Abstract
P-glycoprotein (P-gp) is a protein belonging to the ATP-binding cassette (ABC) transporters superfamily that has clinical relevance due to its role in drug metabolism and multi-drug resistance (MDR) in several human pathogens and diseases. P-gp is a major cause of drug resistance in cancer, parasitic diseases, epilepsy and other disorders. This review article aims to summarize the research findings on the marine natural products with P-glycoprotein inhibitor properties. Natural compounds that modulate P-gp offer great possibilities for semi-synthetic modification to create new drugs and are valuable research tools to understand the function of complex ABC transporters.
1. Introduction
1.1. P-Glycoprotein
P-glycoprotein (P-gp), belonging to the large ATP-binding cassette (ABC) family, is a transmembrane protein encoded by the ATP-binding cassette sub-family B member 1 (ABCB1) gene. This protein is composed of 1280 amino acids (170 kDa) organized in two transmembrane domains, each one comprised of twelve highly hydrophobic α-helices and two intracellular nucleotide binding regions with ATPase activity. The drug-binding site (DBS) is found in the intracellular part of the protein, and when ATP activates P-gp, the substrate is extruded by a “flip-flop” mechanism to the luminal side. Its subsequent dephosphorylation leads to the transformation of the protein back to the initial state [1,2,3,4].
1.2. Functions of P-gp
P-gp can transport a wide range of xenobiotics out of the cell at the apical membrane of many secretory cell types, including adrenal gland, brain, kidney, liver, placenta, small and large intestine and the testes. Not all functions of the P-gp are known; however, there is a growing understanding of the role of P-gp in many organisms [1,2,3,4,5,6,7,8,9,10].
In the blood-brain barrier and blood-placenta barrier, P-gp prevents xenobiotic accumulation in the brain and pregnant uterus, respectively. Usually, P-gp excretes xenobiotics that are taken along with nutrients through the urine, bile and intestinal lumen and translocates hormones. The expression of P-gp in normal gastrointestinal tract cells prevents drug absorption after oral administration. Similarly, P-gp in the brain blocks the entrance of antiviral drugs [5,6,7,8,9,10].
1.3. Mechanism of P-gp Efflux Function
The mechanism by which P-gp performs its efflux pump function is not completely understood; however, there are several models to explain this process, two of which are most accepted. The first model states that P-gp exerts its effect through a flippase type mechanism (Figure 1) [11,12]. This mechanism is based on the assumption that drugs in the external and internal medium of the membrane should be at equilibrium with the outer and inner leaflet of the membrane. Based on this equilibrium, P-gp exchanges drugs from the inner leaflet of the membrane to the outer leaflet [11].
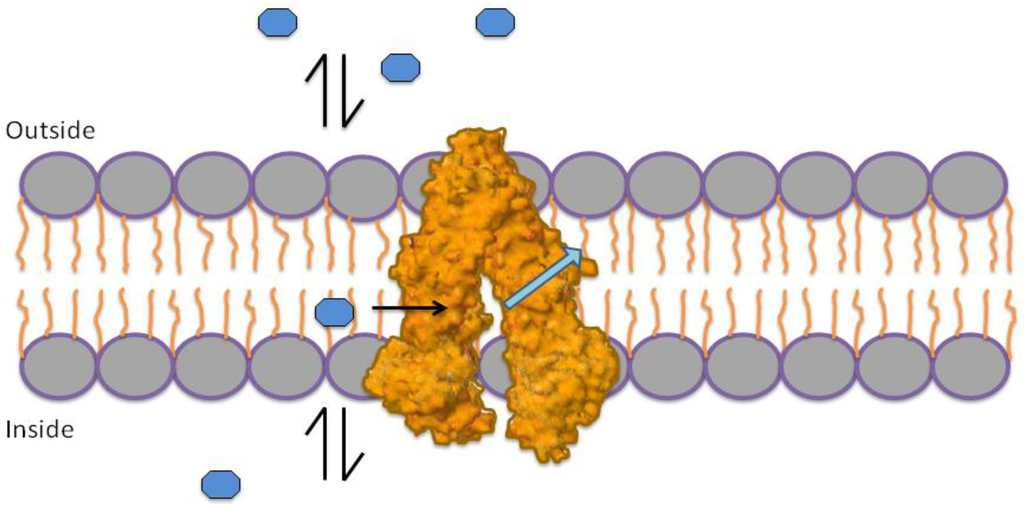
Figure 1.
Flippase mechanism. P-gp interchanges molecules from the inner leaflet of the membrane to the outer leaflet, in order to maintain a concentration balance on both sides.
Conversely, the “hydrophobic vacuum cleaner” model (currently the most accepted) suggests that P-gp removes hydrophobic molecules that are within in the membrane by a “vacuum cleaner” device (Figure 2) [12,13,14]. The high-resolution crystal structure of a mouse P-gp does not differentiate between the models, but rather, could be used to support either of them. The protein structure contains two portals within the P-gp cavity that connect to the inner leaflet of the membrane and would allow hydrophobic drugs to pass directly from the lipid bilayer to the cavity [14].
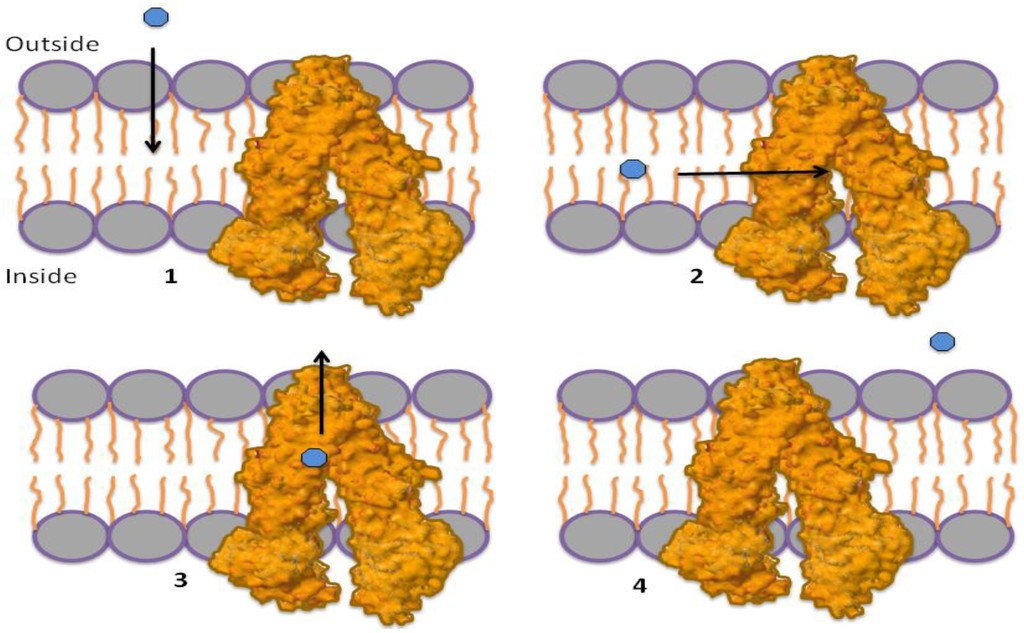
Figure 2.
