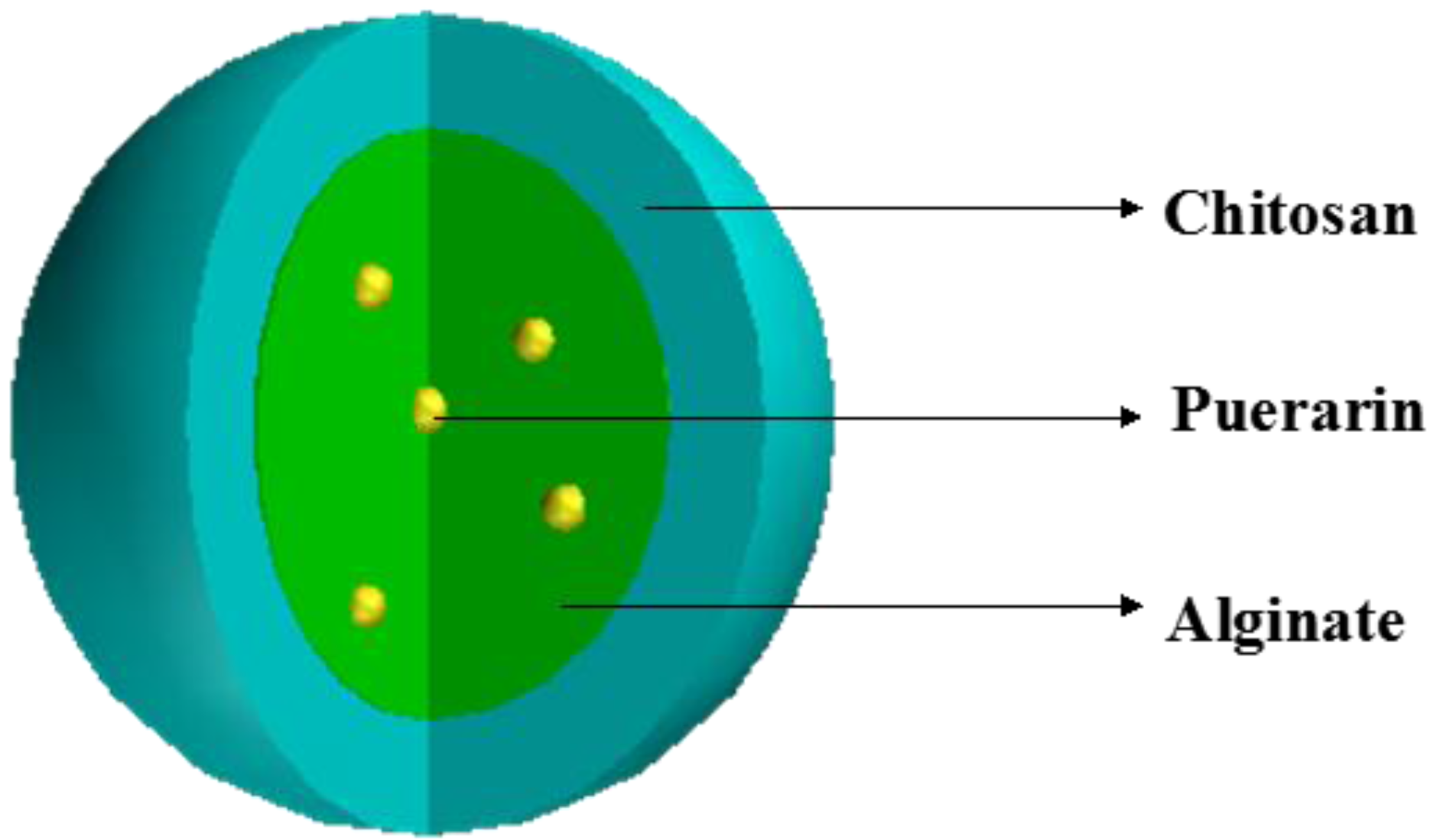
2.2. Preparation of Mucoadhesive Microparticles
| Formulation | Composition (as(w/w) Ratio) | Coagulation Fluid (%(w/v)) | Mean Size (μm) (±S.D.) | |
|---|---|---|---|---|
| Alginate | Puerarin | Chitosan | ||
| A1 | 1 | 0.5 | 0 | 50.3 ± 11.2 |
| A2 | 1 | 0.5 | 0.4 | 69.3 ± 17.6 |
| A3 | 1 | 0.5 | 0.7 | 86.3 ± 20.5 |
| A4 | 1 | 0.5 | 1 | 112.3 ± 7.8 |
| A5 | 1 | 0.5 | 1.3 | 124.7 ± 25.6 |
2.3. Characterization of the Microparticles
2.3.1. Morphological Examination
2.3.2. Particle Size Measurement
2.3.3. Differential Scanning Calorimetry (DSC) Analysis
2.3.4. Fourier Transform Infrared Spectroscopy (FT-IR)
2.4. Determination of Drug Loading, Encapsulation Efficiency and Swelling Ratio
2.5. Drug Release Study in Vitro
2.6. Evaluation of Mucoadhesiveness in Vitro
2.7. Fluorescence Imaging of the Gastrointestinal Tract
2.8. Gastro-Protective Studies in Rats
2.8.1. Ethanol-Induced Gastric Injury in Rats
2.8.2. Determination of the Ulcer Index and Percent Inhibition
2.8.3. Histopathology
2.8.4. Measurement of Cytokine, Production (TNF-α, 1L-1β, IL-6 and PGE2)
2.9. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the Puerarin Microparticles
3.1.1. Morphological Observations of Puerarin Microparticles
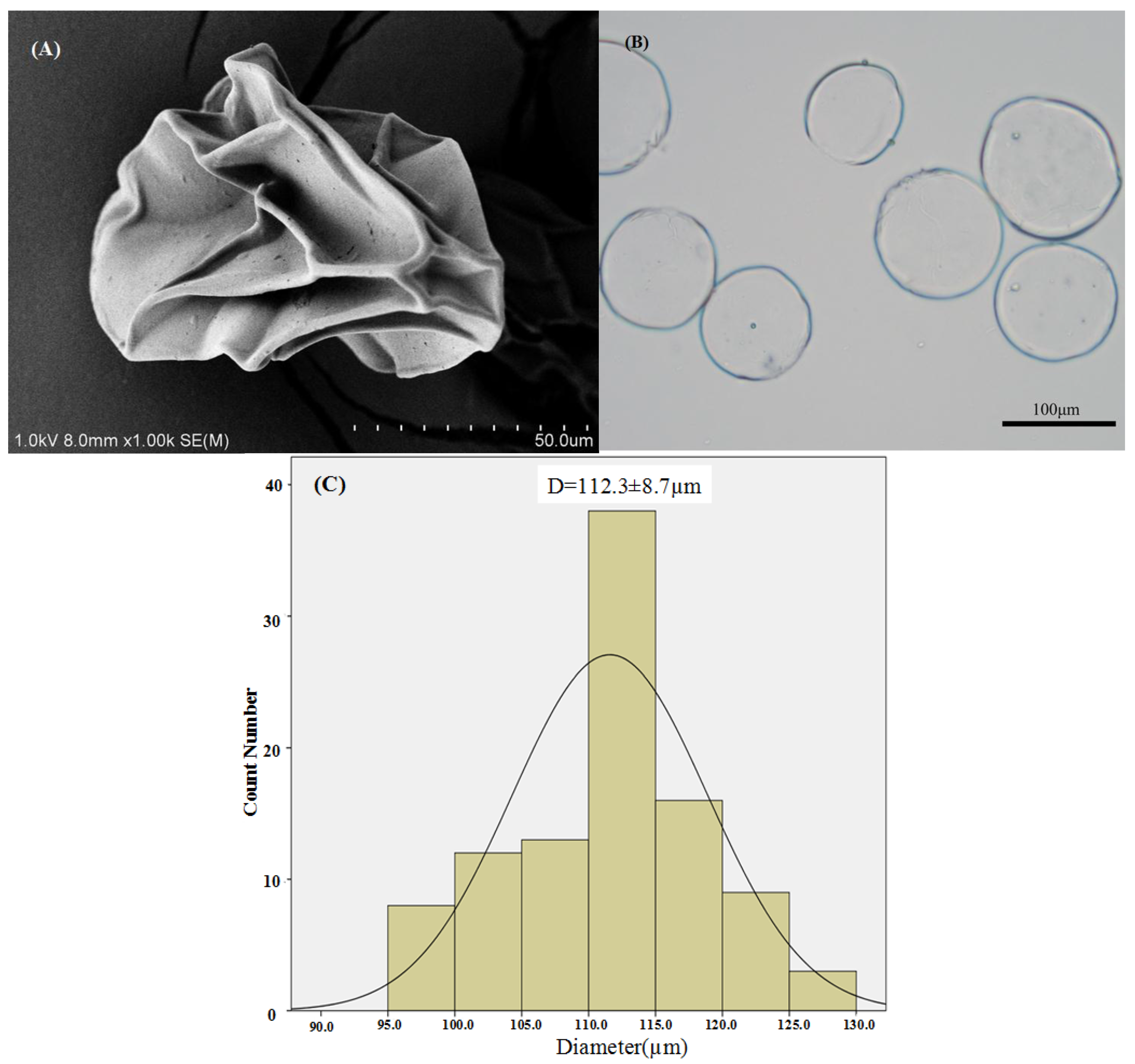
3.1.2. Particle Size
3.1.3. DSC analysis
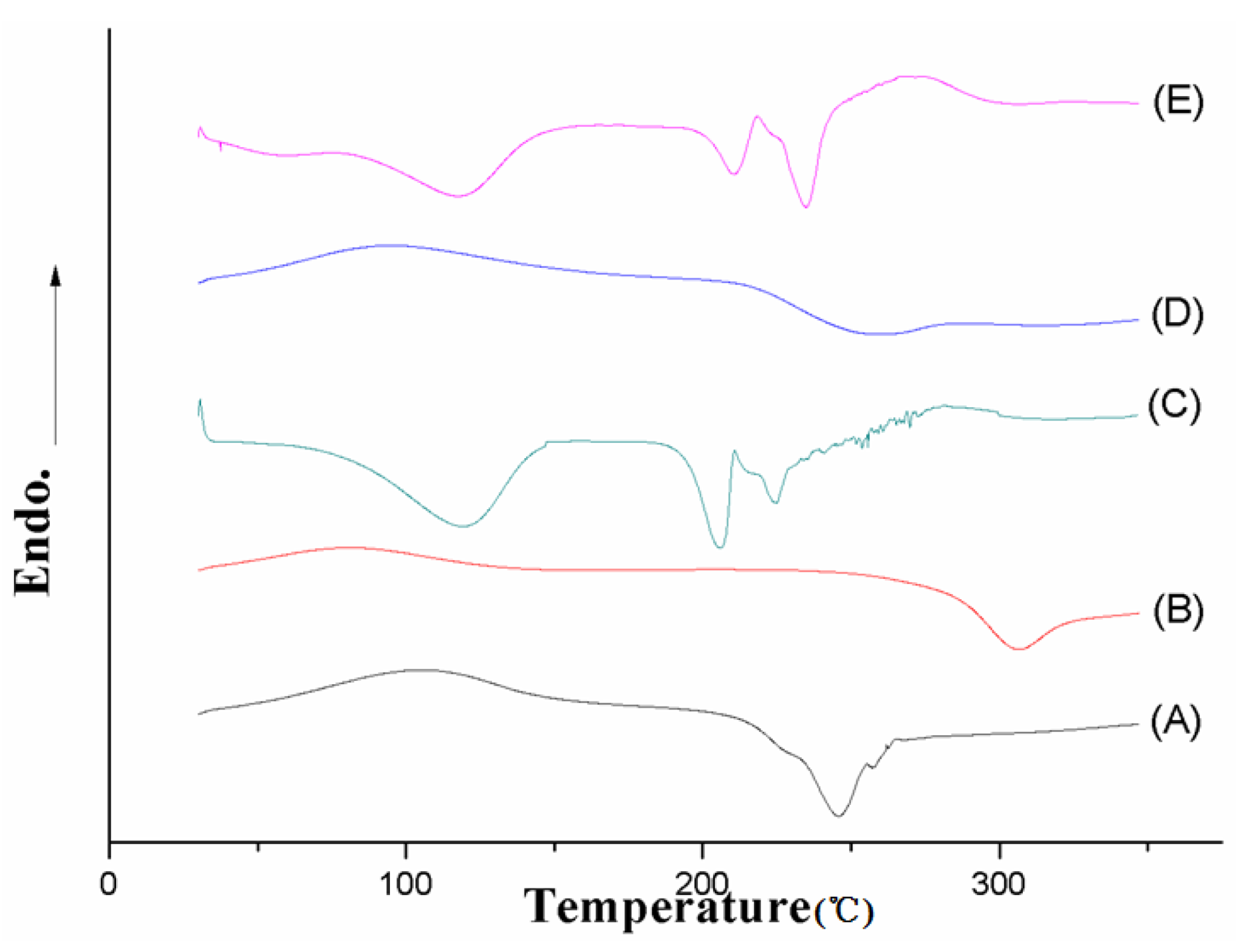
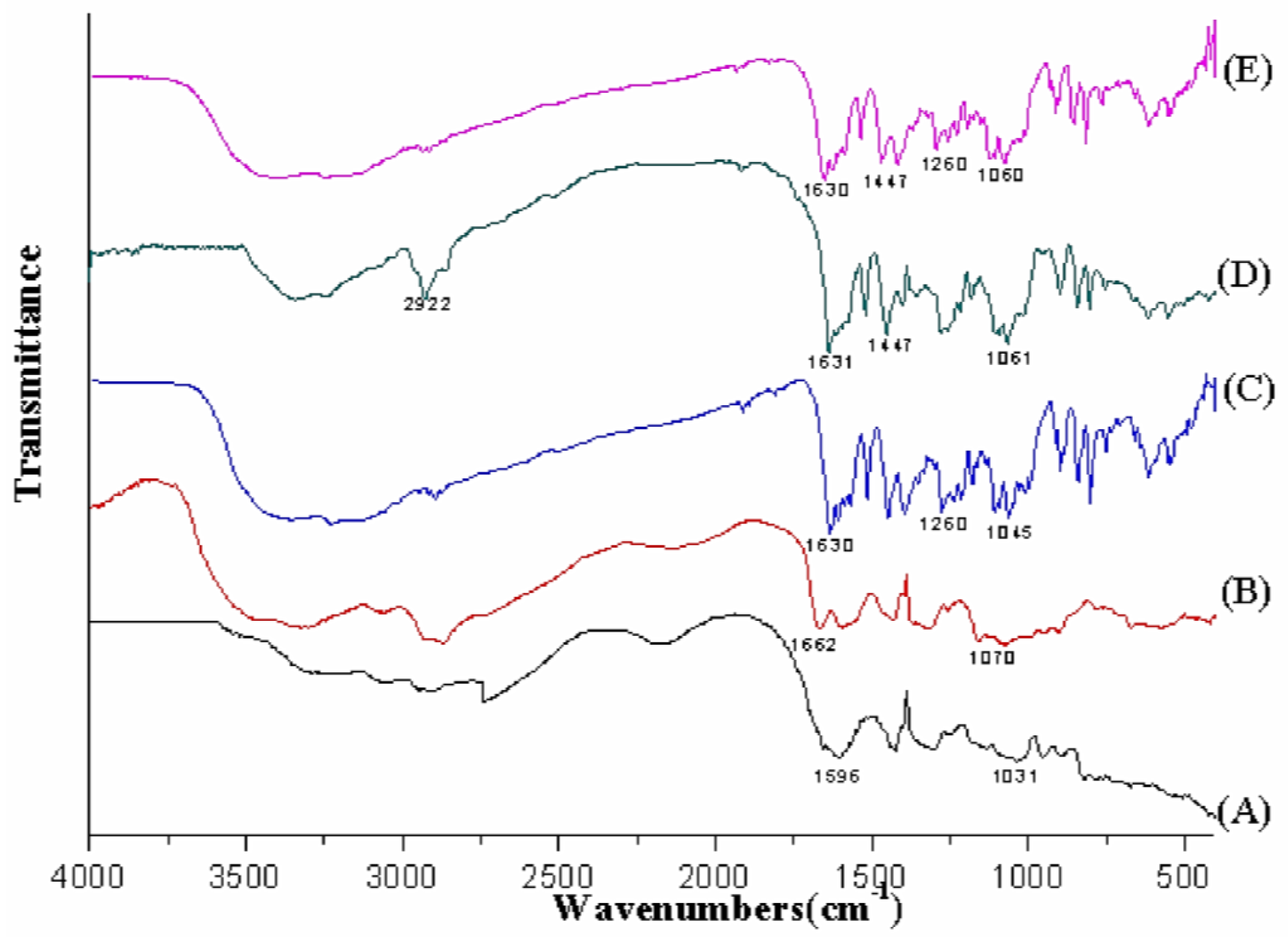
3.1.4. FT-IR Spectroscopy
3.2. Drug Loading, Encapsulation Efficiency and Swelling
| Formulation | Entrapment Efficiency (%) | Drug Loading (%) | Swelling Ratio (%) (±S.D.) |
|---|---|---|---|
| A1 | 70.3 | 5.1 | 69 ± 11 |
| A2 | 84.3 | 10.4 | 132 ± 18 |
| A3 | 90.8 | 15.9 | 179 ± 12 |
| A4 | 98.5 | 34.7 | 222 ± 2 |
| A5 | 99.2 | 23.2 | 175 ± 11 |
3.3. In Vitro Drug Release Results

3.4. In Vitro Evaluation of Mucoadhesiveness
| Number | % of Microparticles Remaining | |
|---|---|---|
| Stomach SGF | Small Intestine SIF | |
| 1 | 92.5 | 30.5 |
| 2 | 91.0 | 29.0 |
| 3 | 94.5 | 32.5 |
| Average | 92.6 ± 1.7 | 30.6 ± 1.7 |
3.5. Fluorescence Imaging of the Gastrointestinal Tract
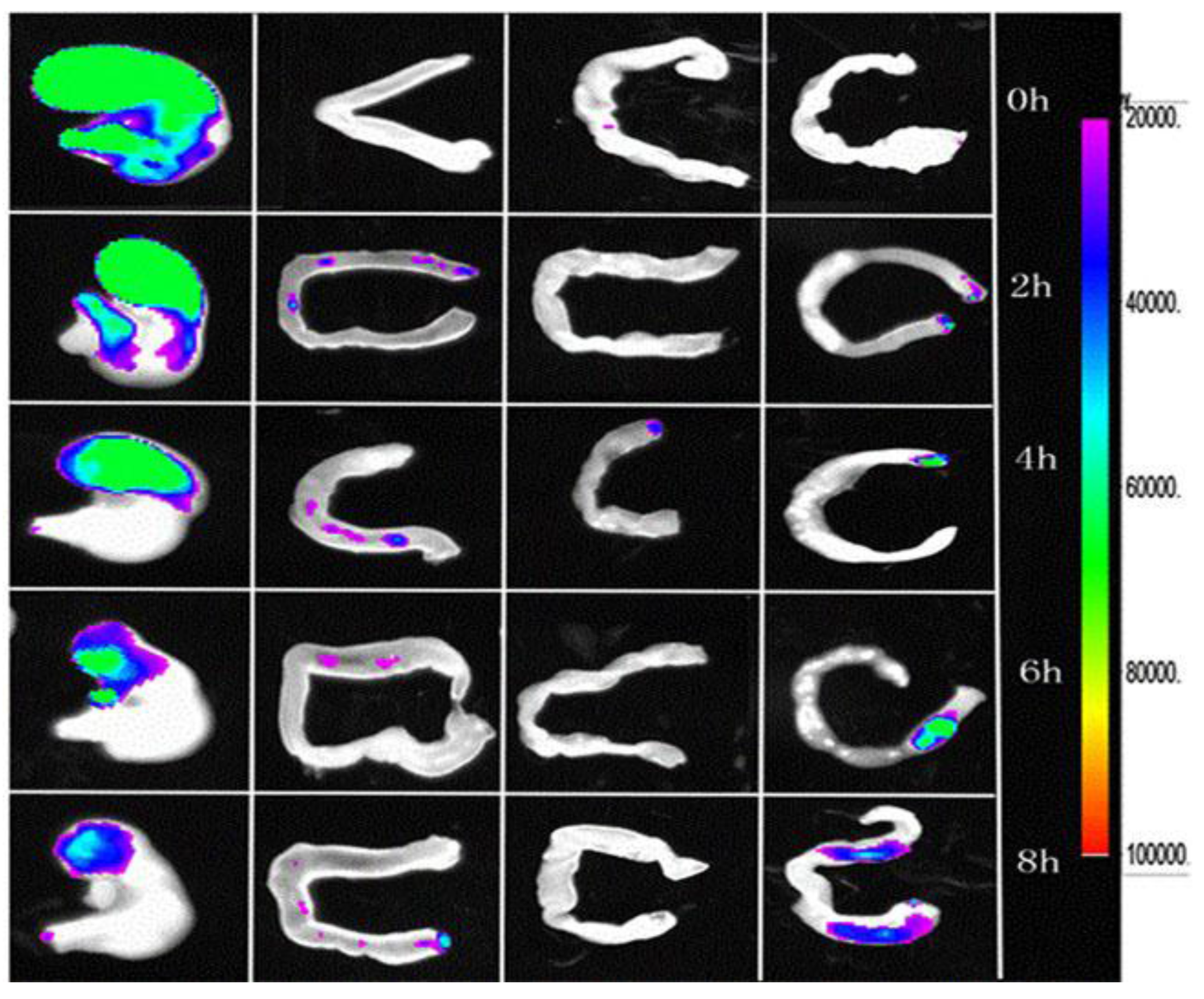
3.6. Gastro-Protective Study Results
