Abstract
The glyoxylate cycle is a sequence of anaplerotic reactions catalyzed by the key enzymes isocitrate lyase (ICL) and malate synthase (MLS). Mutants of Candida albicans lacking ICL are markedly less virulent in mice than the wild-type. Suvanine sesterterpenes (1−9) isolated from a tropical sponge Coscinoderma sp. were evaluated for their inhibitory activities toward recombinant ICL from C. albicans. These studies led to the identification of a potent ICL inhibitor, suvanine salt (2), which possesses a sodium counterion and displays an inhibitory concentration value (IC50) of 6.35 μM. The growth phenotype of ICL deletion mutants and semi-quantitative reverse transcription-polymerase chain reaction (RT-PCR) analyses indicated that compound 2 inhibits the ICL mRNA expression in C. albicans under C2-carbon-utilizing conditions. The present data highlight the potential for suvanine sesterterpenes treatment of C. albicans infections via inhibition of ICL activity.
1. Introduction
The glyoxylate cycle, a modified form of the tricarboxylic acid (TCA) cycle, is well documented in archaea, bacteria, protists, plants, fungi, and nematodes [1]. Discovered initially in microorganisms, this cycle plays a fundamental role in the nutrient-limited environment by providing the means for microorganisms to grow on acetate, ethanol or fatty acids [2]. The cycle function has been confirmed by analyzing mutants of pathogenic microorganisms that lack isocitrate lyase (ICL) and malate synthase (MLS), key enzymes in the glyoxylate cycle [3,4]. The genetic regulation of the glyoxylate cycle during microbial growth on acetate has been investigated, and in the last several years it has become evident that this pathway is important in microbial pathogenesis. The expression of ICL is upregulated during infection of macrophages by the pulmonary bacterium Mycobacterium tuberculosis [5,6]. Infection of rice with Magnaporthe grisea leads to the expression of genes involved in the glyoxylate cycle [7]. In addition, ICL-deletion mutants of these microorganisms show virulence attenuation.
Research on candidiasis in mice has shown that Candida albicans, the most serious human pathogenic fungus, requires ICL to be fully virulent [8,9]. The genes of the glyoxylate cycle are strongly induced upon phagocytosis of C. albicans by macrophages. The interior environment of the phagolysosome is abundant in carbon sources such as fatty acids or their breakdown products, which allows C. albicans to utilize the enzymes of the glyoxylate cycle and permits the use of C2 carbon sources. The C. albicans mutant strain lacking the glyoxylate cycle enzyme ICL is markedly less virulent in a mouse model of systemic candidiasis and less persistent in internal organs than the wild-type strain [8,9,10]. As this cycle does not operate in humans, the key enzymes of the glyoxylate cycle represent promising targets for the control of fungal infection and the development of antifungal drugs. In previous years, a wide array of works developing potential ICL inhibitors have been reported. Various 3-nitropropionamides, pyruvate-isoniazid analogs, salicylanilide and benzanilide derivatives showed a potential to inhibit M. tuberculosis ICL [11,12]. As part of efforts to discover pharmacologically effective ICL inhibitors, many marine-derived natural compounds were isolated and evaluated against C. albicans and M. grisea ICL [13,14].
Several of the sponge-derived sesterterpenes and related pentaprenyl hydroquinones [15], represented by the halisulfates and suvanine, possess sulfate groups and exhibit diverse bioactivities such as cytotoxic, antimicrobial [16] and anti-inflammatory properties [17], as well as inhibitory effects on serine protease [18] and CDC25 phosphatase [19]. In addition, recent biological study has shown that HSP60, a chaperone involved in the inflammatory response, is the main cellular target of suvanine [20]. In the course of searching for secondary metabolites of biological significance from marine organisms, we encountered the sponge Coscinoderma sp., collected from Chuuk Island, Micronesia. Chemical investigation of this animal led to the isolation of new compounds, suvanine salts and related derivatives [21]. In this study, we investigated the potential for isolated suvanine sesterterpenes as inhibitors of C. albicans ICL.
2. Results and Discussion
Compound 1−9 were obtained as mentioned previously [21] (Figure 1). The expression and purification of recombinant ICL from the genomic DNA of C. albicans (ATCC 10231) were carried out by a method described previously [22]. The inhibitory effects of the isolated compounds on ICL were evaluated according to a procedure documented previously [23,24]. The basic concept of this method was to measure spectrophotometrically the formation of glyoxylate phenylhydrazone in the presence of phenylhydrazine and isocitrate. The effect of the inhibitor on ICL was calculated as a percentage relative to dimethyl sulfoxide (DMSO)-treated control. Mixture of ICL, substrate, phenyhydrazine was incubated for 30 min with various concentrations of suvanine sesterterpenes (100 to 0.1 μg/mL). The formation of glyoxylate phenylhydrazone was followed spectrophotometrically at 324 nm. Data were scaled to internal controls, and a four- parameter logistic model (GraphPad ver. 5.0, Prism) was used to fit the measured data and determine IC50 (inhibitory concentration for 50% activity) values [25]. The representative dose–response curves of suvanine sesterterpenes (1, 2, and 4) against the ICL enzyme were compared to that of known ICL inhibitors, 3-nitropropinate and itaconate [12,26] (Figure 2).