Hydrophobic vacuum cleaner mechanism. (1) Substrates pass through the membrane to the lipid bilayer; (2) substrates can enter the P-gp through “portals” that pass the substrate from the lipid bilayer in to the P-gp internal cavity; (3) substrates bind to the drug-binding site (DBS); (4) P-gp transports the substrate to the outside of the cell.
1.4. Role of ATP in Protein Activation
In the absence of exogenous substrates, P-gp has an intrinsic ATPase activity [15]. Moreover, P-gp substrates can positively or negatively modulate this activity and, consequently, the rate of hydrolysis of ATP [16]. Compounds that modulate the P-gp ATPase activity have been categorized into three groups: (1) Compounds that stimulate basal ATPase activity at low concentrations and inhibit the activity at high concentrations (e.g., vinblastine, verapamil and paclitaxel); (2) compounds that increase ATPase activity in a dose-dependent manner (e.g., bisantrene, valinomycin and tetraphenylphosphonium); and (3) compounds that inhibit both basal and compound-stimulated ATPase activity (e.g., cyclosporin A, rapamycin and gramicidin D) [16].
A particularly important aspect to consider in the mechanism of P-gp efflux function is the role of ATP in protein activation. Higgins and coworkers proposed that two molecules of ATP are required in the first step of protein activation [17]. Siarheyeva and colleagues suggested a mechanism that also involves two molecules of ATP, but one ATP molecule is strongly bound and the other weakly bound to the protein, affording two specific substrate-binding domains, one of high affinity and one of low affinity [18]. Sauna and coworkers proposed another model in which one molecule of ATP activates the efflux pump of P-gp to move drugs out of the protein, and a second ATP molecule returns P-gp to its original conformation [19].
All these models predict that inhibiting the protein ATPase activity can disrupt P-gp efflux function. However, this is not a general rule, because some P-gp inhibitors increase the protein ATPase activity [20,21,22,23]. Furthermore, initial investigations have suggested that P-gp inhibitors should bind to the protein nucleotide binding site (NBS) to inhibit ATPase activity, but one study of a flavonoid-type inhibitor found that the compound stimulated the activity of ATPase without binding to the protein NBS [22].
In general, P-gp inhibitors work in one of the three ways:
- Promoting a conformational change in P-gp that blocks the ATP binding site and, subsequently, ATPase function;
- Promoting a conformational change in P-gp that enhances ATP binding, but concurrently blocks the substrate binding site;
- Inactivating the substrate binding site without inducing any conformational changes, e.g., stereo-isomers of cyclic hexapeptide inhibitors QZ59-RRR and QZ59-SSS [24].
1.5. Importance in Therapy
Due to its role in drug metabolism, P-gp has considerable clinical relevance; it affects the absorption, distribution and secretion of drugs, and it has a major role in multidrug resistance (MDR) in cancer [4,5,6,7,8,9]. P-gp reduces the clinical efficacy of several drugs (anticancer, antibiotics, antidepressants, antiepileptics, antihistamines, antihypertensives, antiarrhythmics, calcium channel blockers, cardiac glycosides, immunosuppressants, HIV protease inhibitors, hypocholesterolaemiants and steroids) by modifying their absorption and distribution in tissues [1,2,6,8,9,10].
P-gp was first discovered in 1976 for its role in MDR in cancer; it is overexpressed in several human tumors and is an important barrier to success in cancer treatments [3,4,5,6,7,8,10]. Interestingly, P-gp in tumors also appears to provide resistance to apoptosis induced by different stimuli, including serum starvation, Fas, TNF and UVB- and γ-irradiation [5,8]. The mechanism whereby P-gp inhibits apoptosis is still unclear [5,8].
In AIDS patients, P-gp contributes to the resistance to protease inhibitors, such as indinavir, ritonavir, nelfinavir and saquinavir [2,25]. P-gp is also involved in MDR in some human parasitic infections, including those caused by Plasmodium falciparum [26], Leishmania tropica [27], Leishmania amazonensis [28], Trypanosoma cruzi [29] and Entamoeba histolytica [30].
1.6. P-gp Inhibitors
Over the years, identifying small molecules that interfere with the activity of P-gp has taken relevance, because blocking its pump function could reduce the effective concentration of drugs administered in the treatment of cancer, HIV, parasitic diseases and other diseases. On the basis of their specificity, affinity and toxicity, P-gp inhibitors are categorized into three generations (Table 1). The first generation of inhibitors are metabolites that already have a clinical use, e.g., verapamil (calcium channel blocker drug) and cyclosporine A (immunosuppressant drug), and, subsequently, were tested against P-gp and found to inhibit the enzyme. These drugs required high concentrations to inhibit P-gp, and for this reason, they were not approved as inhibitor P-gp drugs [31,32].
Second generation inhibitors are compounds without previous therapeutic use and that have a higher affinity for P-gp than the first generation compounds. The problem with these metabolites is that they are quickly metabolized by the CYPA4 enzyme, thus altering their pharmacokinetics and reducing their efficacy. It is important to point out that these inhibitors are designed to have lower toxicity than the compounds belonging to the first generation, despite retaining certain undesirable toxicity features that limit their pharmacological use [33,34,35].

Table 1.
Selected examples of classical inhibitors of P-gp by generation.
| First Generation | Second generation | Third Generation |
|---|---|---|
| Verapamil Cyclosporine A Vincristine Reserpine Quinidine Tamoxifen Trifluoperazine | (R)-verapamil Valspodar (PSC-833) Dexniguldipine Elacridar (GF120918) Biricodar Dofequidar | Tariquidar (XR9576) Zosuquidar (LY335979) Laniquidar (R101933) ONT-093 (OC-144-093) Mitotane (NSC-38721) Annamycin |
The third generation inhibitors are compounds that were obtained using combinatorial chemistry and subsequent structure-activity relationship studies to identify compounds that inhibit P-gp with high specificity and low toxicity. These P-gp inhibitors have a potency of about 10-fold more than the earlier generations of inhibitors. These compounds are not inhibited by the CYPA4 enzyme, and therefore, they do not exhibit altered pharmacokinetics [36,37].
Inhibitors belonging to one of the three generations exert their effect by one of the following mechanisms (Table 2): (1) Disrupting the hydrolysis of ATP [38,39,40,41,42,43,44,45,46,47]; (2) altering P-gp expression [48,49,50,51,52,53,54,55]; and (3) reversible inhibition or competition for a binding site, as demonstrated by photoaffinity labelling [56,57,58,59,60,61,62,63,64,65].

Table 2.
