Anti-Inflammation Activities of Mycosporine-Like Amino Acids (MAAs) in Response to UV Radiation Suggest Potential Anti-Skin Aging Activity
Abstract
:1. Introduction
2. Results and Discussion
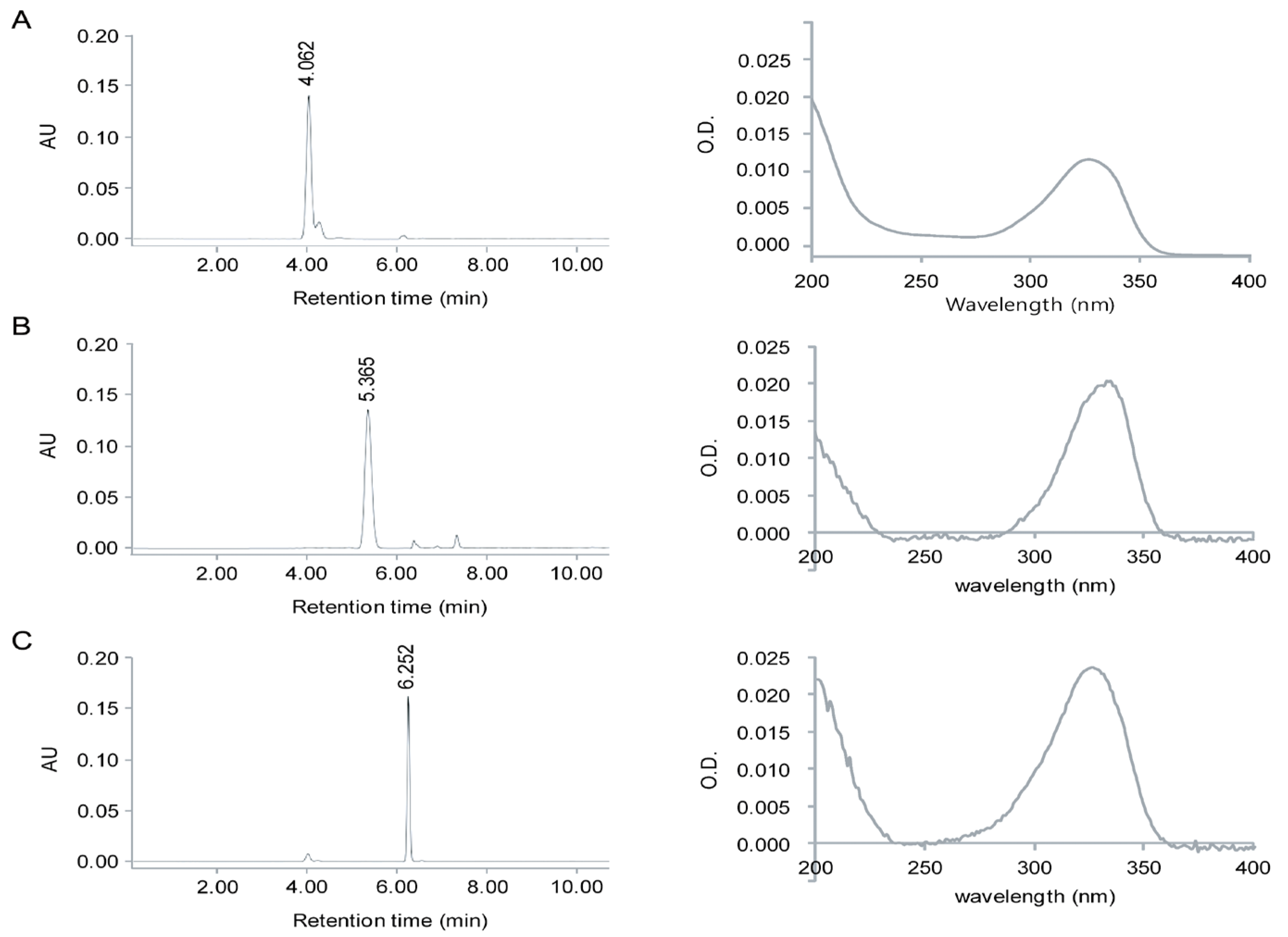
2.1. Identification of MAAs from Chlamydomonas hedleyi by HPLC Assay

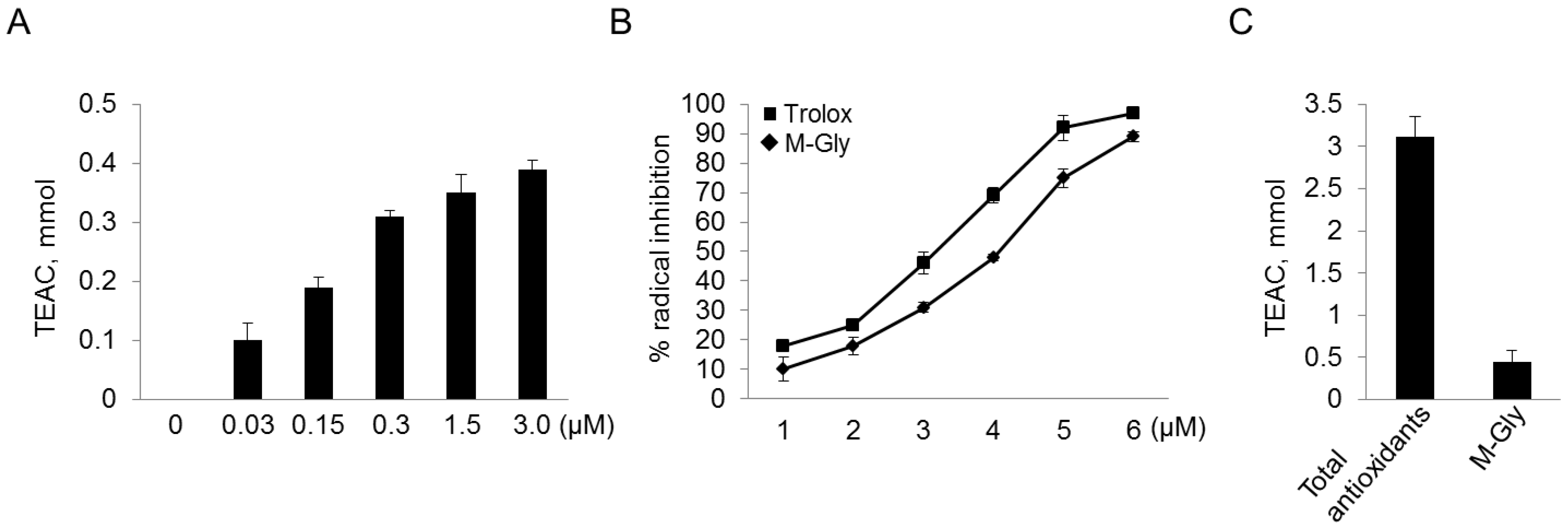
2.2. Antioxidant Activity of MAAs with Free Radical-Trapping Abilities
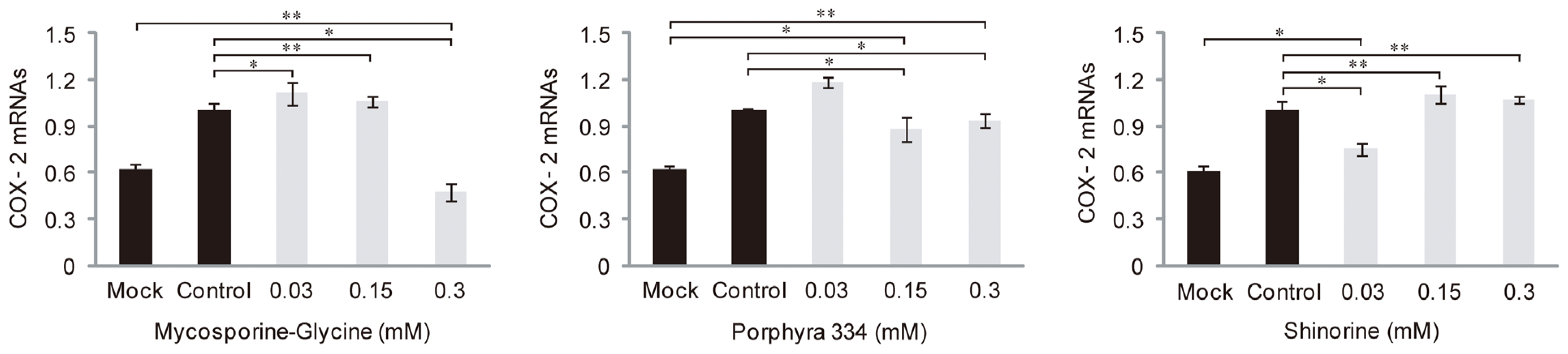
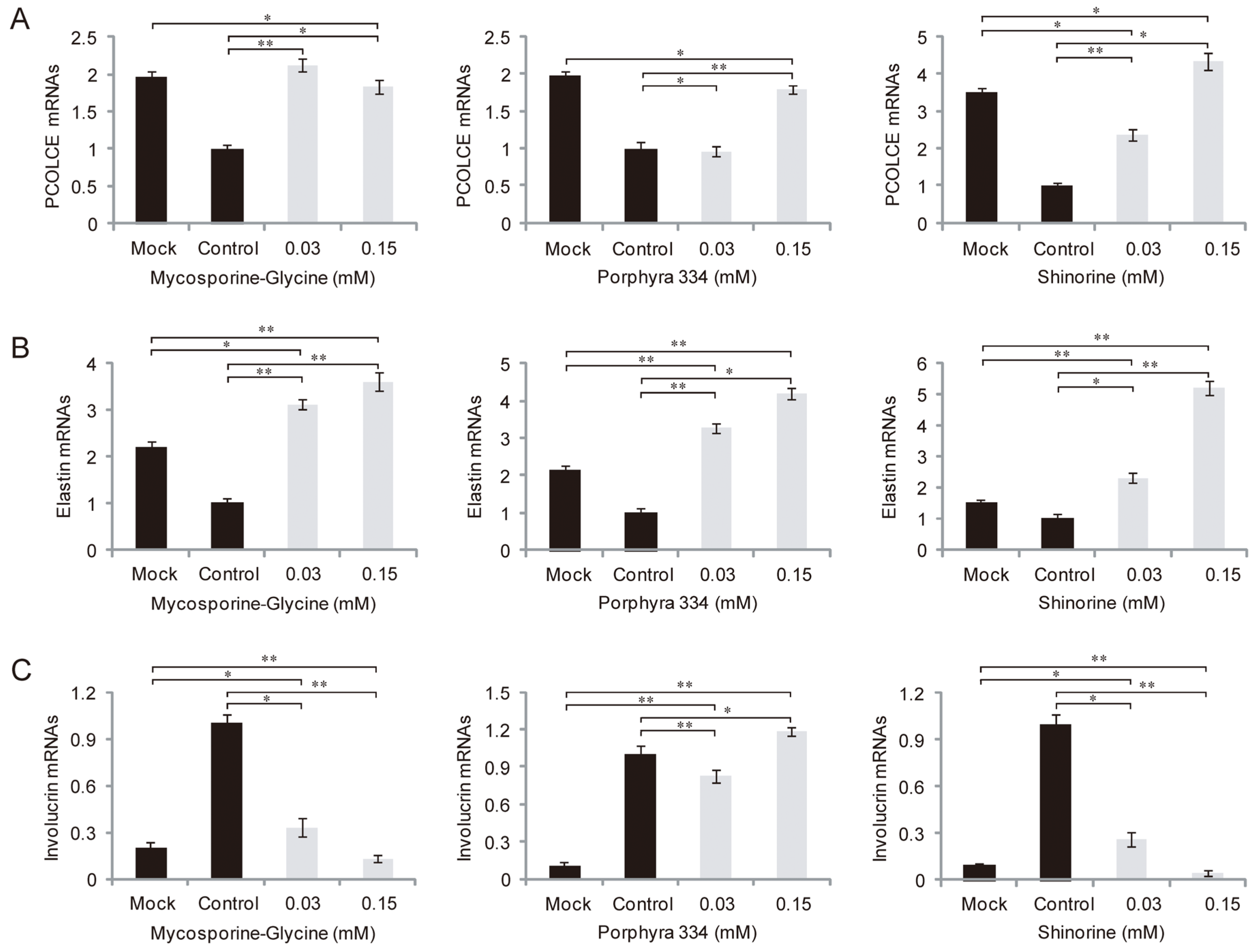
2.3. Role of MAAs in Skin Inflammation and Aging
3. Experimental Section
3.1. Growth of Chlamydomonas hedleyi
3.2. Identification and Characterization of MAAs
3.3. MS/MS Analysis
3.4. Assay for Antioxidant Activity
3.5. Cell Culture
3.6. UV Exposure Procedures
3.7. RNA Extraction and qRT-PCR
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yagura, T.; Makita, K.; Yamamoto, H.; Menck, C.F.M.; Schuch, A.P. Biological sensors for solar ultraviolet radiation. Sensors 2011, 11, 4277–4294. [Google Scholar] [CrossRef] [PubMed]
- Solomon, K.R. Effect of ozone depletion and UV-B radiation on humans and the environment. Atmos. Ocean 2008, 46, 185–202. [Google Scholar] [CrossRef]
- Weatherhead, E.C.; Andersen, S.B. The search for signs of recovery of the ozone layer. Nature 2006, 441, 39–45. [Google Scholar] [CrossRef]
- Gao, K.; Xu, J. Effects of solar UV radiation on diurnal photosynthetic performance and growth of Gracilaria lemaneiformis (Rhodophyta). Eur. J. Phycol. 2008, 43, 297–307. [Google Scholar] [CrossRef]
- Bhandari, R.; Sharma, P.K. Effect of UV-B and high visual radiation on photosynthesis in freshwater (Nostoc spongiaeforme) and marine (Phormidium corium) cyanobacteria. Indian J. Biochem. Biophys. 2007, 44, 231–239. [Google Scholar] [PubMed]
- Wu, H.; Gao, K.; Villafane, V.E.; Watanabe, T.; Helbling, E.W. Effects of solar UV radiation on morphology and photosynthesis of filamentous cyanobacterium Arthrospira platensis. Appl. Environ. Microbiol. 2005, 71, 5004–5013. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Zhang, Y.; Zhang, T.; An, L.; Wang, X. Effects of enhanced ultraviolet-B radiation on algae and cyanobacteria. Crit. Rev. Microbiol. 2005, 31, 79–89. [Google Scholar] [CrossRef]