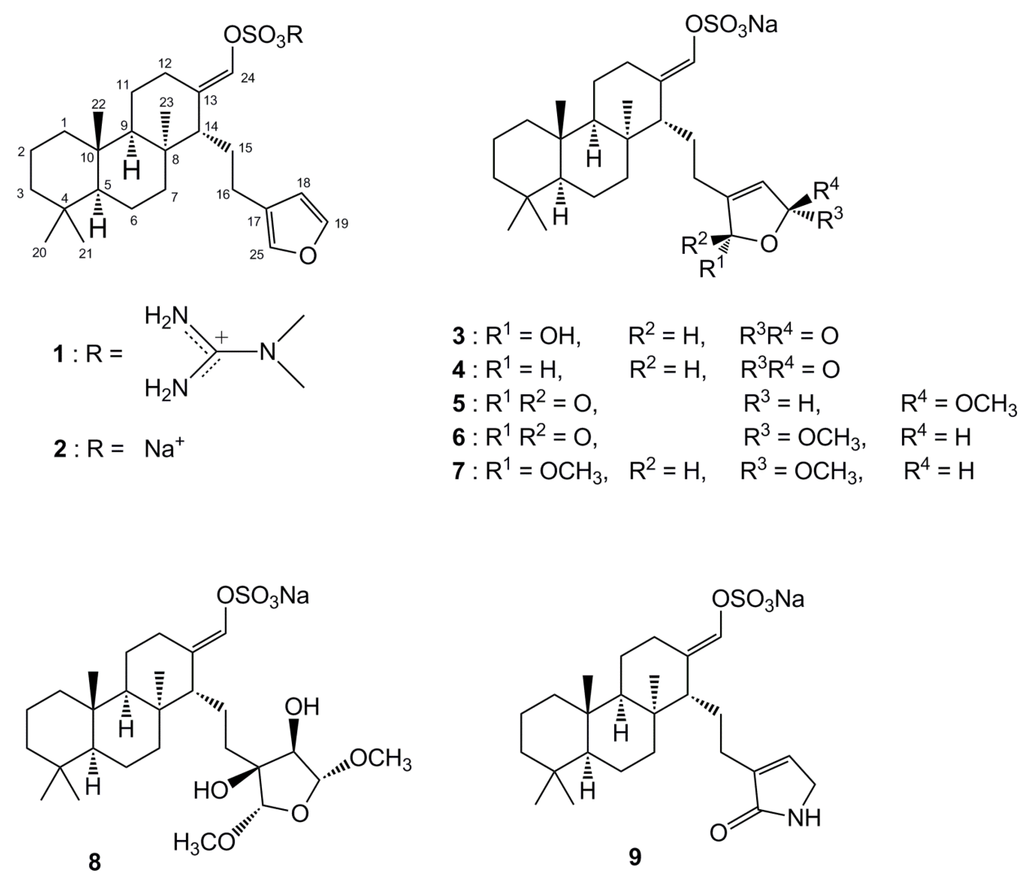
Figure 1.
The structures of suvanine sesterterpenes (1–9).
The ICL inhibitory potencies (IC50) of the isolated compounds 1−9 are shown in Table 1. Among the suvanine sesterterpenes, suvanine salts (1 and 2) and a butenolide-containing derivative of suvanine (4) were found to be strong ICL inhibitors, with IC50 values of 22.43, 6.35, and 26.26 μM, respectively. Compound 2 in particular was more effective than 3-nitropropinate (IC50 = 17.27 μM) and itaconate (IC50 = 13.94 μM). Regarding the suvanine salts, compound 2 with a sodium counterion exhibited much more potent inhibition than 1, which possessed an N,N-dimethylguanidium counterion [18]. One possible explanation for the lower potency of compound 1 is that, not only the distance, the spatial orientation of the enolsulfate group relative to that of the furan moiety also plays an important role in ICL inhibitory activity. Compounds 3–9 possessed modified furan moieties with varying degrees of oxidation and exhibited lower inhibitory activities than compound 2. By comparing the chemical structures of the isolated compounds, we found that the ICL inhibitory activities of compounds 3–9 were affected markedly by the degree of oxidation of the furan ring (Figure 1). Overall, these results provided important insight regarding the structure−activity relationships of suvanine sesterterpenes.