3.6.1. Ulcer Index and Percent Inhibition
| Animal Group | Pre-Treatment | Ulcer Index (Score) (Mean ± S.E.) | Inhibition (%) |
|---|---|---|---|
| A | Normal control | -- | -- |
| B | Ulcer control | 24.72 ± 1.32 | 0 |
| C | Omeprazole (20 mg/kg) | 4.93 ± 0.36 | 80.0 |
| D | Rebamipide (100 mg/kg) | 5.61 ± 0.55 | 77.3 |
| E | Microspheres (150 mg/kg) | 19.96 ± 1.15 | 19.3 |
| F | Microspheres (300 mg/kg) | 12.53 ± 0.98 | 49.3 |
| G | Microspheres (450 mg/kg) | 7.92 ± 0.76 | 68.0 |
| H | Microspheres (600 mg/kg) | 5.87 ± 0.61 | 76.2 |

3.6.2. Histological Results

3.6.3. Measurement of TNF-α, 1L-1β, IL-6 Expression and PGE2 Production

4. Conclusions
Acknowledgments
Author Contributions
Conflict of Interest
References
- Yeomans, N.; Naesdal, J. Systematic review: Ulcer definition in NSAID ulcer prevention trials. Aliment. Pharmacol. Ther. 2008, 27, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Anal, A.K.; Bhopatkar, D.; Tokura, S.; Tamura, H.; Stevens, W.F. Chitosan-alginate multilayer beads for gastric passage and controlled intestinal release of protein. Drug Dev. Ind. Pharm. 2003, 29, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Arao, T.; Udayama, M.; Kinjo, J.; Nohara, T.; Funakoshi, T.; Kojima, S. Preventive effects of saponins from puerariae radix (the root of Pueraria lobata Ohwi) on in vitro immunological injury of rat primary hepatocyte cultures. Biol. Pharm. Bull. 1997, 20, 988–991. [Google Scholar] [CrossRef] [PubMed]
- Artursson, P.; Lindmark, T.; Davis, S.S.; Illum, L. Effect of chitosan on the permeability of monolayers of intestinal epithelial cells (Caco-2). Pharm. Res. 1994, 11, 1358–1361. [Google Scholar] [CrossRef] [PubMed]
- Axon, A. The role of acid inhibition in the treatment of Helicobacter pylori infection. Scand. J. Gastroenterol. 1994, 29, 16–23. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Guggi, D.; Pinter, Y. Thiolated chitosans: development and in vitro evaluation of a mucoadhesive, permeation enhancing oral drug delivery system. J. Control. Release 2004, 94, 177–186. [Google Scholar] [CrossRef]
