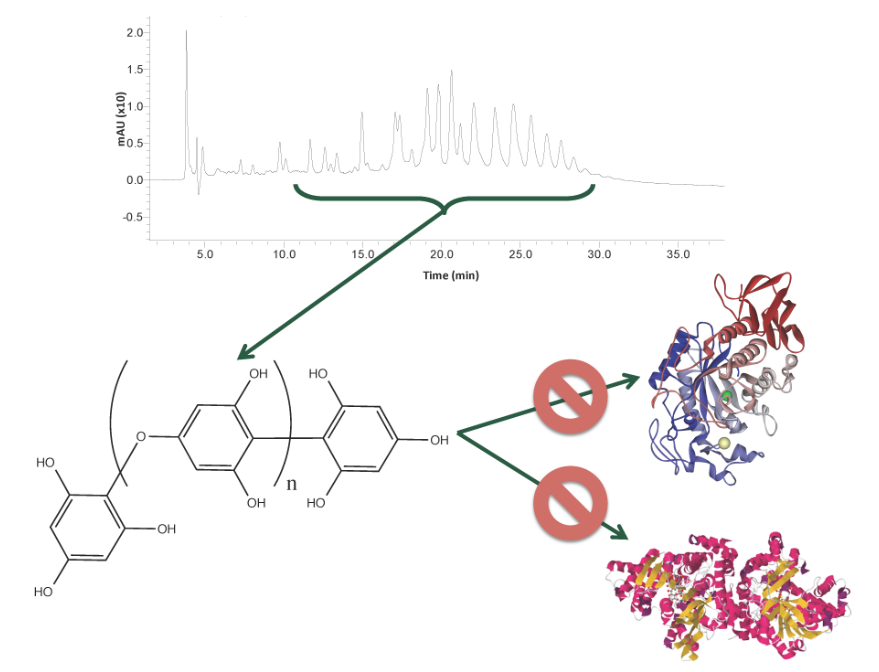
Phlorotannins from Alaskan Seaweed Inhibit Carbolytic Enzyme Activity
Abstract
:1. Introduction
2. Results and Discussion
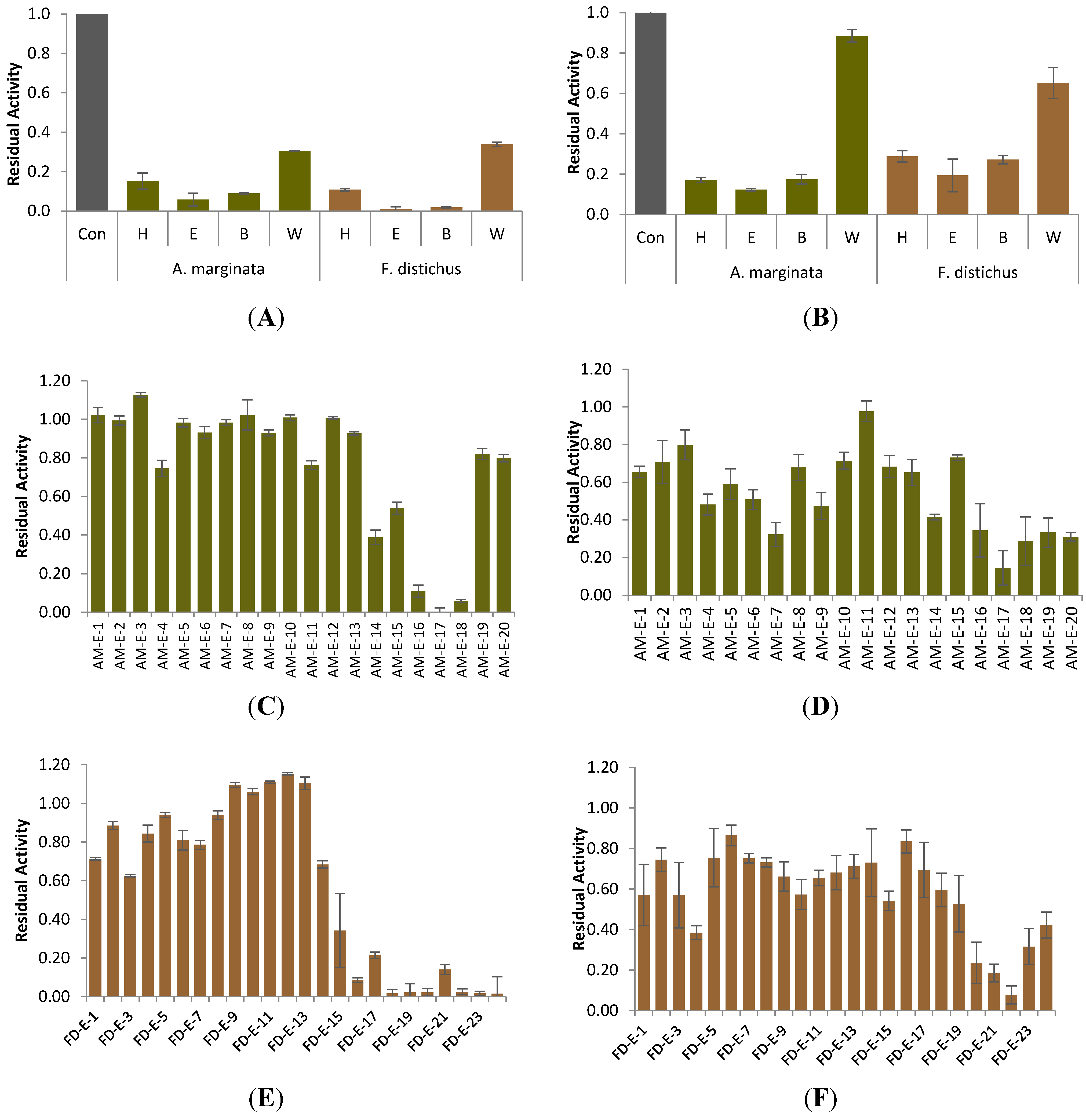
2.1. Carbolytic Enzyme Inhibition
| Sample | Crude Extract Yield (g) | α-Glucosidase | α-Amylase |
|---|---|---|---|
| Alaria marginata | 9.537 | 6.4 ± 0.8 a *** | 17.9 ± 4.1 a *** |
| Fucus distichus | 11.198 | 3.0 ± 1.2 a *** | 18.4 ± 5.3 a *** |
| Saccharina groenlandica | 11.595 | 76.1 ± 5.0 b *** | 65.5 ± 3.2 b * |
| Saccharina latissima | 13.011 | 75.1 ± 3.1 b *** | 56.3 ± 9.8 b * |
| Pyropia fallax | 7.780 | 86.6 ± 6.1 c | 62.2 ± 8.2 b * |
| Ulva lactuca | 7.982 | 88.0 ± 6.7 c | 82.4 ± 9.4 c |
2.2. Comparison of Inhibitory Activity with Acarbose
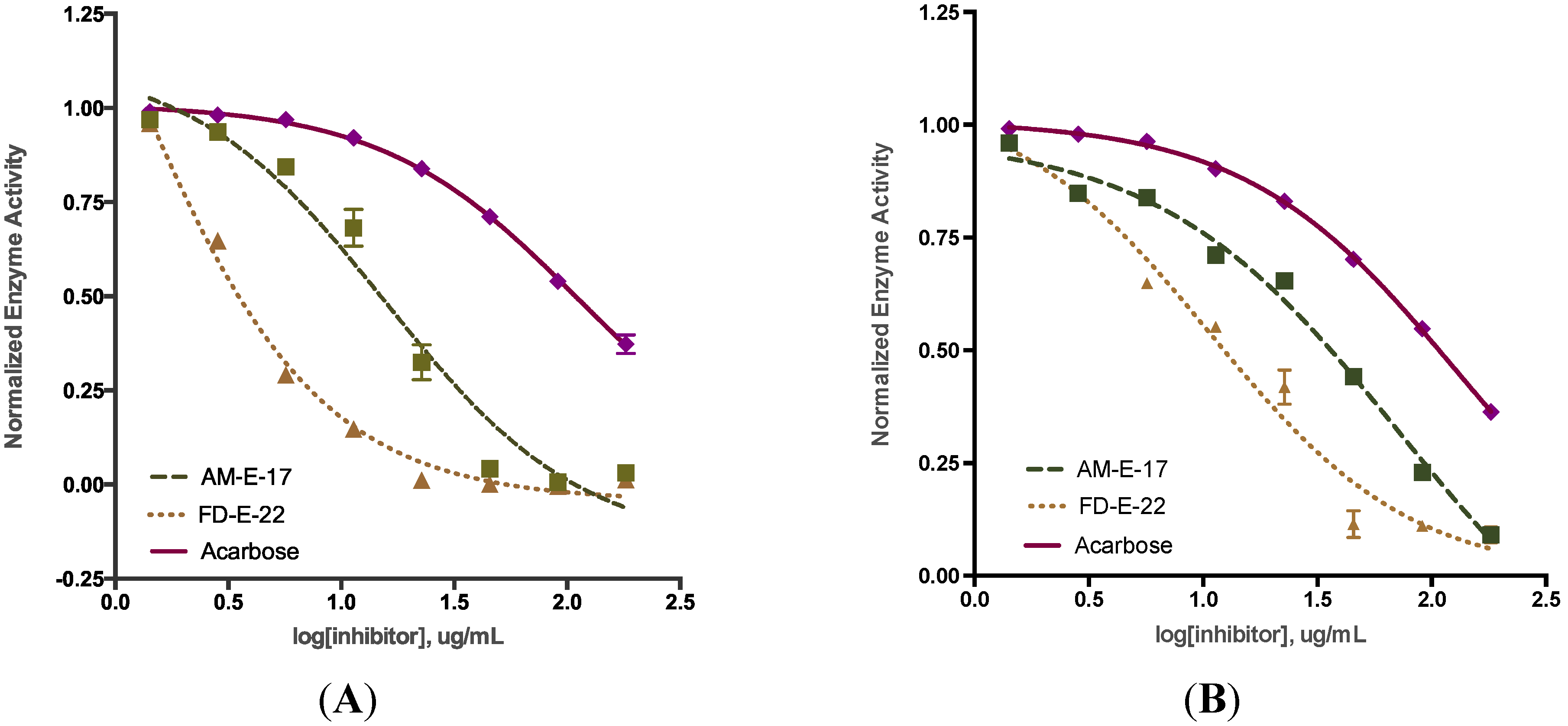
| Sample a | α-Glucosidase | α-Amylase |
|---|---|---|
| AM-E-17 | 15.66 ± 0.82 *** | 63.28 ± 0.87 *** |
| FD-E-22 | 0.89 ± 0.08 *** | 13.98 ± 1.32 *** |