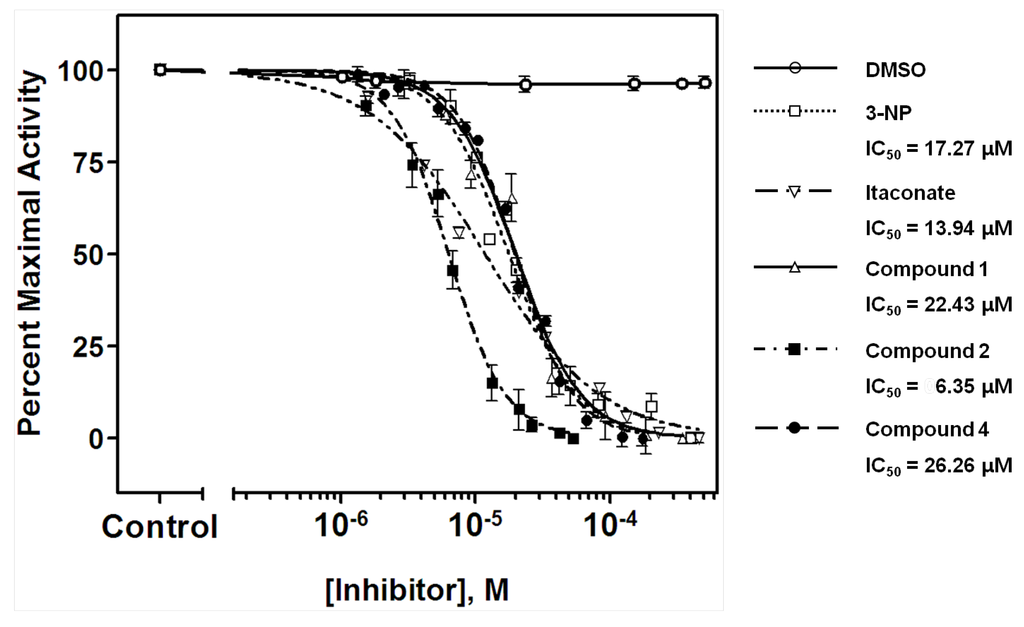
Figure 2.
A comparison of the dose–response curves of suvanine sesterterpenes (1, 2, and 4) against the ICL enzyme from C. albicans ATCC 10231. Data were scaled to internal controls (0.5% DMSO-treated), and GraphPad ver. 5.0 was used to fit the measured data and determine the IC50 values. The results are presented as means ± SD (n = 3). 3-Nitropropinate and itaconate were used as the positive controls.

Table 1.
Inhibitory activity of compounds 1–9 against ICL from C. albicans ATCC 10231 and growth of C. albicans SC5314.
| Compound | ICL IC50, µM (µg/mL) | MIC (µg/mL) | |
| Glucose | Acetate | ||
| 1 | 22.43 ± 1.49 (12.03 ± 0.80) | 100 | 25 |
| 2 | 6.35 ± 1.37 (3.00 ± 0.65) | 100 | 12.5 |
| 3 | 56.19 ± 7.10 (28.33 ± 3.58) | >100 | >100 |
| 4 | 26.26 ± 3.69 (12.82 ± 1.80) | >100 | 100 |
| 5 | 50.23 ± 6.27 (26.03 ± 3.25) | >100 | >100 |
| 6 | 96.15 ± 1.54 (49.83 ± 0.80) | >100 | 100 |
| 7 | 59.10 ± 3.45 (27.30 ± 1.85) | >100 | >100 |
| 8 | 67.64 ± 4.88 (38.44 ± 2.77) | >100 | >100 |
| 9 | 59.15 ± 1.11 (28.82 ± 0.54) | >100 | >100 |
| 3-NP | 17.27 ± 1.04 (2.06 ± 0.12) | >100 | >100 |
| Itaconate | 13.94 ± 0.64 (1.66 ± 0.08) | >100 | >100 |
| Amph B | ND | 1.56 | 0.39 |
3-Nitropropinate (3-NP) and itaconate were used as reference inhibitors of ICL; Amphotericin B (Amph B) was used as a standard antifungal drug; 0.5% DMSO was used as a negative control; ND, not determined.
To determine the type of inhibition, kinetic studies were performed with compounds 1, 2 and 4 at IC50 or twofold IC50 based on Lineweaver and Burk plot [27] (Figure 3). Inhibitor constants were obtained by Dixon plot. Inhibitory kinetics show that compound 1 behaved as an uncompetitive inhibitor (Ki = 17.38 μM). Compound 2 (Ki = 14.43 μM) and 4 (Ki = 62.57 μM) behaved as mixed noncompetitive inhibitors. Moreover, the binding of compounds 1, 2 and 4 to enzyme was reversible because the enzyme activity was indeed recovered by dialysis within 2 h, excluding the possible existence of a covalent bond between inhibitor and enzyme.
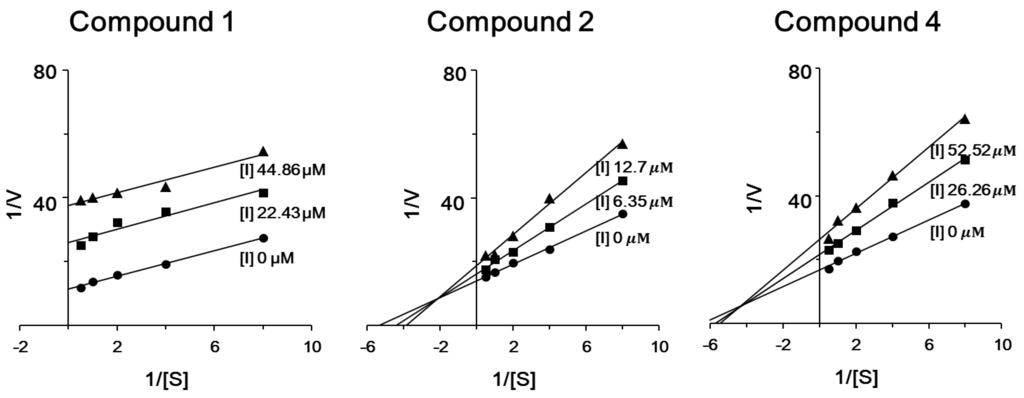
Figure 3.