- Fontana, G.; Licciardi, M.; Mansueto, S.; Schillaci, D.; Giammona, G. Amoxicillin-loaded polyethylcyanoacrylate nanoparticles: Influence of PEG coating on the particle size, drug release rate and phagocytic uptake. Biomaterials 2001, 22, 2857–2865. [Google Scholar] [CrossRef] [PubMed]
- Hunt, R.H.; Yuan, Y. Acid-NSAID/Aspirin Interaction in Peptic Ulcer Disease. Dig. Dis. 2011, 29, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, S.; Jain, S.; Singh, H.P.; Tiwary, A. Mucoadhesive microspheres for gastroretentive delivery of acyclovir: In vitro and in vivo evaluation. AAPS J. 2008, 10, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Le Bail, P.; Morin, F.G.; Marchessault, R.H. Characterization of a crosslinked high amylose starch excipient. Int. J. Biol. Macromol. 1999, 26, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Hasani, S.; Pellequer, Y.; Lamprecht, A. Selective adhesion of nanoparticles to inflamed tissue in gastric ulcers. Pharm. Res. 2009, 26, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.K.; Chavda, J.R. Formulation and evaluation of stomach-specific amoxicillin-loaded carbopol-934P mucoadhesive microspheres for anti-Helicobacter pylori therapy. J. Microencapsul. 2009, 26, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Schmidgall, J.; Hensel, A. Bioadhesive properties of polygalacturonides against colonic epithelial membranes. Int. J. Biol. Macromol. 2002, 30, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, N.; Akiyama, Y.; Nakao, M.; Tada, M.; Kitano, M.; Ogawa, Y. Mucoadhesive microspheres containing amoxicillin for clearance of Helicobacter pylori. Antimicrob. Agents Chemother. 1998, 42, 2492–2494. [Google Scholar] [PubMed]
- Han, J.; Zhou, Z.; Yin, R.; Yang, D.; Nie, J. Alginate–chitosan/hydroxyapatite polyelectrolyte complex porous scaffolds: Preparation and characterization. Int. J. Biol. Macromol. 2010, 46, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Patel, Y.L.; Sher, P.; Pawar, A.P. The effect of drug concentration and curing time on processing and properties of calcium alginate beads containing metronidazole by response surface methodology. AAPS PharmSciTech 2006, 7, E24–E30. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.-H.; Song, Y.-B.; Chang, P.-S.; Lee, H.G. Microencapsulation of α-tocopherol using sodium alginate and its controlled release properties. Int. J. Biol. Macromol. 2006, 38, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Smrdel, P.; Bogataj, M.; Mrhar, A. The influence of selected parameters on the size and shape of alginate beads prepared by ionotropic gelation. Sci. Pharm. 2008, 76, 77. [Google Scholar] [CrossRef]