- Helbling, E.W.; Farias, M.E.; Zenoff, M.V.F.; Villafane, V.E. In situ responses of phytoplankton from the subtropical Lake La Angostura (Tucuman, Argentina) in relation to solar ultraviolet radiation exposure and mixing conditions. Hydrobiologia 2006, 559, 123–134. [Google Scholar] [CrossRef]
- Grimbaldeston, M.A.; Simpson, A.; Finlay-Jones, J.J.; Hart, P.H. The effect of ultraviolet radiation exposure on the prevalence of mast cells in human skin. Br. J. Dermatol. 2003, 148, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Shih, M.F.; Cherng, J.Y. Potential protective effect of fresh grown unicellular green algae component (resilient factor) against PMA- and UVB-induced MMP-1 expression in skin fibroblasts. Eur. J. Dermatol. 2008, 18, 303–307. [Google Scholar] [PubMed]
- Steiglitz, B.M.; Kreider, J.M.; Frankenburg, E.P.; Pappano, W.N.; Hoffman, G.G.; Meganck, J.A.; Liang, X.; Hook, M.; Goldstein, D.E.; Greenspan, D.S. Procollagen C proteinase enhancer 1 genes are important determinants of the mechanical properties and geometry of bone and the ultrastructure of connective tissues. Mol. Cell. Biol. 2006, 26, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Bosset, S.; Bonnet-Duguennoy, M.; Barre, P.; Chalon, A.; Lazou, K.; Kurfurst, R.; Bonte, F.; Schnebert, S.; Disant, F.; le Varlet, B.; Nicolas, J.F. Decreased expression of keratinocyte beta1 integrins in chronically sun-exposed skin in vivo. Br. J. Dermatol. 2003, 148, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Rice, R.H.; Qin, Q.; Pilato, A.; Rubin, A.L. Keratinocyte differentiation markers: Involucrin, transglutaminase, and toxicity. J. Natl. Cancer Inst. Monogr. 1992, 13, 87–91. [Google Scholar] [PubMed]