Lineweaver-Burk plot of ICL inhibition by compounds 1, 2 and 4. [S], substrate concentration [mM]; V, reaction velocity (∆absorbance unit/min). Each data point represents the mean of three experiments.
The microbial strategy for survival during growth in a nutrient-free environment entails a metabolic shift in the carbon source to C2 substrates generated by β-oxidation of fatty acids [1,2]. Under these conditions, glycolysis is decreased and the glyoxylate shunt is upregulated significantly to allow anaplerotic maintenance of the TCA cycle and assimilation of carbon via gluconeogenesis. To investigate whether ICL inhibitors affected C2 substrate use, we cultured C. albicans strain SC5314 in YNB broth containing either 2% glucose or 2% potassium acetate as the sole carbon source, and inhibition was evaluated based on the MIC. C. albicans strain SC5314 was used in this test as the ICL sequence alignment of this strain (GenBank accession number AF222905) showed 100% identity with that of C. albicans ATCC 10231 over its entire length (data not shown). As shown in Table 1, fungal growth inhibition tests indicated that suvanine sesterterpenes 1–9 at concentrations up to 100 μg/mL had weak to no inhibitory effects on SC5314 cultured in glucose. Compounds 1 and 2 at concentration 100 μg/mL showed antifungal activities on SC5314 cultured in glucose. Therefore, at high concentrations, it is possible that these compounds could affect other molecular targets in C. albicans cells [16,17,18,19,20]. A similar trend was observed in SC5314 cultured in acetate with all compounds, except for compounds 1 and 2. Compound 2, which showed particularly potent inhibitory activity against SC5314 cultured in acetate, with an MIC value of 12.5 μg/mL. The antifungal activity of compounds 1–9 was also evaluated against C. albicans strains ATCC 11006 (from human virginal tracts), ATCC 10261 and ATCC 18804 (from human skin lesin). As expected, these compounds at concentrations up to 100 μg/mL had no inhibitory effects on C. albicans strains cultured in glucose, but were inhibitory to these strains cultured in acetate. These results indicated that ICL is involved in the proliferation of C. albicans using C2 substrates.
The genes encoding the glyoxylate cycle are required for virulence in both M. tuberculosis and C. albicans that can survive within macrophages [5,8]. It was expected that inhibitors of the glyoxylate cycle pathway would block the nutrient availability and prevent survival of these pathogens inside the macrophage. Based on these findings, we next investigated the effects of compound 2 on the growth phenotype and ICL mRNA expression of C. albicans strain SC5314 (wild-type), MRC10 (Δicl), and MRC11 (Δicl + ICL) [9]. As shown in Figure 4A, these strains grew normally on YNB agar plates containing glucose or glucose plus 12.5 μg/mL compound 2. However, the ICL-deletion mutant MRC10 failed to grow on acetate as the sole carbon source. In addition, the growth of all tested strains was inhibited markedly on YNB agar plates containing acetate plus 12.5 μg/mL compound 2. The effects of compound 2 on ICL mRNA expression in C. albicans were investigated using semi-quantitative RT-PCR analysis. As shown in Figure 4B, the ICL transcript levels in SC5314 and MRC11 were undetectable when the YNB liquid medium contained glucose, but were strongly induced when the medium contained acetate. Interestingly, under the ICL expression conditions, the transcript levels of ICL in both strains were diminished with increasing compound 2 concentrations. At 12.5 μg/mL, compound 2 reduced ICL mRNA expression by seventeenfold. These findings indicated that compound 2 inhibits ICL expression in C. albicans under C2-carbon-utilizing conditions.
In this study, however, we investigated the effect of suvanine salt on the ICL mRNA expression in C. albicans. Further studies are required to identify the relationship between enzyme inhibition and inhibition of gene expression, and the main cellular target of this compound. In previous works, suvanine sesterterpenes functionally inhibited enzyme activity such as serine protease [18], CDC25 phosphatase [19]. We also found that these sesterterpenes (1–9) have potent Na+/K+-ATPase inhibitory activities and these activities were influenced by structure bearing sulfonate group [21]. There is no direct evidence to identify how sulfonate of compounds inhibits Na+/K+-ATPase. However, strong supposition is that sulfonate containing compound modifies a specific conformationally sensitive amino residue on Na+/K+-ATPase, resulting in loss of enzyme activity [28]. Sulfur-containing natural products have unique chemical and biochemical properties linked to redox process, metal binding and catalytic reactions [29]. Therefore, suvanine sesterterpenes are expected to have various bioactivities.