- Liu, Q.; Rauth, A.M.; Liu, J.; Babakhanian, K.; Wang, X.; Bendayan, R.; Wu, X.Y. Characterization of a microsphere formulation containing glucose oxidase and its in vivo efficacy in a murine solid tumor model. Pharm. Res. 2009, 26, 2343–2357. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Solanki, S.; Chaudhari, R.; Bahadur, D.; Aslam, M.; Srivastava, R. Multifunctional alginate microspheres for biosensing, drug delivery and magnetic resonance imaging. Acta Biomater. 2011, 7, 3955–3963. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; Ma, H.; Li, X.; Yang, H.; Wang, A. pH-sensitive sodium alginate/poly (vinyl alcohol) hydrogel beads prepared by combined Ca2+ crosslinking and freeze-thawing cycles for controlled release of diclofenac sodium. Int. J. Biol. Macromol. 2010, 46, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.; Nayak, A.K. Development, optimization, and anti-diabetic activity of gliclazide-loaded alginate–methyl cellulose mucoadhesive microcapsules. AAPS PharmSciTech 2011, 12, 1431–1441. [Google Scholar] [CrossRef] [PubMed]
- Janes, K.; Calvo, P.; Alonso, M. Polysaccharide colloidal particles as delivery systems for macromolecules. Adv. Drug Deliv. Rev. 2001, 47, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Finne-Wistrand, A.; Albertsson, A.-C. Facile Synthesis of Degradable and Electrically Conductive Polysaccharide Hydrogels. Biomacromolecules 2011, 12, 2601–2609. [Google Scholar] [CrossRef] [PubMed]
- Whang, H.S.; Kirsch, W.; Zhu, Y.H.; Yang, C.Z.; Hudson, S.M. Hemostatic agents derived from chitin and chitosan. J. Macromol. Sci. Polym. Rev. 2005, 309–323. [Google Scholar] [CrossRef]