- Grether-Beck, S.; Muhlberg, K.; Brenden, H.; Felsner, I.; Brynjolfsdottir, A.; Einarsson, S.; Krutmann, J. Bioactive molecules from the Blue Lagoon: In vitro and in vivo assessment of silica mud and microalgae extracts for their effects on skin barrier function and prevention of skin ageing. Exp. Dermatol. 2008, 17, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.J.; Ham, Y.M.; Kim, S.S.; Yoo, B.S.; Moon, J.Y.; Baik, J.S.; Lee, N.H.; Hyun, C.G. Suppression of pro-inflammatory cytokines, iNOS, and COX-2 expression by brown algae Sargassum micracanthum in RAW 264.7 macrophages. EurAsia. J. BioSci. 2009, 3, 130–143. [Google Scholar] [CrossRef]
- Sinha, R.P.; Häder, D.-P. UV-induced DNA damage and repair. Photochem. Photobiol. Sci. 2002, 1, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, R.; Paris, D.; Melck, D.; Fulgentini, L.; Colombetti, G.; Motta, A. In vivo NMR metabolic profiling of Fabrea salina reveals sequential defense mechanisms against ultraviolet radiation. Biophys. J. 2011, 100, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, M.F. Algae and aquatic macrophytes response to cope with ultraviolet radiation—A review. Emir. J. Food Agric. 2012, 24, 527–545. [Google Scholar] [CrossRef]
- Karsten, U.; Sawell, T.; Wiencke, C. A survey of the distribution of UV-absorbing substances in the tropical macroalgae. Phycol. Res. 1998, 46, 271–279. [Google Scholar] [CrossRef]
- Sinha, R.P.; Klisch, M.; Groniger, A.; Häder, D.-P. Ultraviolet-absorbing/screening substances in cyanobacteria, phytoplankton and macroalgae. J. Photochem. Photobiol. B 1998, 47, 83–94. [Google Scholar] [CrossRef]
- Torres, A.; Enk, C.D.; Hochberg, M.; Srebnik, M. Porphyra-334, a potential natural source for UVA protective sunscreens. Photochm. Photobiol. Sci. 2006, 4, 432–435. [Google Scholar] [CrossRef]
- Tsujino, I.; Yabe, K.; Sekikawa, I. Isolation and structure of a new amino acid, shinorine, from the red alga Chondrus yendoi Yamada Mikami. Bot. Mar. 1980, 23, 65–68. [Google Scholar]