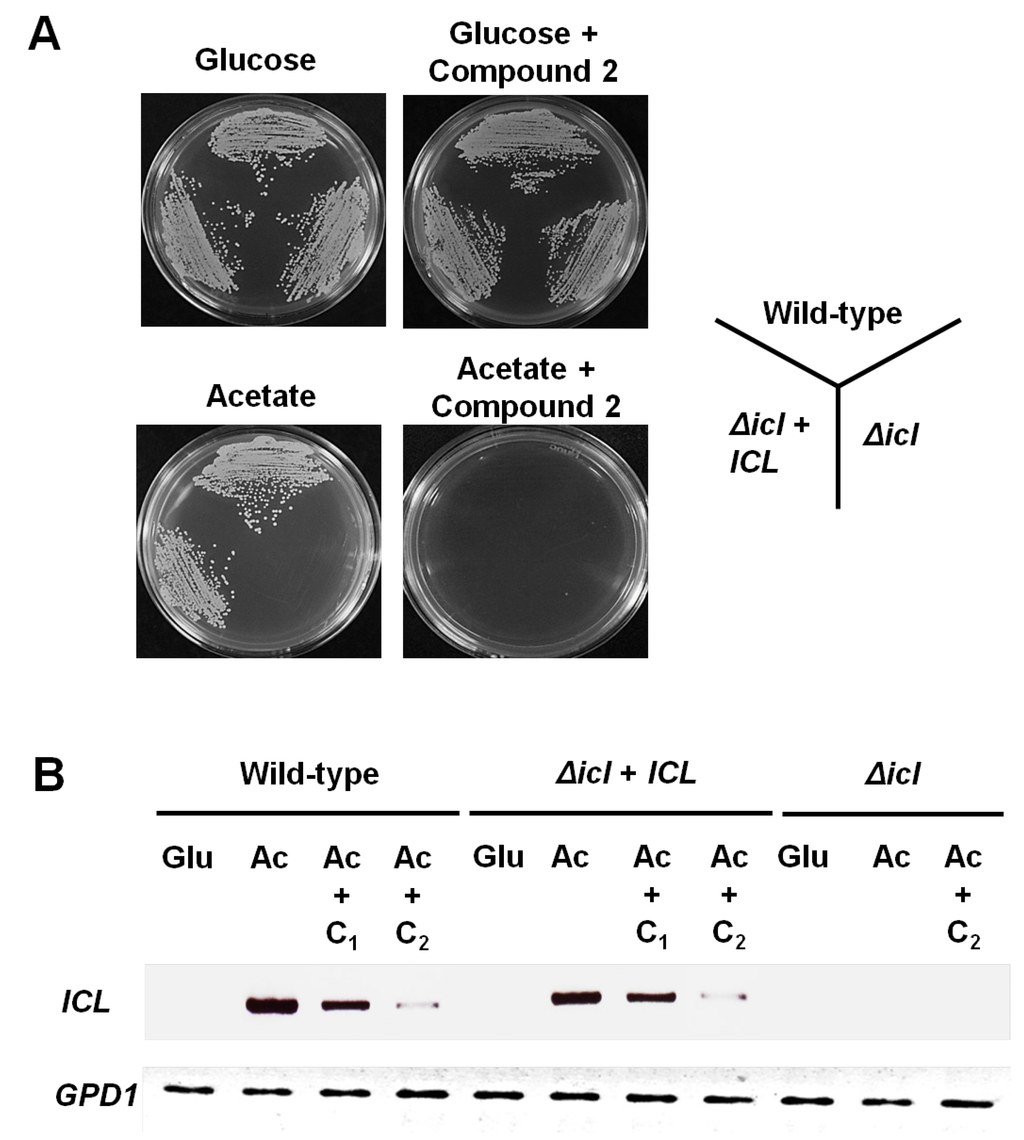
Figure 4.
Inhibitory activity of compound 2 against the growth and ICL mRNA expression of the wild-type and Δicl mutants. (A) C. albicans strain SC5314 (wild-type), MRC10 (Δicl), and MRC11 (Δicl + ICL) were cultured on YNB agar plates containing the indicated carbon source (2% glucose or 2% sodium acetate) with or without compound 2 (12.5 µg/mL) for 3 days at 28 °C; (B) Strains were grown to mid-log phase in minimal YNB liquid medium containing 2% glucose. Cells were collected by centrifugation and shifted to the same medium containing 2% glucose (Glu), 2% sodium acetate (Ac), or 2% sodium acetate (Ac) plus compound 2 (C1, 6.25 µg/mL; C2, 12.5 µg/mL) for 4 h at 28 °C. Total RNA was prepared from these cells and ICL mRNA expression was determined by semi-quantitative RT-PCR analysis. The GPD1 housekeeping gene was used as a loading control.
3. Experimental Section
3.1. General Experimental Procedure
Optical rotations were measured on a JASCO P-1020 polarimeter (JASCO Co., Tokyo, Japan) using a 1-cm cell. Ultraviolet (UV) spectra were recorded on a Hitachi U-3010 spectrophotometer (Hitachi High-Technologies Co., Tokyo, Japan). Infrared (IR) spectra were recorded on a JASCO 300E FT-IR spectrometer (JASCO Co., Tokyo, Japan). Bruker Avance 600 MHz spectrometer (Bruker, Rheinstetten, Germany) was used to obtain 1H, 13C and two-dimensional nuclear magnetic resonance (NMR) spectra. Mass spectrometric data were obtained at the Korea Basic Science Institute (Daegu, Korea) and were acquired using a JEOL JMS 700 mass spectrometer (JEOL Ltd., Tokyo, Japan) using meta-nitrobenzyl alcohol as a matrix for the fast atom bombardment mass spectrometry (FABMS). High-performance liquid chromatography (HPLC) was performed using a Spectrasystem p2000 (Thermo Scientific, Waltham, MA, USA) equipped with a refractive index detector, Spectrasystem RI-150. All solvents were spectroscopic grade or distilled from glass prior to use.