- Ji, C.; Khademhosseini, A.; Dehghani, F. Enhancing cell penetration and proliferation in chitosan hydrogels for tissue engineering applications. Biomaterials 2011, 32, 9719–9729. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.-Y.; Wu, J.; Moochhala, S.M.; Tan, M.-H.; Lu, J. Development of a chitosan-based wound dressing with improved hemostatic and antimicrobial properties. Biomaterials 2008, 29, 4323–4332. [Google Scholar] [CrossRef] [PubMed]
- Tronci, G.; Ajiro, H.; Russell, S.J.; Wood, D.J.; Akashi, M. Tunable drug-loading capability of chitosan hydrogels with varied network architectures. Acta Biomater. 2014, 10, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Strand, S.P.; Lelu, S.; Reitan, N.K.; Davies, C.D.L.; Artursson, P.; Varum, K.M. Molecular design of chitosan gene delivery systems with an optimized balance between polyplex stability and polyplex unpacking. Biomaterials 2010, 31, 975–987. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Maeda, T.; Miyamoto, E.; Kawashima, S. Preparation of chitosan-reinforced alginate gel beads—Effects of chitosan on gel matrix erosion. Int. J. Pharm. 1993, 96, 139–145. [Google Scholar] [CrossRef]
- Liu, L.-S.; Liu, S.-Q.; Ng, S.Y.; Froix, M.; Ohno, T.; Heller, J. Controlled release of interleukin-2 for tumour immunotherapy using alginate/chitosan porous microspheres. J. Control. Release 1997, 43, 65–74. [Google Scholar] [CrossRef]
- Porporatto, C.; Bianco, I.D.; Correa, S.G. Local and systemic activity of the polysaccharide chitosan at lymphoid tissues after oral administration. J. Leukocyte Biol. 2005, 78, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Grabovac, V.; Guggi, D.; Bernkop-Schnürch, A. Comparison of the mucoadhesive properties of various polymers. Adv. Drug Deliv. Rev. 2005, 57, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Liang, T.; Duan, X.; Xu, L.; Zhang, K.; Li, R. Anti-diabetic effects of puerarin, isolated from Pueraria lobata (Willd.), on streptozotocin-diabetogenic mice through promoting insulin expression and ameliorating metabolic function. Food Chem. Toxicol. 2013, 60, 341–347. [Google Scholar]
- Xiao, C.; Li, J.; Dong, X.; He, X.; Niu, X.; Liu, C.; Zhong, G.; Bauer, R.; Yang, D.; Lu, A. Anti-oxidative and TNF-α suppressive activities of puerarin derivative (4AC) in RAW264. 7 cells and collagen-induced arthritic rats. Eur. J. Pharmacol. 2011, 666, 242–250. [Google Scholar]