- Llewellyn, C.A.; Airs, R.L. Distribution and abundance of MAAs in 33 species of microalgae across 13 classes. Mar. Drugs 2010, 8, 1273–1291. [Google Scholar] [CrossRef]
- Ito, S.; Hirata, Y. Isolation and structure of a mycosporine from the zoanthidian Palythoa tuberculosa. Tetrahedron Lett. 1977, 28, 2429–2430. [Google Scholar] [CrossRef]
- Dunlap, W.C.; Yamamoto, Y. Small-molecule antioxidants in marine organisms: Antioxidant activity of mycosporine-glycine. Comp. Biochem. Physiol. 1995, 112, 105–114. [Google Scholar] [CrossRef]
- Conde, F.R.; Churio, M.S.; Previtali, C.M. The deactivation pathways of the excited-states of the mycosporine-like amino acids shinorine and porphyra-334 in aqueous solution. Photochem. Photobiol. Sci. 2004, 3, 960–967. [Google Scholar] [CrossRef]
- Daniel, S.; Cornelia, S.; Fred, Z. UV-A sunscreen from red algae for protection against premature skin aging. Cosmet. Toilet. Manufact. Worldw. 2004, 129, 139–143. [Google Scholar]
- Carreto, J.I.; Carignan, M.O. Mycosporine-like amino acids: Relevant secondary metabolites chemical and ecological aspects. Mar. Drugs. 2011, 9, 387–446. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Incharoensakdi, A. UV radiation-induced biosynthesis, stability and antioxidant activity of mycosporine-like amino acids (MAAs) in a unicellular cyanobacterium Gloeocapsa sp. CU2556. J. Phothochem. Photobiol. B 2014, 130, 287–292. [Google Scholar] [CrossRef]
- Singh, S.P.; Kumari, S.; Rastogi, R.P.; Singh, K.L.; Sinha, R.P. Mycosporine-like amino acids (MAAs): Chemical structure, biosynthesis and significance as UV-absorbing/screening compounds. Indian J. Exp. Biol. 2008, 46, 7–17. [Google Scholar] [PubMed]
- Rosic, N.N. Phylogenetic analysis of genes involved in mycosporine-like amino acid biosynthesis in symbiotic dinoflagellates. Appl. Microbiol. Biotechnol. 2012, 94, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Yoshiki, M.; Tsuge, K.; Tsuruta, Y.; Yoshimura, T.; Koganemaru, K.; Sumi, T.; Matsui, T.; Matsumoto, K. Production of new antioxidant compound from mycosporine-like amino acid, porphyra-334 by heat treatment. Food Chem. 2009, 113, 1127–1132. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Lipid oxidation and improving the oxidative stability. Chem. Soc. Rev. 2010, 4067, 4067–4079. [Google Scholar] [CrossRef]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotech. Advan. 2011, 29, 483–501. [Google Scholar] [CrossRef]
- Coba, F.; Aguilera, J.; Figueroa, F.L.; Galvez, M.V.; Herrera, E. Antioxidant activity of mycosporine-like amino acids isolated from three red macroalgae and one marine lichen. J. Appl. Phycol. 2009, 21, 161–169. [Google Scholar] [CrossRef]
- Tao, C.; Sugawara, T.; Maeda, S.; Wang, X.; Hirata, T. Antioxidative activities of a mycosporine-like amino acid, porphyra-334. Fish. Sci. 2008, 74, 1166–1172. [Google Scholar] [CrossRef]
- Oyamada, C.; Kaneniwa, M.; Ebitani, K.; Murata, M.; Ishihara, K. Mycosporine-like amino acids extracted from scallop (Painopecten yessoensis) ovaries: UV protection and growth stimulation activities on human cells. Mar. Biotechnol. 2008, 10, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Mushir, S.; Fatma, T. Ultraviolet Radiation-absorbing Mycosporine-like Amino Acids in Cyanobacterium Aulosira fertilissima: Environmental Perspective and Characterization. Curr. Res. J. Biol. Sci. 2011, 3, 165–171. [Google Scholar]
- Peinado, N.K.; Diaz, R.T.A.; Figueroa, F.L.; Helbling, E.W. Ammonium and UV radiation stimulate the accumulation of mycosporine-like amino acids in Porphyra columbina (Rhodophyta) from Patagonia, Argentina. J. Phycol. 2004, 40, 248–259. [Google Scholar] [CrossRef]
- Arbeloa, E.M.; Carignan, M.O.; Acuna, F.H.; Churio, M.S.; Carreto, J.I. Mycosporine-like amino acid content in the sea anemones Aulactinia marplatensis, Oulactis muscosa and Anthothoe chilensis. Comp. Biochem. Physiol. B 2010, 156, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Bertrand-Vallery, V.; Belot, N.; Dieu, M.; Delaive, E.; Ninane, N.; Demazy, C.; Raes, M.; Salmon, M.; Poumay, Y.; Debacq-Chainiaux, F.; et al. Proteomic profiling of human keratinocytes undergoing UVB-induced alternative differentiation reveals tripartite motif protein 29 as a survival factor. PLoS One 2010, 5, e10462. [Google Scholar]