Mechanism of P-gp classical inhibitors.
| ATPase Activity | P-gp Expression | Competition for Binding Site | ||
|---|---|---|---|---|
| Inhibitor | Stimulator | Down Regulator | Up Regulator | |
| Valspodar Tariquidar Elacridar ONT-093 | Verapamil Cyclosporine A Vincristine Quinidine Tamoxifen Toremifene Trifluoperazine Dexverapamil Biricodar | Verapamil Cyclosporine A Reserpine Toremifene Trifluoperazine Dexverapamil Valspodar | Vincristine | Verapamil Cyclosporine A Vincristine Reserpine Quinidine Valspodar Dexniguldipine Biricodar Elacridar Dofequidar Tariquidar Zosuquidar |
One of the most common mechanisms displayed by classical P-gp inhibitors is competition for drug binding sites. However, P-gp has multiple binding sites, making it difficult to design target-based inhibitors. The poor selectivity shown by inhibitors with this mechanism could be due to P-gp having multiple binding sites. It is also difficult to detect general functional groups that modulate inhibitory activity against P-gp; however, it has been possible to obtain active functional groups from specific pharmacophores.

Table 3.
P-gp inhibitors that have been evaluated in clinical trials.
| P-gp Inhibitor | Phase | Trial | Protocols Identification |
|---|---|---|---|
| Tariquidar (XR9576) | II | Tariquidar and Docetaxel to Treat Patients With Lung, Ovarian, Renal and Cervical Cancer | 03-C-0284, NCI-03-C-0284, NCT00072202, NCT00069160 |
| II | Surgery Plus Chemotherapy (Doxorubicin, Vincristine and Etoposide), Mitotane and Tariquidar to Treat Adrenocortical Cancer | 040011, 04-C-0011, NCT00071058 | |
| I | Study of XR9576 and Vinorelbine in Patients with Advanced Cancer | NCI-00-C-0044 | |
| I | Trial of Tariquidar (XR9576) in Combination with Doxorubicin, Vinorelbine or Docetaxel in Pediatric Patients with Solid Tumors | NCT00011414 | |
| Zosuquidar (LY335979) | III | Daunorubicin and Cytarabine ± Zosuquidar in Treating Older Patients with Newly Diagnosed Acute Myeloid Leukemia or Refractory Anemia | CDR0000257122 E3999, U10CA021115, ECOG-E3999, NCT00046930 |
| II | Zosuquidar in Combination With Daunorubicin and Cytarabine in Patients Ages 55–75 with Newly Diagnosed Acute Myeloid Leukemia (AML) | KAN-979-01 NCT00129168 | |
| II | A Trial of Gemtuzumab Ozogamicin (GO) in Combination with Zosuquidar in Patients with CD33 Positive Acute Myeloid Leukemia | KAN-979-02 NCT00233909 | |
| Laniquidar (R101933) | II | R101933 Combined with Chemotherapy in Treating Patients with Metastatic Breast Cancer That Has Not Responded to Previous Chemotherapy | EORTC-10003-16004 EORTC-16004, ECSG-EORTC-16004, IDBBC-10003, NCT00028873 |
| Elacridar (GF120918) | I | A Phase I, Randomized, Open-Label, Parallel-Cohort, Dose-Finding Study of Elacridar (GF120918) in Combination with 2.0 mg Oral Topotecan in Cancer Patients | BCR10001 |
| Mitotane (NSC-38721) | III | Trial in Locally Advanced and Metastatic Adrenocortical Carcinoma Treatment (FIRM-ACT) | CO-ACT-001 NCT00094497 |
| II | Phase II Study of Continuous-Infusion DOX/VCR/VP-16 with Daily Oral Mitotane for Renal Cell Cancer | NCI-94-C-0156 | |
| II | Phase II Mitotane plus Cortisone Acetate/Fludrocortisone and ADR for Residual, Recurrent or Metastatic Adrenal Cortical Carcinoma | EST-1879 | |
| II | Phase II Study of Continuous-Infusion DOX/VCR/VP-16 with Daily Oral Mitotane Before and After Surgery in Patients with Adrenocortical Carcinoma | NCI-93-C-0200D NCI-93-C-0200B | |
| Annamycin | II | Chemotherapy in Treating Patients with Breast Cancer | CDR0000068486 NYU-9851, NCI-G01-1914, NCT00012129 |
FIRM-ACT: First International Randomized trial in locally advanced and Metastatic Adrenocortical Carcinoma Treatment; DOX: Doxorubicin; VCR: Vincristine; VP-16: Etoposide; ADR: Adriamycin.
One of the most interesting studies focused on identifying functional groups of active molecules, which was performed with 27 digoxin transport inhibitors in Caco-2 cells, found that two hydrophobic groups along with a hydrogen-bond acceptor group and an aromatic core were required for P-gp inhibition [66] This finding is relevant, because many previously reported P-gp inhibitors also possesses the majority of these features. It is also important to highlight that peptides similar to cyclosporine A (in size and amino acid composition) could have the inhibitory properties of P-gp, as in the case of valspodar and kendarimide A.
Many inhibitors of P-gp, especially within the third generation compounds, have been tested in clinical trials to assess their pharmacological potential (Table 3). Unfortunately, most of them have failed, because they displayed non-specific toxicity [8]. Among the negative factors that prevented success are: (1) High variability in the response rate associated with P-gp inhibitors, which is related to levels of P-gp expression and the co-expression of other ABC transporters [1,8,67]; (2) the pharmacokinetic interaction between the P-gp inhibitor and the other co-administered drugs, which lead to an increase in drug toxicity [8]; (3) the increase in plasma concentrations of a co-administered drug by interfering with its metabolism or excretion [8]; and (4) the increase in the toxicity of a co-administered drug in healthy tissues by inhibiting the basal activity of P-gp [8]. Therefore, there is an urgent need for identifying new, more effective and non-toxic P-gp inhibitors.
2. Inhibitors from Marine Sources
Oceans cover around seventy percent of the Earth’s surface and represent a resource of huge dimensions for natural product chemistry. This media contains nearly eighty percent of the biological diversity of life on the planet. Studies over the past 30 years have led to the discovery of thousands of new compounds from marine sources, which have shown a wide range of biological activities [68,69,70].
Several marine compounds or analogs inspired by marine natural products have been approved for clinical use, including vidarabine (for the treatment of a recurrent epithelial keratitis caused by herpes simplex virus type 1 and 2 and superficial keratitis), cytarabine (for cancer), ziconotide (for the treatment of severe chronic pain in patients with cancer or AIDS), trabectedin (for use as an anticancer agent against soft tissue sarcoma) and halaven (for metastatic breast cancer) [68,69,70].
In this article, we systematically review several marine natural products with P-gp inhibitor properties (Table 4). As mentioned, this protein is a major cause of drug resistance in cancer, some parasitic diseases, epilepsy and other disorders. The modulation of targets of P-gp by natural or synthetic compounds offers great possibilities for the discovery of new drugs and valuable research tools to understand the complex ABC transporters.