- Cheng, Y.-F.; Zhu, G.-Q.; Wang, M.; Cheng, H.; Zhou, A.; Wang, N.; Fang, N.; Wang, X.-C.; Xiao, X.-Q.; Chen, Z.-W. Involvement of ubiquitin proteasome system in protective mechanisms of Puerarin to MPP+-elicited apoptosis. Neurosci. Res. 2009, 63, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Keung, W.M.; Klyosov, A.A.; Vallee, B.L. Daidzin inhibits mitochondrial aldehyde dehydrogenase and suppresses ethanol intake of Syrian golden hamsters. Proc. Natl. Acad. Sci. USA 1997, 94, 1675–1679. [Google Scholar] [CrossRef] [PubMed]
- Keung, W.-M.; Vallee, B.L. Daidzin and daidzein suppress free-choice ethanol intake by Syrian golden hamsters. Proc. Natl. Acad. Sci. USA 1993, 90, 10008–10012. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ji, G.; Liu, J. Reversal of chemical-induced liver fibrosis in Wistar rats by puerarin. J. Nutr. Biochem. 2006, 17, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, J.; Hu, Z.; Xie, Y. Protective effect of puerarin on stress-induced gastric mucosal injury in rats. China J. Chin. Mater. Med. 2006, 31, 504–506. [Google Scholar]
- Poncelet, D.; Lencki, R.; Beaulieu, C.; Halle, J.; Neufeld, R.; Fournier, A. Production of alginate beads by emulsification/internal gelation. I. Methodology. Appl. Microbiol. Biotechnol. 1992, 38, 39–45. [Google Scholar]
- Rao, K.; Buri, P. A novel in situ method to test polymers and coated microparticles for bioadhesion. Int. J. Pharm. 1989, 52, 265–270. [Google Scholar] [CrossRef]
- Huang, M.; Khor, E.; Lim, L.-Y. Uptake and cytotoxicity of chitosan molecules and nanoparticles: Effects of molecular weight and degree of deacetylation. Pharm. Res. 2004, 21, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Nwafor, P.A.; Okwuasaba, F.; Binda, L. Antidiarrhoeal and antiulcerogenic effects of methanolic extract of Asparagus pubescens root in rats. J. Ethnopharmacol. 2000, 72, 421–427. [Google Scholar] [CrossRef] [PubMed]
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan—A review. J. Control. Release 2006, 114, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Buri, P.A. Surface, interfacial and molecular aspects of polymer bioadhesion on soft tissues. J. Control. Release 1985, 2, 257–275. [Google Scholar] [CrossRef]