3.2. C. albicans Strains and Growth Media
C. albicans strain ATCC 10231 was the source of the ICL gene. C. albicans strains used for growth assay were SC5314 (ATCC MYA-2876) (wild-type), MRC10 (Δicl), MRC11 (Δicl +ICL), ATCC 10261, ATCC 18804, and ATCC 11006. The ICL-deletion mutant strains MRC10 and MRC11 were kindly provided by Prof. Michael C. Lorenz (The University of Texas Health Science Center at Houston, USA) [9]. These strains were subcultured in yeast nitrogen base (YNB) broth (Difco Laboratories, Detroit, MI, USA) containing 2% glucose at 28 °C. Stock solutions of test compounds were prepared by dissolution in DMSO and stored at −20 °C until use. The final concentration of DMSO was 0.5% in all assays, which was found to have no effect on the enzyme activity at a concentration of less than 1%. Other general reagents were obtained from commercial suppliers and were of the highest grade available.
3.3. Preparation of Recombinant ICL
The preparation of recombinant ICL protein from the genomic DNA of C. albicans ATCC 10231 was carried out using a method described previously [22]. Briefly, ICL was PCR-amplified using two synthetic primers: 5′-AGAATTCCTACCATGCCTTACACTCC-3′ (forward) and 5′-CTTCGTCGACTCAAAATTAAGCCTTG-3′ (reverse). The PCR product was cloned into the pBAD/Thio-TOPO vector (Invitrogen, Carlsbad, CA, USA) and transformed into Escherichia coli TOP10 (Invitrogen). After arabinose (0.02%) induction of cultures at 25 °C for 8 h, cells were lysed by lysozyme treatment and sonication, and the recombinant protein was purified using a Ni-NTA affinity column (Qiagen, Hilden, Germany) according to the manufacturer’s protocol.
3.4. ICL Inhibitor Potency Determination
The effect of isolated compounds on ICL was evaluated according to a procedure documented previously [23,24]. A 1-mL aliquot of the reaction mixture contained 20 mM sodium phosphate buffer (pH 7.0), 1.27 mM threo-dl (+) isocitrate, 3.75 mM MgCl2, 4.1 mM phenylhydrazine and 2.5 μg/mL purified ICL. The reaction was performed at 37 °C for 30 min with and without a prescribed concentration of the inhibitor dissolved in DMSO (final concentration, 0.5%). The formation of glyoxylate phenylhydrazone was followed spectrophotometrically at 324 nm. The effect of the inhibitor on ICL was calculated as a percentage relative to the solvent-treated control. A 12-point, twofold serial dilution dose–response assay was performed in triplicate. The resultant dose–response concentration range was 100 to 0.1 μg/mL of inhibitor in a 1-mL final reaction volume. Data were scaled to internal controls, and a four-parameter logistic model (GraphPad ver. 5.0, Prism) was used to fit the measured data and determine the IC50 values [25]. The ICL inhibitor 3-nitropropionate was used as a positive control [26]. Protein concentration was determined by the method of Bradford [30] using the Bio-Rad protein assay kit (Bio-Rad, Hercules, CA, USA) and bovine serum albumin as a standard.
3.5. In Vitro Growth Assays
C. albicans strains were grown in YNB (6.7% yeast nitrogen base) broth containing 2% glucose at 28 °C for 24 h, harvested by centrifugation, and washed twice with sterile distilled water. Each test compound was dissolved in DMSO and diluted with YNB containing 2% glucose or 2% potassium acetate to prepare serial twofold dilutions in the range 100–0.1 μg/mL. The final DMSO concentration was maintained at 0.5% by adding DMSO to the medium. Twenty microliters of the broth containing test fungus was added to each well of a 96-well microtiter plate (final concentration of 1 × 104 cells/mL). Culture plates were incubated for 3 days at 28 °C. The minimum inhibitory concentration (MIC) values were determined to be the lowest concentration at which the test compounds inhibited fungal growth. Amphotericin B was used as a reference compound.
3.6. Semi-Quantitative RT-PCR Analysis
C. albicans strain SC5314 (wild-type), MRC10 (Δicl), and MRC11 (Δicl +ICL) were grown into the mid-log phase in YNB broth (2% glucose), collected by centrifugation and washed twice with sterile distilled water. Cells were resuspended in YNB media containing 2% glucose, 2% acetate, or 2% acetate plus compound 2 (6.25 and 12.5 µg/mL) and grown for 4 h at 28 °C. Total RNA from each sample was isolated by using RNeasy Mini Kit (Qiagen), and 1 μg of total RNA was reverse transcribed into cDNA using the Superscript III First-Strand Synthesis System (Invitrogen). Semi-quantitative RT-PCR was conducted using the ICL-specific primers: 5′-ATGCCTTACACTCCTATTGACATTCAAAA-3′ (forward) and 5′-TAGATTCAGCTTCAGCCATCAAAGC-3′ (reverse). The GPD1 (glycerol-3-phosphate dehydrogenase) housekeeping gene was used as a loading control with the specific primers: 5′-AGTATGTGGAGCTTTACTGGGA-3′ (forward) and 5′-CAGAAACACCAGCAACATCTTC-3′ (reverse). PCR amplification was started with an initial incubation at 98 °C for 10 min followed by 30 cycles of 30 s at 98 °C for, 30 s at 56 °C, and 30 s at 72 °C and then performed at 72 °C for 5 min. The ICL mRNA expression level was determined by densitometric analysis using the ImageJ software (NIH, Bethesda, MD, USA).