- Seite, S.; Moyal, D.; Richard, S.; de Rigal, J.; Leveque, J.L.; Hourseau, C.; Fourtanier, A. Mexoryl SX: A broad absorption UVB filter protects human skin from the effects of repeated suberythemal doses of UVA. J. Photochem. Photobiol. B 1998, 4, 69–76. [Google Scholar] [CrossRef]
- Enujiugha, V.N.; Talabi, J.Y.; Malomo, S.A.; Olagunju, A.I. DPPH radical scavenging capacity of phenolic extracts from African yam bean (Sphenostylis stenocarpa). Food Nutr. Sci. 2012, 3, 7–13. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suh, S.-S.; Hwang, J.; Park, M.; Seo, H.H.; Kim, H.-S.; Lee, J.H.; Moh, S.H.; Lee, T.-K. Anti-Inflammation Activities of Mycosporine-Like Amino Acids (MAAs) in Response to UV Radiation Suggest Potential Anti-Skin Aging Activity. Mar. Drugs 2014, 12, 5174-5187. https://doi.org/10.3390/md12105174
Suh S-S, Hwang J, Park M, Seo HH, Kim H-S, Lee JH, Moh SH, Lee T-K. Anti-Inflammation Activities of Mycosporine-Like Amino Acids (MAAs) in Response to UV Radiation Suggest Potential Anti-Skin Aging Activity. Marine Drugs. 2014; 12(10):5174-5187. https://doi.org/10.3390/md12105174
Chicago/Turabian StyleSuh, Sung-Suk, Jinik Hwang, Mirye Park, Hyo Hyun Seo, Hyoung-Shik Kim, Jeong Hun Lee, Sang Hyun Moh, and Taek-Kyun Lee. 2014. "Anti-Inflammation Activities of Mycosporine-Like Amino Acids (MAAs) in Response to UV Radiation Suggest Potential Anti-Skin Aging Activity" Marine Drugs 12, no. 10: 5174-5187. https://doi.org/10.3390/md12105174
APA StyleSuh, S.-S., Hwang, J., Park, M., Seo, H. H., Kim, H.-S., Lee, J. H., Moh, S. H., & Lee, T.-K. (2014). Anti-Inflammation Activities of Mycosporine-Like Amino Acids (MAAs) in Response to UV Radiation Suggest Potential Anti-Skin Aging Activity. Marine Drugs, 12(10), 5174-5187. https://doi.org/10.3390/md12105174