| Acarbose | 112.0 ± 2.85 | 138.7 ± 0.65 |
2.3. Inhibition Kinetics

2.4. Phlorotannin Characterization
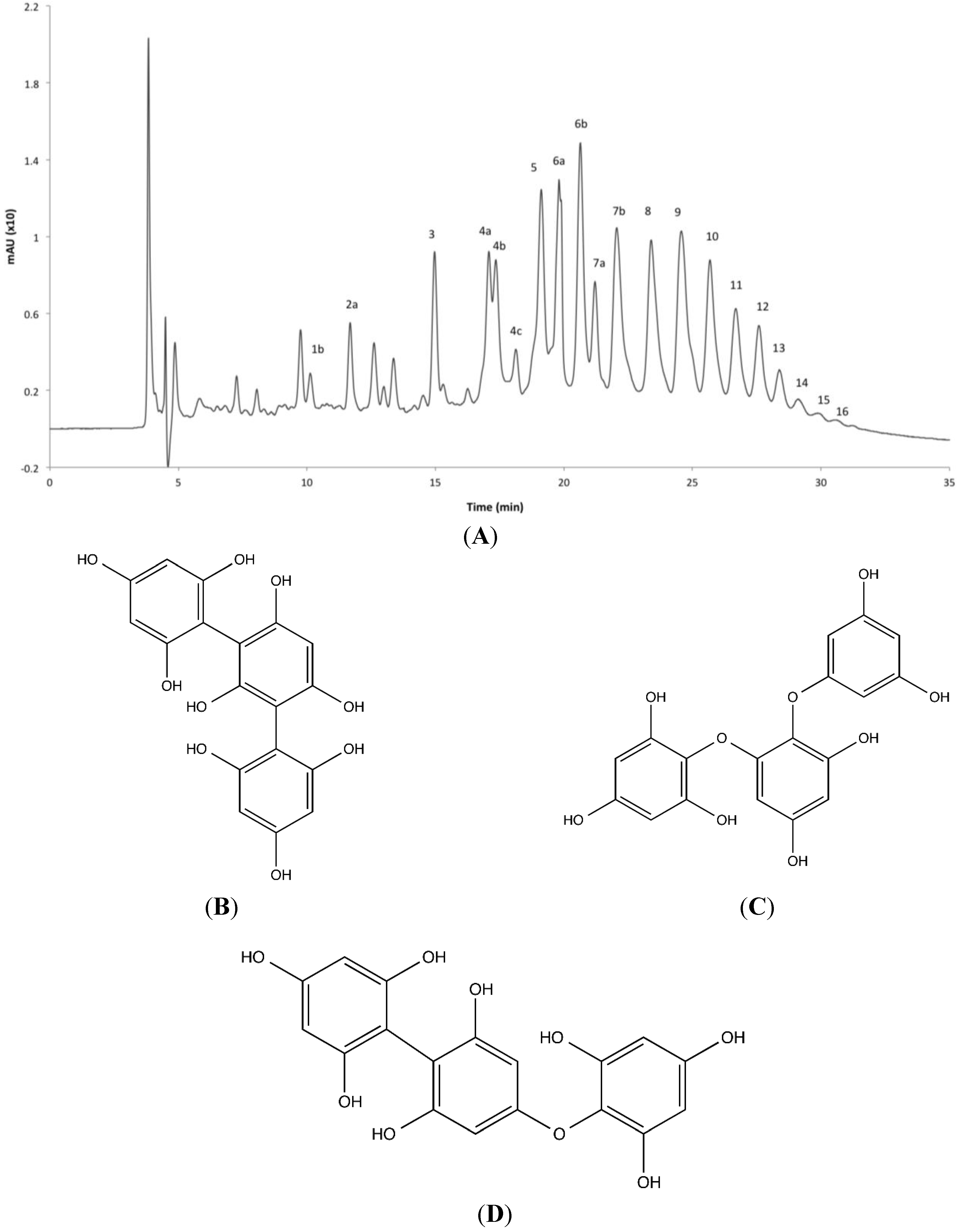
| Peak No. | RT (min) | Molecular Formula | ESI | Measured Mass (m/z) | Predicted Mass (m/z) | Δm (ppm) | DP a | MS/MS Ions (m/z) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 9.752 | C18H14O9 | (+) | 375.0710; [M + 1]+ | 375.0711 | −0.1 | 3 | 357 | 231 | ||||
| 2a | 11.860 | C24H18O12 | (+) | 499.0907; [M + 1]+ | 499.0871 | 3.6 | 4 | 481 | 463 | 355 | 337 | 231 | |
| 2b | 12.679 | C24H18O12 | (+) | 499.0880; [M + 1]+ | 499.0871 | 0.9 | 4 | 481 | 463 | 355 | 338 | 231 | |
| 2c | 12.996 | C24H18O12 | (+) | 499.0880; [M + 1]+ | 499.0871 | 0.9 | 4 | 481 | 463 | 355 | 337 | 231 | |
| 2d | 13.259 | C24H18O12 | (+) | 499.0892; [M + 1]+ | 499.0871 | 2.1 | 4 | 481 | 463 | 356 | 337 | 231 | |
| 3 | 14.822 | C30H22O15 | (+) | 623.1007; [M + 1]+ | 623.1031 | −2.4 | 5 | 605 | 587 | 479 | 461 | 355 | 231 |