Table 4.
Marine compounds with P-gp inhibitor properties. ET-743, ecteinascidin 743; MDR, multi-drug resistance.
| Inhibitor | Intracellular Accumulation of Substrates | ATPase Activity | Photoaffinity Labelling | Cell Line Tested | Drug with Enhanced Activity | P-gp Expression | Selective to MDR1 or ABCB1 |
|---|---|---|---|---|---|---|---|
| Sipholenol A | Increased | Stimulated | Inhibited | KB-C2, KB-V1 | colchicine, vinblastine, paclitaxel | Not altered | Yes |
| Lamellarin | Increased | xx | xx | P338/Schabel | doxorubicin, daunorubicin, vinblastine | xx | xx |
| Agosterol A | xx | xx | Inhibited | KB-C2 | colchicine | xx | No |
| ET-743 + | Increased | xx | Not inhibited. | KB-8-5, KB-C2 | doxorubicin, vincristine | Downregulated | xx |
| N-Methylwelwitin-dolinone C isothiocyanate | Increased | xx | Inhibited | NCI/ADR-RES | vinblastine, taxol, actinomycin D, daunomycin, colchicine | xx | xx |
| Parguerenes | Increased | xx | xx | SW620AD-300, HEK293/ABCB1, CEM/VLB100 | vinblastine, doxorubicin and paclitaxel | Not altered | No |
| Patellamide d | xx | xx | xx | CEM/VLB100 | vinblastine, colchicine and adriamycin | xx | xx |
| Kendarimide A | xx | xx | xx | KB-C2 | colchicine | xx | xx |
| Bryostatin 1 | Increased | xx | Inhibited | KB-C1, HeLa-MDR1-V185 | vinblastine, colchicine | xx | |
| ISA, ISA B | xx | xx | xx | KB/VJ300 | vincristine | xx | xx |
| Nocardioazines | xx | xx | xx | SW620AD-300 | doxorubicin | xx | xx |
| Discodermolide * | xx | xx | xx | SW620AD-300, A2780AD | xx | xx | No |
| Polyoxygenated steroids # | xx | xx | xx | KB-C2 | xx | xx | xx |
+ Inhibits the expression of MDR1; * the authors expressed only a reduction in resistance to paclitaxel; # the authors expressed only an inhibition in the growth of MDR cells; xx: Information not reported; P388/Shabel: MDR murine leukemia cells; HEK293/ABCB1: Human primary embryonic kidney stable gene-transfected cell line.
2.1. Inhibitors from Tunicates
The compound, ecteinascidin 743 (ET-743, 1) (Figure 3), isolated from the Caribbean tunicate, Ecteinascidia turbinata [71,72], showed good anti-cancer in vitro activity against mouse lymphocytic leukemia (L1210) cells with a half maximal inhibitory concentration (IC50) values of 0.5 ng/mL. ET-743 partially reverses resistance to doxorubicin and vincristine in MDR epidermal carcinoma (KB-C2 and KB-8-5) P-gp/multidrug resistance 1 (MDR1) overexpressing cancer cell lines. A greater intracellular accumulation of doxorubicin and vincristine (up to 122 and 22 fold, respectively) were observed in both cells when pretreated with non-toxic concentrations of 1. However, photoaffinity labeling experiments showed that overcoming doxorubicin/vincristine resistance was not a result of the direct inhibition of P-gp activity [73]. Because of these beneficial effects in cancer treatments, 1 has received orphan drug designation specifically for soft tissue sarcoma treatment in the United States and ovarian cancer treatment in the United States and Europe [74].
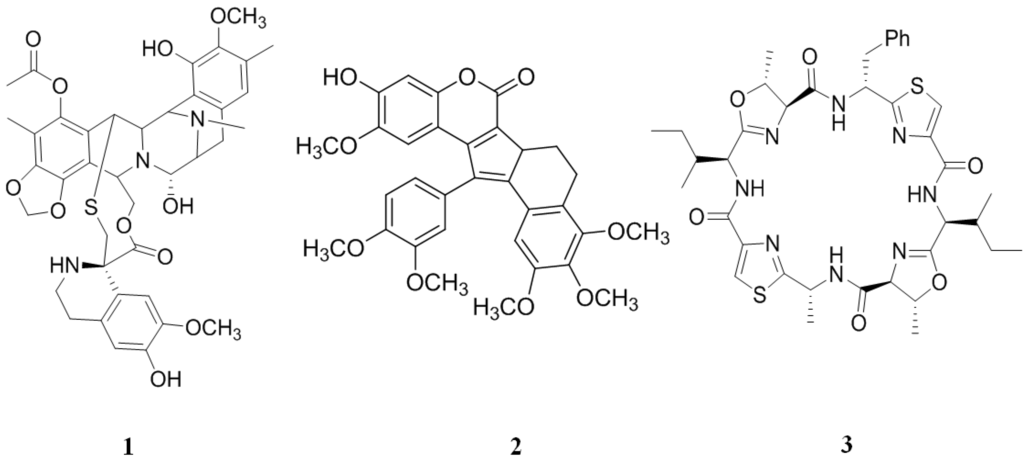
Figure 3.
Inhibitors of P-gp that have been isolated from tunicates.
Lamellarins are a group of polyaromatic alkaloids originally isolated from Lamellaria sp. [75] and later from the ascidian, D. chartaceum [76,77], the sponge, Dendrilla cactos [78,79], and some species of unidentified ascidians [80,81,82]. This class of compounds has shown diverse biological activities, including cytotoxicity [75,83,84], immunomodulating activity [77], inhibition of HIV integrase [83] and, critically, the ability to render some MDR cancer cell lines susceptible to anti-cancer treatments [84].
Lamellarin I (2) (Figure 3) presented a better chemo-sensitizing activity than verapamil (nine to 16 fold higher) in doxorubicin-resistant human colon adenocarcinoma (Lo Vo/Dx) cell line. In addition, 2 increases the cytotoxicity of doxorubin, vinblastine and daunorubicin in a concentration-dependent manner in MDR cells. Compound 2 exerts this effect through a direct inhibition of the P-gp pump function, as demonstrated by the accumulation of Rhodamine 123 in Lo Vo/Dx cells [84].
The patellamides are thiazole- and oxazoline-containing cyclic octapeptides isolated from Lissoclinum patella that show several biological activities, including cytotoxicity and reversing resistance in the MDR human leukemic (CEM/VLB100) cell line against vinblastine, colchicine and adriamycin [85,86]. The cytotoxicity of patellamide-type compounds may be because of conformational restrictions set by the presence of the heterocycles and their ability to intercalate DNA [86]. Of this family of compounds, patellamide D (3) (Figure 3) showed the best activity in reversing MDR; it enhanced by 66, 2.8 and 1.4 fold the activity of vinblastine, adriamycin and colchicine, respectively. The activity of 3 is similar to verapamil, a well-known P-gp inhibitor [87].