4. Conclusions
Suvanine sesterterpenes (1–9) were isolated from the sponge Coscinoderma sp., and their inhibitory activities were investigated against ICL from C. albicans. These studies led to the identification of compounds 1, 2, and 4 as potent inhibitors. Compound 2 in particular, which possesses a sodium counterion, was most effective. The preliminary structure–activity relationship study suggested that the ICL inhibitory activities of suvanine sesterterpenes are affected markedly by the distance and spatial orientation of the enolsulfate group relative to that of the furan ring. This analysis provides potentially useful information on advanced drug design strategies to identify novel ICL inhibitors. The growth phenotype of ICL deletion mutants and semi-quantitative RT-PCR analysis indicated that compound 2 inhibited ICL mRNA expression in C. albicans under C2-carbon-utilizing conditions. As the enzymes of the glyoxylate cycle are not found in mammals, this compound shows promise as an antifungal agent in terms of suppressing C. albicans virulence. Further in vivo studies on this compound are underway in our laboratory.
Acknowledgments
We would like to thank Michael C. Lorenz for providing us with C. albicans ICL mutant strains. This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A1A2039659) and the Korean Government (MEST) (NRF-M1A5A1-2010-0020433).
Author Contributions
So-Hyoung Lee and Ki-Bong Oh designed the experiment, analyzed the data, and wrote the manuscript. Heegyu Kim also performed most of the experiments. Chan-Hong Ahn evaluated the antifungal activity of the isolated compounds. Tae Hyung Won and Jongheon Shin collected the sponge sample and performed characterization of the suvanine sesterterpenes.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vanni, P.; Giachetti, E.; Pinzuati, G.; McFadden, B.A. Comparative structure, function and regulation of isocitrate lyase, an important assimilatory enzyme. Comp. Biochem. Physiol. B 1990, 95, 431–458. [Google Scholar]
- Dunn, M.F.; Ramirez-Trujillo, J.A.; Hernandez-Lucas, I. Major roles of isocitrate lyase and malate synthase in bacterial and fungal pathogenesis. Microbiology 2009, 155, 3166–3175. [Google Scholar] [CrossRef]
- Kunze, M.; Pracharoenwattana, I.; Smith, S.M.; Hartig, A. A central role for the peroxisomal membrane in glyoxylate cycle function. Biochim. Biophys. Acta 2006, 1763, 1441–1452. [Google Scholar] [CrossRef]
- Strijbis, K.; Distel, B. Intracellular acetyl unit transport in fungal carbon metabolism. Eukaryot. Cell 2010, 9, 1809–1815. [Google Scholar] [CrossRef]
- McKinney, J.D.; Honer Zu Bentrup, K.; Munoz-Elias, E.J.; Miczak, A.; Chen, B.; Chan, W.T.; Swenson, D.; Sacchettini, J.C.; Jacobs, W.R., Jr.; Russell, D.G. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 2000, 406, 735–738. [Google Scholar] [CrossRef]
- Munoz-Elias, E.J.; McKinney, J.D. Mycobacterium tuberculosis isocitrate lyases 1 and 2 are jointly required for in vivo growth and virulence. Nat. Med. 2005, 11, 638–644. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Thornton, C.R.; Kershaw, M.J.; Debao, L.; Talbot, N.J. The glyoxylate cycle is required for temporal regulation of virulence by the plant pathogenic fungus Magnaporthe grisea. Mol. Microbiol. 2003, 47, 1601–1612. [Google Scholar] [CrossRef]
- Lorenz, M.C.; Fink, G.R. The glyoxylate cycle is required for fungal virulence. Nature 2001, 412, 83–86. [Google Scholar] [CrossRef]
- Ramirez, M.A.; Lorenz, M.C. Mutations in alternative carbon utilization pathways in Candida albicans attenuate virulence and confer pleiotropic phenotypes. Eukaryot. Cell 2007, 6, 280–290. [Google Scholar] [CrossRef]
- Goldstein, A.L.; McCusker, J.H. Development of Saccharomyces cerevisiae as a model pathogen. A system for the genetic identification of gene products required for survival in the mammalian host environment. Genetics 2001, 159, 499–513. [Google Scholar]
- Sharma, R.; Das, O.; Damle, S.G.; Sharma, A.K. Isocitrate lyase: A potential target for anti-tubercular drugs. Recent Pat. Inflamm. Allergy Drug Discov. 2013, 7, 114–123. [Google Scholar] [CrossRef]