| 4a | 16.255 | C36H26O18 | (+) | 747.1121; [M + 1]+ | 747.1119 | 0.2 | 6 | 729 | 711 | 585 | 571 | 479 | 355 |
| 4b | 16.943 | C36H26O18 | (+) | 747.1135; [M + 1]+ | 747.1119 | 1.6 | 6 | 729 | 711 | 585 | 571 | 479 | 355 |
| 4c | 17.345 | C36H26O18 | (+) | 747.1089; [M + 1]+ | 747.1119 | −3.0 | 6 | 729 | 711 | 585 | 571 | 479 | 355 |
| 5a | 17.922 | C42H30O21 | (+) | 871.1296; [M + 1]+ | 871.1328 | −3.2 | 7 | 853 | 745 | 601 | 479 | ||
| 5b | 19.113 | C42H30O21 | (+) | 871.1343; [M + 1]+ | 871.1328 | 1.5 | 7 | 853 | 745 | 601 | 479 | ||
| 6a | 19.809 | C48H34O24 | (+) | 995.1530; [M + 1]+ | 995.1513 | 1.7 | 8 | 977 | 959 | 869 | 729 | 581 | 461 |
| 6b | 20.632 | C48H34O24 | (+) | 995.1532; [M + 1]+ | 995.1513 | 1.9 | 8 | 977 | 959 | 869 | 729 | 581 | 461 |
| 7a | 21.204 | C54H38O27 | (+) | 1119.1647; [M + 1]+ | 1119.1673 | −2.6 | 9 | 1101 | 993 | 853 | 709 | 461 | |
| 7b | 22.050 | C54H38O27 | (+) | 1119.1716; [M + 1]+ | 1119.1673 | 4.3 | 9 | 1101 | 993 | 853 | 709 | 461 | |