2.2. Inhibitors from Sponge
A novel polyhydroxylated sterol acetate, agosterol A (4) (Figure 4), was isolated from the marine sponge, Spongia sp. [88]. This compound completely reversed MDR in human KB carcinoma cells overexpressing an MRP1 (a membrane glycoprotein) [88,89]. In order to obtain the mechanism of action of 4, accumulation and efflux experiments were performed using KB-C2 and human carcinoma overexpressing MRP1 (KB-CV60) cell lines [89]. Compound 4 interrupted the ATP-dependent active efflux of vincristine in both cells by increasing intracellular concentrations of this Vinca alkaloid. In other experiments, 4 inhibited the [3H] azidopine photolabeling of P-gp and the uptake of [3H]S-(2,4-dinitrophenyl)glutathione in inside-out membrane vesicles from KB-CV60 cells [90]. Taken together, the data indicate that 4 inhibits the drug efflux modulated by P-gp and MRP1.
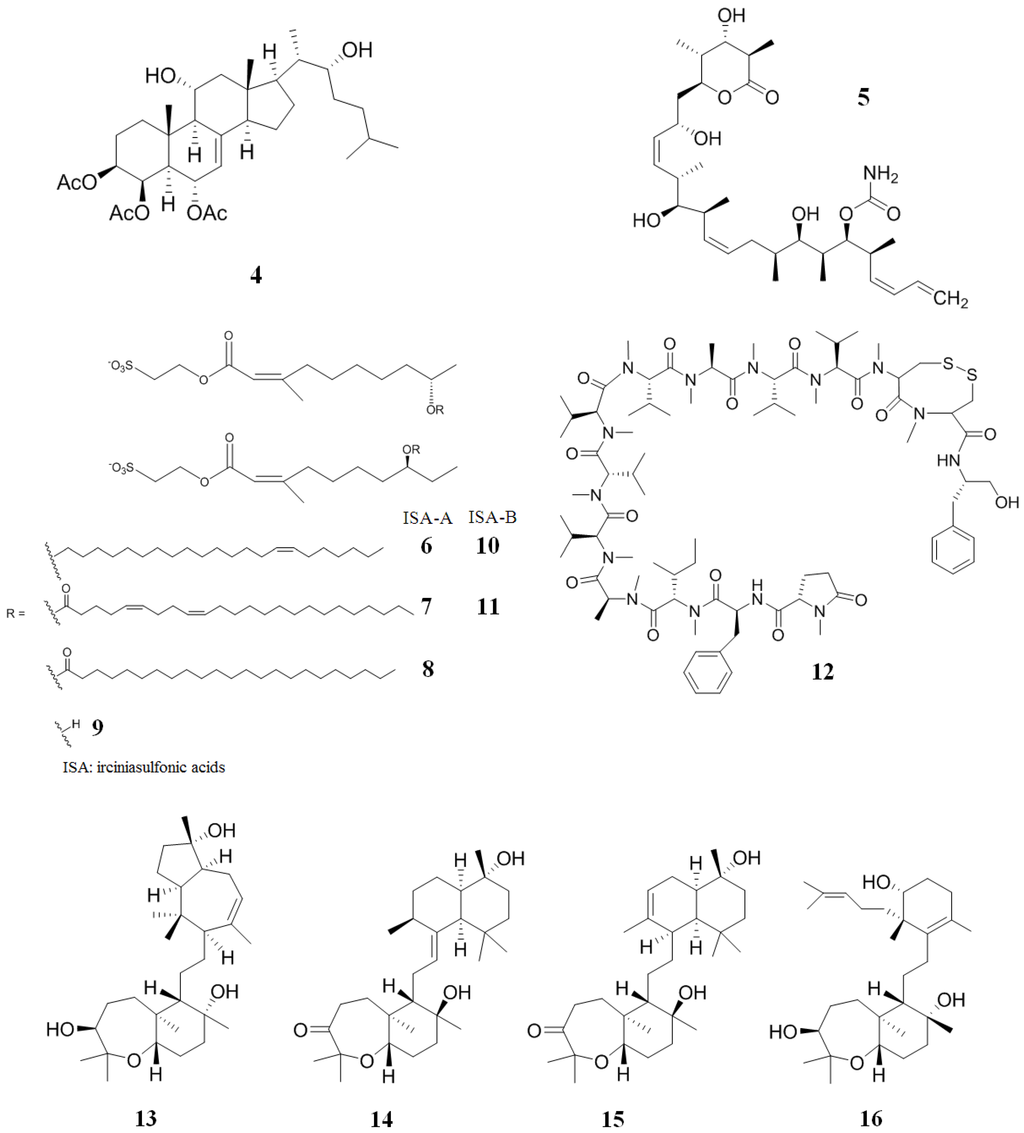
Figure 4.
Inhibitors of P-gp that have been isolated from sponges.
Discodermolide (5) (Figure 4) is a polyketide that was discovered in the marine sponge, Discodermia dissoluta, due to its immunosuppressive and anti-tumor activities [91,92,93]. Interestingly, 5 has the same mechanism of action as taxol (it blocks the cell cycle at the G"/M checkpoint and induces apoptosis), but is more potent against several types of cancer cell [94]. Additionally, 5 is active against taxol-resistant cells. Normally, MDR ovarian carcinoma (A2780AD) and paclitaxel-resistant colon carcinoma (SW620AD-300) cell lines are 25–89 fold more drug-resistant than their parenteral lines. Compound 5 drastically decreased multidrug resistance to taxol in both cell lines, with IC50 values in the range of 70 nM for SW620AD-300 (compared to 260 nM for taxol) and 580 nM for A2780AD cells (compared to 3900 nM for taxol) [94].
A mixture of three structurally-related esters (irciniasulfonic acids, 6–11) (Figure 4) were isolated from the marine sponge Ircinia sp. The irciniasulfonic acids mixture are found to reverse MDR at 33 µg/mL against P-gp overexpressing derived from human cancer KB cells (KB/VJ300) cells in the presence of 10 ng/mL of vincristine. It was subsequently discovered that a simple chain of deacylirciniasulfonic acid (9) (Figure 4) was 13 fold more potent than the other irciniasulfonic acids (IC50 values of 3 μM and 38 μM, respectively) [95,96].
Kendarimide A (12) (Figure 4), isolated from the marine sponge, Haliclona sp., reversed multidrug resistance in a human carcinoma cell line overexpressing P-gp [97]. Compound 12 completely reversed the resistance to colchicine in KB-C2 cells at a 6 μM concentration. A mixture of 6 µM of 12 and 0.1 µg/mL of colchicine inhibited the growth of KB-C2 cells by 87%, while 12 showed no inhibitory activity against human epidermoid carcinoma (KB-3-1) cells at a 6 µM concentration. It is important to point out that cyclosporin A, a potent peptide with activity against multi-drug resistant P-gp, is composed of a similar number of amino acid residues as 12. This might indicate that peptides having an analogous amino acid length have the ability to reverse multidrug resistance [97].