- Torelli-Souza, R.R.; Cavalcante Bastos, L.A.; Nunes, H.G.; Camara, C.A.; Amorim, R.V.S. Sustained release of an antitumoral drug from alginate-chitosan hydrogel beads and its potential use as colonic drug delivery. J. Appl. Polym. Sci. 2012, 126, E409–E418. [Google Scholar] [CrossRef]
- Mortazavi, S.A.; Smart, J.D. An investigation into the role of water movement and mucus gel dehydration in mucoadhesion. J. Control. Release 1993, 25, 197–203. [Google Scholar] [CrossRef]
- Duchêne, D.; Ponchel, G. Principle and investigation of the bioadhesion mechanism of solid dosage forms. Biomaterials 1992, 13, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Sahasathian, T.; Praphairaksit, N.; Muangsin, N. Mucoadhesive and floating chitosan-coated alginate beads for the controlled gastric release of amoxicillin. Arch. Pharm. Res. 2010, 33, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Amiji, M.M. Tetracycline-containing chitosan microspheres for local treatment of Helicobacter pylori infection. Cellulose 2007, 14, 3–14. [Google Scholar] [CrossRef]
- Li, C.-Y.; Xu, H.-D.; Zhao, B.-T.; Chang, H.-I.; Rhee, H.-I. Gastroprotective effect of cyanidin 3-glucoside on ethanol-induced gastric lesions in rats. Alcohol 2008, 42, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.K.-S.; Cho, C.-H.; Lam, S.-K. Adaptive cytoprotection through modulation of nitric oxide in ethanol-evoked gastritis. World J. Gastroenterol. 2004, 10, 2503–2508. [Google Scholar] [PubMed]
- Gómez-Burgaz, M.; García-Ochoa, B.; Torrado-Santiago, S. Chitosan–carboxymethylcellulose interpolymer complexes for gastric-specific delivery of clarithromycin. Int. J. Pharm. 2008, 359, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Fatovic-Ferencic, S.; Banic, M. No Acid, No Ulcer: Dragutin (Carl) Schwarz (1868–1917), the Man Ahead of His Time. Dig. Dis. 2011, 29, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Ohta, Y.; Kobayashi, T.; Inui, K.; Yoshino, J.; Nakazawa, S. Protective effect of ebselen, a seleno-organic compound, against the progression of acute gastric mucosal lesions induced by compound 48/80, a mast cell degranulator, in rats. Jpn. J. Pharmacol. 