| 8 | 23.390 | C60H42O30 | (+) | 1243.1853; [M + 1]+ | 1243.1834 | 1.9 | 10 | 1225 | 959 | 851 | 469 | ||
| 9 | 24.561 | C66H46O33 | (+) | 1367.1960; [M + 1]+ | 1367.2000 | −4.0 | 11 | 1351 | 1241 | 1227 | 705 | 683 | |
| 10 | 25.677 | C72H50O36 | (+) | 1491.2123; [M + 1]+ | 1491.2155 | −3.2 | 12 | 1473 | 1347 | 829 | 807 | 745 | |
| 11 | 26.681 | C78H54O39 | (−) | 1613.2214; [M − 1]− | 1613.2174 | 4.0 | 13 | 806 | 797 | 599 | |||
| 12 | 27.581 | C84H58O42 | (−) | 1737.2298; [M − 1]− | 1737.2336 | −3.8 | 14 | 868 | 859 | 806 | 797 | ||
| 13 | 28.385 | C90H62O45 | (−) | 1861.2540; [M − 1]− | 1861.2496 | 4.4 | 15 | 931 | 922 | 868 | 859 | 735 | 643 |
| 14 | 29.113 | C96H66O48 | (−) | 1985.2614; [M − 1]− | 1985.2656 | 4.2 | 16 | 993 | 984 | 930 | |||
| 15 | 29.905 | C102H70O51 | (−) | 2109.2806; [M − 1]− | 2109.2806 | 0.0 | 17 | 1055 | 1046 | 983 | |||
| 16 | 30.578 | C108H74O54 | (−) | 2233.2928; [M − 1]− | 2233.2966 | −3.8 | 18 | 1116 | 1107 | 1044 | 783 | 715 | 540 |
3. Experimental Section
3.1. Chemicals
3.2. Instrumentation
3.3. Sample Material
| Phylum | Classification | Species | Abbreviation | Common Name |
|---|---|---|---|---|
| Phaeophyta | Brown seaweed | Alaria marginata | AM | Winged kelp |
| Fucus distichus | FD | Bladder wrack | ||
| Saccharina groenlandica | SG | Kelp | ||
| Saccharina latissima | SL | Sugar kelp | ||
| Rhodophyta | Red seaweed | Pyropia fallax | PF | Laver |
| Chlorophyta | Green seaweed | Ulva lactuca | UL | Sea lettuce |
3.4. Extraction and Isolation
3.5. Biochemical Assays
3.5.1. α-Glucosidase Assay
3.5.2. Kinetics of α-glucosidase Inhibitors
3.5.3. α-Amylase Assay
3.5.4. Statistics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Centers for Disease Control & Prevention (CDC). Diabetes Data & Trends. Available online: http://www.cdc.gov/diabetes/statistics/prev/national/figpersons.htm (accessed on 19 June 2012).