Sipholenol A (13) (Figure 4) is a triterpenoid isolated from the Red Sea sponge, Callyspongia siphonella [98]. This sponge is a prolific producer of about 30 different triterpenes grouped into four major families: Sipholane, siphonellane, neviotane and dahabane. The sipholane family has the ability to reverse P-gp-mediated MDR in some cancer cells [98,99]. Compound 13 enhanced the cytotoxicity of three well-known P-gp substrates (colchicines, vinblastine and paclitaxel) in KB-C2 and MDR human cervix carcinoma subclone derived from KB-3-1 (KB-V1) cells. In this experiment, 13 decreased the multidrug resistance of these cells in a concentration-dependent manner [100]. Finally, it was found that 13 increases the accumulation of paclitaxel by inhibiting the P-gp efflux function, stimulates ATPase activity, inhibits the photolabeling of P-gp using [125I]-iodoarylazidoprazosin as the transport substrate and does not affect P-gp expression. Further research demonstrated that sipholenone E (14), sipholenol L (15) and siphonellinol D (16) (Figure 4) showed similar activity to 13 [101].
2.3. Inhibitors from Cyanobacteria and Alga
Welwitindolinones are a group of alkaloids isolated from the cyanobacteria, Hapalosiphon welwitschii. N-Methylwelwitindolinone C isothiocyanate (17) (Figure 5) enhanced the cytotoxicity of two anticancer drugs, actinomycin D and daunomycin, in vinblastine-resistant ovarian carcinoma (SK-VLB-1) cell lines. Additionally, 17 increased the activity of anticancer drugs vinblastine, taxol, actinomycin D, colchicine and daunomycin in MDR ovarian adenocarcinoma (NCI/ADR-RES) cell lines, formerly known as MDR breast carcinoma (MCF-7/ADR) cells. Another member of this alkaloid family, welwitindolinone C isothiocyanate (18) (Figure 5), presented a weak activity, indicating that the methyl group is important for biological activity. Moreover, when an isonitrile group replaced the isothiocyanate group, the compound was inactive, demonstrating that the isothiocyanate group is also important for the biological activity shown by these alkaloids [102].
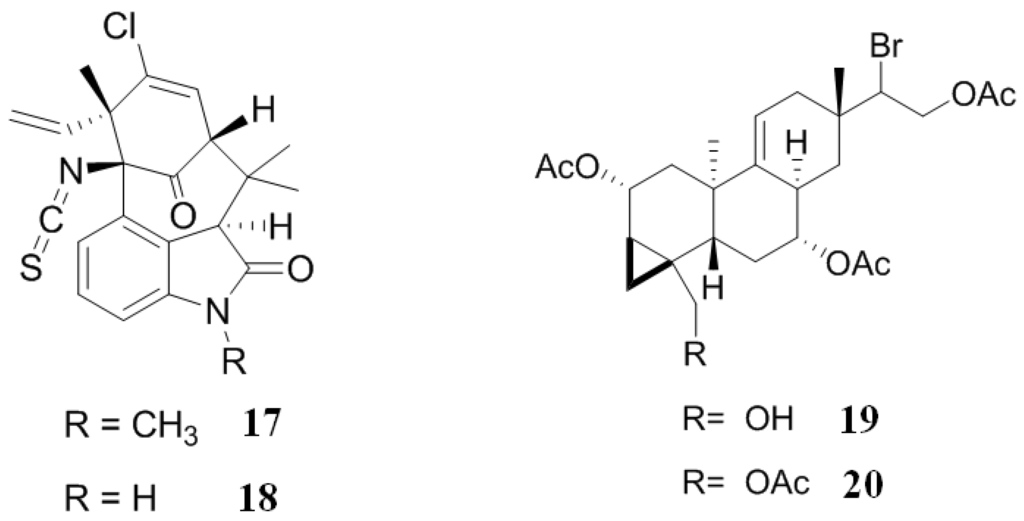
Figure 5.
Inhibitors of P-gp that have been isolated from cyanobacteria and algae.
Two unusual brominated diterpenes, parguerenes I (19) and II (20) (Figure 5), obtained from the Australian marine red alga, Laurencia filiformis, are non-cytotoxic inhibitors of P-gp and MRP1 mediated drug efflux. These two small molecules exhibited a dose-dependent reversal of P-gp mediated efflux of vinblastine, doxorubicin and paclitaxel MDR, without altering the levels expression of P-gp. This suggests that 19 and 20 inhibit P-gp efflux by a novel mechanism of action or at least by a mechanism this is very different to other known inhibitors, such as verapamil and cyclosporin A, due to 19 and 20 interacting with and upsetting the extracellular antibody binding epitope of P-gp. A detailed analysis of the structure-activity relationship between 19 and 20 indicates that it is possible to manipulate and optimize the core pharmacophore of these molecules in order to increase their activity [103].
2.4. Inhibitors from Bryozoans
From a species of bryozoan, Bugula neritina, have been isolated a group of macrolide lactones called bryostatins. These compounds are potent modulators of protein kinase C, and they are anticancer and memory enhancing agents [104,105]. Probably the most important member of this compounds family is bryostatin 1 (21) (Figure 6), which is a modulator of protein kinase C with a potency similar to that of the tumor-promoting phorbol ester, 12-O-tetradecanoylphorbol-13-acetate (TPA) [106,107]. Compound 21 also has the ability to modulate the P-gp mediated MDR. In an experiment using two cell lines overexpressing a mutant MDR1 encoded P-gp, colchicine-resistant MDR clone of KB cells (KB-C1) and human epitheloid cervix carcinoma (HeLa) cells transfected with an MDR1-V185 build (HeLa-MDR1-V185) that contains a valine instead of a glycine in position 185, it is found that 21 reversed the resistance developed against vinblastine and colchicine by both cells [108]. Additionally, 21 is capable of reversing P-gp mediated MDR in HeLa cells transfected with MDR1-V185 by an increase of the intracellular accumulation of rhodamine 123. From these experiments, it was evident that 21 joins to sites G185 and V185 P-gp, and thus, this compound is able to reverse a mutant P-gp specifically [108].