- Kratky, M.; Vinsova, J. Advances in mycobacterial isocitrate lyase targeting and inhibitors. Curr. Med. Chem. 2012, 19, 6126–6137. [Google Scholar] [CrossRef]
- Lee, D.; Shin, J.; Yoon, K.M.; Kim, T.I.; Lee, S.H.; Lee, H.S.; Oh, K.B. Inhibition of Candida albicans isocitrate lyase activity by sesterterpene sulfates from the tropical sponge Dysidea sp. Bioorg. Med. Chem. Lett. 2008, 18, 5377–5380. [Google Scholar] [CrossRef]
- Lee, H.S.; Lee, T.H.; Lee, J.H.; Chae, C.S.; Chung, S.C.; Shin, D.S.; Shin, J.; Oh, K.B. Inhibition of the pathogenicity of Magnaporthe grisea by bromophenols, isocitrate lyase inhibitors, from the red alga Odonthalia corymbifera. J. Argic. Food. Chem. 2007, 55, 6923–6928. [Google Scholar] [CrossRef]
- Kernan, M.R.; Faulkner, D.J. Sesterterpene sulfates from a sponge of the family Halichondriidae. J. Org. Chem. 1988, 53, 4574–4578. [Google Scholar] [CrossRef]
- Shin, D.S.; Lee, T.H.; Lee, H.S.; Shin, J.; Oh, K.B. Inhibition of infection of the rice blast fungus by halisulfate 1, an isocitrate lyase inhibitor. FEMS Microbiol. Lett. 2007, 272, 43–47. [Google Scholar] [CrossRef]
- De Marino, S.; Festa, C.; D’Auria, M.V.; Bourguet-Kondracki, M.L.; Petek, S.; Debitus, C.; Andres, R.M.; Terencio, M.C.; Paya, M.; Zampella, A. Coscinolactams A and B: New nitrogen-containing sesterterpenoids from the marine sponge Coscinoderma mathewsi exerting anti-inflammatory properties. Tetrahedron 2009, 65, 2905–2909. [Google Scholar]
- Kimura, J.; Ishizuka, E.; Nakao, Y.; Yoshida, W.Y.; Scheuer, P.J.; Kelly-Borges, M. Isolation of 1-methylherbipoline salts of halisulfate-1 and of suvanine as serine protease inhibitors from a marine sponge, Coscinoderma mathewsi. J. Nat. Prod. 1998, 61, 248–250. [Google Scholar] [CrossRef]
- Loukaci, A.; Le Saout, I.; Samadi, M.; Leclerc, S.; Damiens, E.; Meijer, L.; Debitus, C.; Guyot, M. Coscinosulfate, a CDC25 phosphatase inhibitor from the sponge Coscinoderma mathewsi. Bioorg. Med. Chem. 2001, 9, 3049–3054. [Google Scholar] [CrossRef]
- Cassiano, C.; Monti, M.C.; Festa, C.; Zampella, A.; Riccio, R.; Casapullo, A. Chemical proteomics reveals heat shock protein 60 to be the main cellular target of the marine bioactive sesterterpene suvanine. ChemBioChem 2012, 13, 1953–1958. [Google Scholar] [CrossRef]
- Bae, J.; Jeon, J.; Lee, Y.J.; Lee, H.S.; Sim, C.J.; Oh, K.B.; Shin, J. Sesterterpenes from the tropical sponge Coscinoderma sp. J. Nat. Prod. 2011, 74, 1805–1811. [Google Scholar] [CrossRef]
- Shin, D.S.; Kim, S.; Yang, H.C.; Oh, K.B. Cloning and expression of isocitrate lyase, a key enzyme of the glyoxylate cycle, of Candida albicans for development of antifungal drugs. J. Microbiol. Biotechnol. 2005, 15, 652–655. [Google Scholar]
- Dixon, G.H.; Kornberg, H.L. Assay methods for key enzymes of the glyoxalate assay. Biochem. J. 1959, 72, 3P. [Google Scholar]
- Hautzel, R.; Anke, H.; Sheldrick, W.S. Mycenon, a new metabolite from a Mycena species TA 87202 (Basidiomycetes) as an inhibitor of isocitrate lyase. J. Antibiot. 1990, 43, 1240–1244. [Google Scholar] [CrossRef]
- Miller, B.; Friedman, A.J.; Choi, H.; Hogan, J.; McCammon, J.A.; Hook, V.; Gerwick, W.H. The marine cyanobacterial metabolite gallinamide A is a potent and selective inhibitor of human cathepsin L. J. Nat. Prod. 2014, 77, 92–99. [Google Scholar] [CrossRef]
- Sharma, V.; Sharma, S.; Hoener Zu Bentrup, K.; McKinney, J.D.; Russell, D.G.; Jacobs, W.R., Jr.; Sacchettini, J.C. Structure of isocitrate lyase, a persistence factor of Mycobacterium tuberculosis. Nat. Struct. Biol. 2000, 7, 663–668. [Google Scholar] [CrossRef]
- Lineweaver, H.; Burk, D. The determination of enzyme dissociation constants. J. Am. Chem. Soc. 1934, 56, 658–666. [Google Scholar] [CrossRef]
- Pedemonte, H.C.; Kaplan, J.H. Inhibition and derivatization of the renal Na,K-ATPase by Dihydro-4,4′-diisothiocyanatostilbene-2,2′-disulfonate. Biochemistry 1988, 27, 7966–7973. [Google Scholar] [CrossRef]
- Jacob, C. A scent of therapy: pharmacological implications of natural products containing redox-active sulfur atoms. Nat. Prod. Rep. 2006, 23, 851–863. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).