- Ceriello, A. Postprandial hyperglycemia and diabetes complications. Diabetes 2005, 54, 1–7. [Google Scholar] [CrossRef]
- Monnier, L.; Colette, C.; Dunseath, G.J.; Owens, D.R. The loss of postprandial glycemic control precedes stepwise deterioration of fasting with worsening diabetes. Diabetes Care 2007, 30, 263–269. [Google Scholar] [CrossRef]
- Cavalot, F.; Petrelli, A.; Traversa, M.; Bonomo, K.; Fiora, E.; Conti, M.; Anfossi, G.; Costa, G.; Trovati, M. Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women: Lessons from the San Luigi Gonzaga diabetes study. J. Clin. Endocrinol. Metab. 2006, 91, 813–819. [Google Scholar] [CrossRef]
- Heo, S.J.; Hwang, J.-H.; Choi, J.-I.; Han, J.S.; Kim, H.-J.; Jeon, Y.-J. Diphlorethohydroxycarmalol isolated from Ishige okamurae, a brown algae, a potent α-glucosidase and α-amylase inhibitor, alleviates postprandial hyperglycemia in diabetic mice. Eur. J. Pharmacol. 2009, 615, 252–256. [Google Scholar] [CrossRef]
- Perfetti, R.; Barnett, P.S.; Mathur, R.; Egan, J.E. Novel therapeutic strategies for the treatment of Type 2 diabetes. Diabetes/Metab. Res. Rev. 1998, 14, 207–225. [Google Scholar] [CrossRef]
- Roy, M.-C.; Anguenot, R.; Fillion, C.; Beaulieu, M.; Bérubé, J.; Richard, D. Effect of a commercially-available algal phlorotannins extract on digestive enzymes and carbohydrate absorption in vivo. Food Res. Int. 2011, 44, 3026–3029. [Google Scholar] [CrossRef]
- Grabitske, H.A.; Slavin, J.L. Gastrointestinal effects of low-digestible carbohydrates. Crit. Rev. Food Sci. Nutr. 2009, 49, 327–360. [Google Scholar] [CrossRef]
- Etxeberria, U.; de la Garza, A.L.; Campión, J.; Martinez, J.A.; Milagro, F.I. Antidiabetic effects of natural plant extracts via inhibition of carbohydrate hydrolysis enzymes with emphasis on pancreatic alpha amylase. Expert Opin. Ther. Targets 2012, 16, 269–297. [Google Scholar] [CrossRef]
- Van de Laar, F.A. Alpha-glucosidase inhibitors in the early treatment of type 2 diabetes. Vasc. Health Risk Manag. 2008, 4, 1189–1195. [Google Scholar]
- Hanefeld, M. The role of acarbose in the treatmet of non-insulin-dependent diabetes mellitus. J. Diabetes Complicat. 1998, 12, 228–237. [Google Scholar] [CrossRef]
- Zhang, J.; Tiller, C.; Shen, J.; Wang, C.; Girouard, G.S.; Dennis, D.; Barrow, C.J.; Miao, M.; Ewart, H.S. Antidiabetic properties of polysaccharide- and polyphenolic-enriched fractions from the brown seaweed Ascophyllum nodosum. Can. J. Physiol. Pharmacol. 2007, 85, 1116–1123. [Google Scholar] [CrossRef]
- Paradis, M.-E.; Couture, P.; Lamarche, B. A randomised crossover placebo-controlled trial investigating the effect of brown seaweed (Ascophyllum nodosum and Fucus vesiculosus) on postchallenge plasma glucose and insulin levels in men and women. Appl. Physiol. Nutr. Metab. 2011, 36, 913–919. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, J.Y.; Choi, W.H.; Lee, S.S. Effects of seaweed supplementation on blood glucose concentration, lipid profile, and antioxidant enzyme activities in patients with type 2 diabetes mellitus. Nutr. Res. Pract. 2008, 2, 62–67. [Google Scholar] [CrossRef]