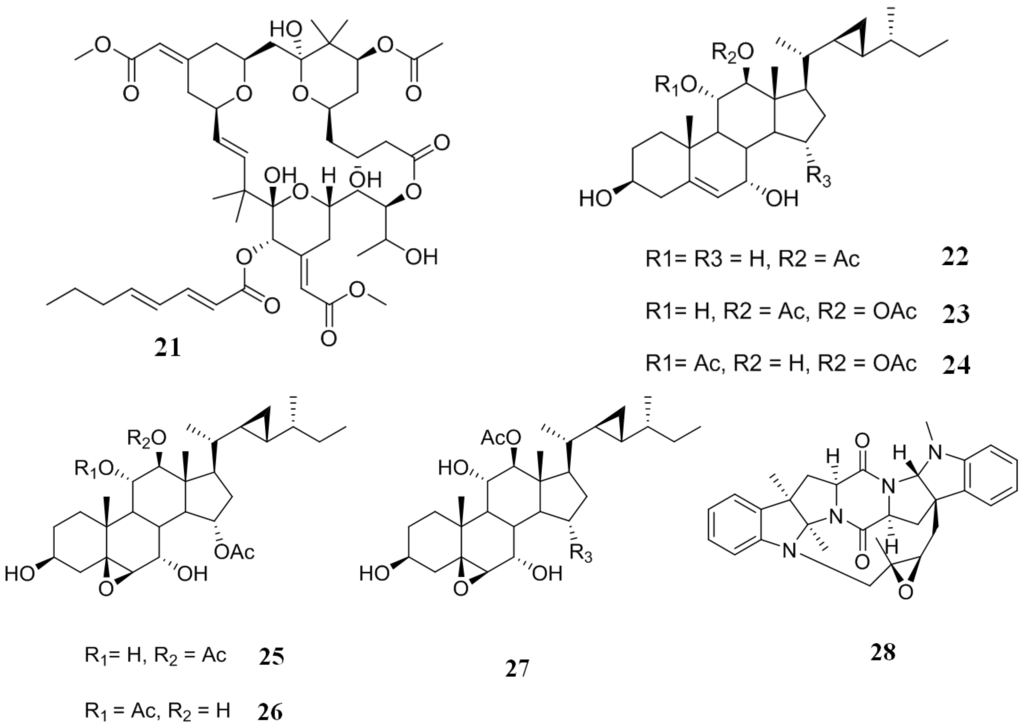
Figure 6.
Inhibitors of P-gp that have been isolated from bryozoans, corals and marine bacteria.
2.5. Inhibitors from Corals
The octocoral, Isis hippuris, is a prolific producer of several polyoxygenated steroids, including gorgosterol, hippuristanol, hippuristerone and hippuristerol types [109]. A few of the first type have been tested against KB-C2 overexpressing P-gp and against KB-CV60 overexpressing multidrug resistance protein-1 cells. Most steroids (22–27) (Figure 6) displayed moderate activity against KB-C2 cells (a percentage growth inhibition of 68.0, 70.0, 87.8, 89.2, 87.4 and 82.1, respectively, at a concentration of 3 µg/mL), but not against KB-CV60 cells (a percentage growth inhibition of 2.1, 16.0, 35.7, 41.0, 18.9 and 16.6, respectively, at a concentration of 3 µg/mL), evidencing some specificity of this type of compound on cell lines that overexpress P-gp [110].
2.6. Inhibitors from Marine Bacteria
The bacterium, Nocardiopsis sp. CMB-M0232, was isolated from a sediment sample collected in Australia. A non-saline liquid culture of this bacterium yielded two prenylated uncommon diketopiperazines (DKP): Nocardioazine A (28) (Figure 6) and nocardioazine B. Compound 28 reversed the resistance exhibited by SW620AD-300 cells equipotent to verapamil. Conversely, nocardioazine B showed no inhibitory activity against P-gp, which could mean that the strange-bridged DKP scaffold formed between one of the nitrogens, and the isoprene external unit might be involved in biological activity [111].
3. Conclusions
P-gp can expel a broad range of structurally different exogenous compounds out of the cells. For this reason, a very active P-gp transporter could potentially diminish drug delivery to the target organ and has been correlated to treatment resistance, despite peripheral drug concentrations that are within their therapeutic range.
Inhibition of P-gp leads to an increase in the permeability of some target organs. This result could permit administering lower drugs oral doses, and it may help to decrease drug toxicity. As a result, P-gp mediated drug efflux is recognized as a desirable target for therapeutic intervention in order to target and optimize the drug delivery of drugs to tumor cells and physiologically/anatomically isolated tissue compartments.
Marine organisms have proven to be an important source in the discovery and development of compounds that inhibit P-gp. Most of these compounds have demonstrated a reversal of multi-drug resistance in certain cancer cells. Importantly, these compounds have a potential application to be administered in conjunction with drug therapies that have been stymied by overexpressed P-gp, including anticancer agents, β-adrenoreceptor blockers, calcium channel blockers, cardiac glycosides, immunosuppressants, steroid hormones and antiparasitic, among others.
Importantly, the majority of the marine inhibitors outlined in the review had been scarcely studied, and very little is known about their mechanism of action. Extensive research, including that of a rational drug design to create derivatives with higher activity, less toxicity and less pharmacokinetic interactions, would lead to more clinically viable drug candidates. Nevertheless, all these agents represent new research tools for the discovery and development of efficient P-gp inhibitors, which may have potential use on their own or in combination with other therapeutic agents for the treatment of various diseases of clinical relevance.
Acknowledgments
This work was supported by funds from the National Secretariat of Science, Technology and Innovation (SENACYT) doctoral grant 270-2011-154 (Dioxelis Lopez). The authors thank Amanda Fenner and Jagannatha Rao for helpful comments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sharom, F.J. Multidrug Resistance Protein (P-Glycoprotein; MDR1). In Drug Transporters: Molecular Characterization and Role in Drug Disposition; You, G., Morris, M.E., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 223–262. [Google Scholar]
- Sharom, F.J. The P-glycoprotein multidrug transporter. Essays Biochem. 2011, 50, 161–178. [Google Scholar] [CrossRef]
- Bansal, T.; Jaggi, M.; Khar, R.K.; Talegaonkar, S. Emerging significance of flavonoids as P-glycoprotein inhibitors in cancer chemotherapy. J. Pharm. Pharm. Sci. 2009, 12, 46–78. [Google Scholar]
- Eckford, P.D.W.; Sharom, F.J. ABC efflux pump-based resistance to chemotherapy drugs. Chem. Rev. 2009, 109, 2989–3011. [Google Scholar] [CrossRef]
- Cordon-Cardo, C.; O’Brien, J.P.; Casals, D.; Rittman-Grauer, L.; Biedler, J.L.; Melamed, M.R.; Bertino, J.R. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc. Natl. Acad. Sci. USA 1989, 86, 695–698. [Google Scholar] [CrossRef]
- Ho, R.H.; Kim, R.B. Transporters and drug therapy: Implications for drug disposition and disease. Clin. Pharmacol. Ther. 2005, 78, 260–277. [Google Scholar] [CrossRef]
- Thiebaut, F.; Tsuruo, T.; Hamada, H.; Gottesman, M.M.; Pastan, I.; Willingham, M.C. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc. Natl. Acad. Sci. USA 1987, 84, 7735–7738. [Google Scholar] [CrossRef]
- Binkhathlan, Z.; Lavasanifar, A. P-glycoprotein inhibition as a therapeutic approach for overcoming multidrug resistance in cancer: Current status and future perspectives. Curr. Cancer Drug Targets 2013, 13, 326–346. [Google Scholar] [CrossRef]