2002, 90, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Kleine, A.; Kluge, S.; Peskar, B.M. Stimulation of prostaglandin biosynthesis mediates gastroprotective effect of rebamipide in rats. Dig. Dis. Sci. 1993, 38, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.P.; Choi, C.Y.; Chung, Y.C.; Jeon, S.S.; Jeong, H.G. Protective effects of puerarin on carbon tetrachloride-induced hepatotoxicity. Arch. Pharm. Res. 2007, 30, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.-H.; Wang, L.; Wang, D.; Jiang, H.; Tang, Q.-Z.; Yan, L.; Bian, Z.-Y.; Wang, X.-A.; Li, H. Puerarin attenuates high-glucose-and diabetes-induced vascular smooth muscle cell proliferation by blocking PKC beta 2/Rac1-dependent signaling. Free Radic. Biol. Med. 2010, 48, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhang, Q.; Yang, X.; Wang, Y.; Sun, L. Puerarin Inhibits Adhesion Molecule Expression in TNF-alpha-Stimulated Human Endothelial Cells via Modulation of the Nuclear Factor kappa B Pathway. Pharmacology 2010, 85, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.S.L.; Cho, C.H. Relationship between ethanol-induced gastritis and gastric ulcer formation in rats. Digestion 2000, 62, 232–239. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, J.-Y.; Gao, L.-N.; Meng, F.-Y.; Cui, Y.-L. Mucoadhesive Microparticles for Gastroretentive Delivery: Preparation, Biodistribution and Targeting Evaluation. Mar. Drugs 2014, 12, 5764-5787. https://doi.org/10.3390/md12125764
Hou J-Y, Gao L-N, Meng F-Y, Cui Y-L. Mucoadhesive Microparticles for Gastroretentive Delivery: Preparation, Biodistribution and Targeting Evaluation. Marine Drugs. 2014; 12(12):5764-5787. https://doi.org/10.3390/md12125764
Chicago/Turabian StyleHou, Jing-Yi, Li-Na Gao, Fan-Yun Meng, and Yuan-Lu Cui. 2014. "Mucoadhesive Microparticles for Gastroretentive Delivery: Preparation, Biodistribution and Targeting Evaluation" Marine Drugs 12, no. 12: 5764-5787. https://doi.org/10.3390/md12125764
APA StyleHou, J.-Y., Gao, L.-N., Meng, F.-Y., & Cui, Y.-L. (2014). Mucoadhesive Microparticles for Gastroretentive Delivery: Preparation, Biodistribution and Targeting Evaluation. Marine Drugs, 12(12), 5764-5787. https://doi.org/10.3390/md12125764