- Goñi, I.; Valdivieso, L.; Garcia-Alonso, A. Nori seaweed consumption modifies glycemic response in healthy volunteers. Nutr. Res. 2000, 20, 1367–1375. [Google Scholar] [CrossRef]
- Eom, S.-H.; Lee, S.-H.; Yoon, N.Y.; Jung, W.-K.; Jeon, Y.-J.; Kim, S.-K.; Lee, M.-S.; Kim, Y.-M. α-Glucosidase- and α-amylase-inhibitory activities of phlorotannins from Eisenia bicyclis. J. Sci. Food Agric. 2012, 92, 2084–2090. [Google Scholar] [CrossRef]
- Kim, K.Y.; Nam, K.A.; Kurihara, H.; Kim, S.M. Potent α-glucosidase inhibitors purified from the red algae Grateloupia elliptica. Phytochemistry 2008, 69, 2820–2825. [Google Scholar] [CrossRef]
- Lee, S.-H.; Li, Y.; Karadeniz, F.; Kim, M.-M.; Kim, S.-K. α-Glucosidase and α-amylase inhibitory activities of phloroglucinal derivatives from edible marine brown alga, Ecklonia cava. J. Sci. Food Agric. 2009, 89, 1552–1558. [Google Scholar] [CrossRef]
- Lordan, S.; Smyth, T.J.; Soler-Vila, A.; Stanton, C.; Ross, R.P. The α-amylase and α-glucosidase inhibitory effects of Irish seaweed extracts. Food Chem. 2013, 141, 2170–2176. [Google Scholar] [CrossRef]
- Garza, D. Common Edible Seaweeds in the Gulf of Alaska; Alaska Sea Grant College Program: Fairbanks, AK, USA, 2005; p. 61. [Google Scholar]
- Turner, N.C.; Bell, M.A.M. The ethnobotany of the Southern Kwakiutl Indians of British Columbia. Econ. Bot. 1973, 27, 257–310. [Google Scholar] [CrossRef]
- Turner, N.J. The ethnobotany of edible seaweed (Porphyra abbottae and related species; Rhodophyta: Bangiales) and its use by First Nations on the Pacific Coast of Canada. Can. J. Bot. 2003, 81, 283–293. [Google Scholar] [CrossRef]
- Wein, E.E.; Freeman, M.M.R.; Makus, J.C. Use of and preference for traditional foods among the Belcher Island Inuit. Arctic 1996, 49, 256–264. [Google Scholar] [CrossRef]
- Nobmann, E.D.; Ponce, R.; Mattil, C.; Devereux, R.; Dyke, B.; Ebbesson, S.O.E.; Laston, S.; MacCluer, J.; Robbins, D.; Romenesko, T.; et al. Dietary intakes vary with age among Eskimo adults of Northwest Alaska in the GOCADAN study, 2000–2003. J. Nutr. 2005, 135, 856–862. [Google Scholar]
- Bersamin, A.; Luick, B.R.; Ruppert, E.; STern, J.S.; Zidenberg-Cherr, S. Diet quality among Yup’ik Eskimos living in rural communities is low: The Center for Alaska Native Health Research Pilot Study. J. Am. Diet. Assoc. 2006, 106, 1055–1063. [Google Scholar] [CrossRef]
- Gahagan, S.; Silverstein, J. Prevention and treatment of type 2 diabetes mellitus in children, with special emphasis on American Indian and Alaska Native children. Pediatrics 2003, 112, e328–e437. [Google Scholar] [CrossRef]
- Ebbesson, S.O.E.; Risica, P.M.; Ebbesson, L.O.E.; Kennish, J.M.; Tejero, E.M. Omega-3 fatty acids imrpove glucose tolerance and components of the metabolic syndrome in Alaskan Eskimos: The Alaska Siberia Project. Int. J. Circumpolar Health 2005, 64, 396–408. [Google Scholar]
- Centers for Disease Control & Prevention (CDC). Racial and ethnic differences in diagnosed diabetes. Available online: http://www.cdc.gov/diabetes/pubs/estimates11.htm-4 (accessed on 19 June 2012).
- Lindberg, M.R.; Lindstrom, S.C. Field Guide to Seaweeds of Alaska; Alaska Sea Grant College Program, University of Alaska Fairbanks: Fairbanks, AK, USA, 2010; p. 188. [Google Scholar]
- Kellogg, J.; Lila, M.A. Chemical and in vitro assessment of Alaskan coastal vegetation antioxidant capacity. J. Agric. Food Chem. 2013, 61, 11025–11032. [Google Scholar] [CrossRef]
- Steevensz, A.J.; MacKinnon, S.L.; Hankinson, R.; Craft, C.; Connan, S.; Stangel, D.B.; Melanson, J.E. Profiling phlorotannins in brown macroalgae by liquid chromatography-high resolution mass spectrometry. Phytochem. Anal. 2012, 23, 547–553. [Google Scholar] [CrossRef]
- Ferreres, F.; Lopes, G.; Gil-Izquierdo, A.; Andrade, P.B.; Sousa, C.; Mouga, T.; Valentão, P. Phlorotannin extracts from Fucales characterized by HPLC-DAD-ESI-MSn: Approaches to hyaluronidase inhibitory capacity and antioxidant properties. Mar. Drugs 2012, 10, 2766–2781. [Google Scholar] [CrossRef]
- Glombitza, K.W.; Pauli, K. Fucols and phlorethols from the brown alga Scytothamnus australis Hook. et Harv. (Chnoosporaceae). Bot. Mar. 2003, 46, 315–320. [Google Scholar] [CrossRef]
- Grace, M.H.; Guzman, I.; Roopchand, D.E.; Moskal, K.; Cheng, D.M.; Pogrebnyak, N.; Raskin, I.; Howell, A.; Lila, M.A. Stable binding of alternative protein-enriched food matrices with concentrated cranberry bioflavonoids for functional food applications. J. Agric. Food Chem. 2013, 61, 6856–6864. [Google Scholar] [CrossRef]
- Xiao, J.; Kai, G.; Yamamoto, K.; Xiaoqing, C. Advance in dietary polyphenols as α-glucosidases inhibitors: a review on structure-activity relationship aspect. Crit. Rev. Food Sci. Nutr. 2013, 53, 818–836. [Google Scholar] [CrossRef]
- Spencer, C.M.; Cai, Y.; Martin, R.E.; Gaffney, S.H.; Goulding, P.N.; Magnolato, D. Polyphenol complexation: Some thoughts and observations. Phytochemistry 1988, 27, 2397–2409. [Google Scholar] [CrossRef]
- Piparo, E.L.; Scheib, H.; Frei, N.; Williamson, G.; Grigorov, M.; Chou, C.J. Flavonoids for controlling starch digestion: structural requirements for inhibiting human α-amylase. J. Med. Chem. 2008, 51, 3555–3561. [Google Scholar] [CrossRef]
- Moon, H.E.; Islam, M.N.; Ahn, B.R.; Chowdhury, S.S.; Sohn, H.S.; Jung, H.A.; Choi, J.S. Protein tyrosine phosphatase 1B and α-glucosidase inhibitory phlorotannins from edible brown algae, Ecklonia stoloniera and Eisenia bicyclis. Biosci. Biotechnol. Biochem. 2011, 75, 1472–1480. [Google Scholar] [CrossRef]
- Koivikko, R.; Loponen, J.; Pihlaja, K.; Jormalainen, V. High-performance liquid chromatographic analysis of phlorotannins from the brown alga Fucus vesiculosus. Phytochem. Anal. 2007, 18, 326–332. [Google Scholar] [CrossRef]
- Kim, T.K.; Rioux, L.E.; Turgeon, S.L. Alpha-amylase and alpha-glucosidase inhibition is diffeentially modulated by fucoidan obtained from Fucus vesiculosus and Ascophyllum nodosum. Phytochemistry 2014, 98, 27–33. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kellogg, J.; Grace, M.H.; Lila, M.A. Phlorotannins from Alaskan Seaweed Inhibit Carbolytic Enzyme Activity. Mar. Drugs 2014, 12, 5277-5294. https://doi.org/10.3390/md12105277
Kellogg J, Grace MH, Lila MA. Phlorotannins from Alaskan Seaweed Inhibit Carbolytic Enzyme Activity. Marine Drugs. 2014; 12(10):5277-5294. https://doi.org/10.3390/md12105277
Chicago/Turabian StyleKellogg, Joshua, Mary H. Grace, and Mary Ann Lila. 2014. "Phlorotannins from Alaskan Seaweed Inhibit Carbolytic Enzyme Activity" Marine Drugs 12, no. 10: 5277-5294. https://doi.org/10.3390/md12105277
APA StyleKellogg, J., Grace, M. H., & Lila, M. A. (2014). Phlorotannins from Alaskan Seaweed Inhibit Carbolytic Enzyme Activity. Marine Drugs, 12(10), 5277-5294. https://doi.org/10.3390/md12105277