- Zhou, S.F. Structure, function and regulation of P-glycoprotein and its clinical relevance in drug disposition. Xenobiotica 2008, 38, 802–832. [Google Scholar] [CrossRef]
- Sharom, F.J. ABC multidrug transporters: Structure, function and role in chemoresistance. Pharmacogenomics 2008, 9, 105–127. [Google Scholar] [CrossRef]
- Higgins, C.F.; Gottesman, M.M. Is the multidrug transporter a flippase? Trends Biochem. Sci. 1992, 17, 18–21. [Google Scholar] [CrossRef]
- Ferreira, R.J.; Ferreira, M.J.U.; dos Santos, D.J.V.A. Molecular docking characterizes substrate-binding sites and efflux modulation mechanisms within P-glycoprotein. J. Chem. Inf. Model. 2013, 53, 1747–1760. [Google Scholar] [CrossRef]
- Rauch, C.; Paine, S.W.; Littlewood, P. Can long range mechanical interaction between drugs and membrane proteins define the notion of molecular promiscuity? Application to P-glycoprotein-mediated multidrug resistance (MDR). Biochim. Biophys. Acta 2013, 1830, 5112–5118. [Google Scholar]
- Aller, S.G.; Yu, J.; Ward, A.; Weng, Y.; Chittaboina, S.; Zhuo, R.; Harrell, P.M.; Trinh, Y.T.; Zhang, Q.; Urbatsch, I.L.; Chang, G. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science 2009, 323, 1718–1722. [Google Scholar] [CrossRef]
- Sharom, F.J.; Yu, X.; Doige, C.A. Functional reconstitution of drug transport and ATPase activity in proteoliposomes containing partially purified P-glycoprotein. J. Biol. Chem. 1993, 268, 24197–24202. [Google Scholar]
- Ambudkar, S.V.; Dey, S.; Hrycyna, C.A.; Ramachandra, M.; Pastan, I.; Gottesman, M.M. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 361–398. [Google Scholar] [CrossRef]
- Higgins, C.F.; Linton, K.J. The ATP switch model for ABC transporters. Nat. Struct. Mol. Biol. 2004, 11, 918–926. [Google Scholar] [CrossRef]
- Siarheyeva, A.; Liu, R.; Sharom, F.J. Characterization of an asymmetric occluded state of P-glycoprotein with two bound nucleotides: Implications for catalysis. J. Biol. Chem. 2010, 285, 7575–7586. [Google Scholar] [CrossRef]
- Sauna, Z.E.; Ambudkar, S.V. Characterization of the catalytic cycle of ATP hydrolysis by human P-glycoprotein. The two ATP hydrolysis events in a single catalytic cycle are kinetically similar but affect different functional outcomes. J. Biol. Chem. 2001, 276, 11653–11661. [Google Scholar] [CrossRef]
- Martin, C.; Berridge, G.; Higgins, C.F.; Callaghan, R. The multi-drug resistance reversal agent SR33557 and modulation of vinca alkaloid binding to P-glycoprotein by an allosteric interaction. Br. J. Pharmacol. 1997, 122, 765–771. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, Z.; Tang, L.; Liu, J.; Zhou, M.; Xie, F.; Wang, Z.; Wang, Y.; Shen, S.; Hu, L.; et al. Reversal of P-gp and MRP1-mediated multidrug resistance by H6, a gypenoside aglycon from Gynostemma pentaphyllum, in vincristine-resistant human oral cancer (KB/VCR) cells. Eur. J. Pharmacol. 2012, 696, 43–53. [Google Scholar] [CrossRef]
- Chan, K.F.; Wong, I.L.K.; Kan, J.W.Y.; Yan, C.S.W.; Chow, L.M.C.; Chan, T.H. Amine linked flavonoid dimers as modulators for P-glycoprotein-based multidrug resistance: Structure-activity relationship and mechanism of modulation. J. Med. Chem. 2012, 55, 1999–2014. [Google Scholar] [CrossRef]
- Chanmahasathien, W.; Ohnuma, S.; Ambudkar, S.V.; Limtrakul, P. Biochemical mechanism of modulation of human P-glycoprotein by stemofoline. Planta Med. 2011, 77, 1990–1995. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Ambudkar, S.V.; Xia, D. Structure of a multidrug transporter: Crystal structures of a mammalian multidrug efflux pump bound to peptide inhibitors may reveal drug binding sites. Nat. Biotechnol. 2009, 27, 546–547. [Google Scholar] [CrossRef]
- Sankatsing, S.U.C.; Beijnen, J.H.; Schinkel, A.H.; Lange, J.M.A.; Prins, J.M. P-glycoprotein in human immunodeficiency virus type 1 infection and therapy. Antimicrob. Agents Chemother. 2004, 48, 1073–1081. [Google Scholar] [CrossRef]
- Wilson, C.M.; Volkman, S.K.; Thaithong, S.; Martin, R.K.; Kyle, D.E.; Milhous, W.K.; Wirth, D.F. Amplification of pfmdr1 associated with mefloquine and halofantrine resistance in Plasmodium falciparum from Thailand. Mol. Biochem. Parasitol. 1993, 57, 151–160. [Google Scholar] [CrossRef]
- Gamarro, F.; Chiquero, M.J.; Amador, M.V.; Légaré, D.; Ouellette, M.; Castanys, S. P-glycoprotein overexpression in methotrexate-resistant Leishmania tropica. Biochem. Pharmacol. 1994, 47, 1939–1947. [Google Scholar] [CrossRef]
- Gueiros, F.J.; Viola, J.P.B.; Gomes, F.C.A.; Farina, M.; Lins, U.; Bertho, A.L.; Wirth, D.F.; Lopes, U.G. Leishmania amazonensis: Multidrug resistance in vinblastine-resistant promastigotes is associated with rhodamine 123 efflux, DNA amplification, and RNA overexpression of a Leishmania mdr1 gene. Exp. Parasitol. 1995, 81, 480–490. [Google Scholar] [CrossRef]
- Campos, M.C.O.; Castro-Pinto, D.B.; Ribeiro, G.A.; Berredo-Pinho, M.M.; Gomes, L.H.F.; da Silva Bellieny, M.S.; Goulart, C.M.; Echevarria, A.; Leon, L.L. P-glycoprotein efflux pump plays an important role in Trypanosoma cruzi drug resistance. Parasitol. Res. 2013, 112, 2341–2351. [Google Scholar] [CrossRef]
- Descoteaux, S.; Ayala, P.; Samuelson, J.; Orozco, E. Increase in mRNA of multiple Eh pgp genes encoding P-glycoprotein homologues in emetine-resistant Entamoeba histolytica parasites. Gene 1995, 164, 179–184. [Google Scholar] [CrossRef]
- Lomovskaya, O.; Bostian, K.A. Practical applications and feasibility of efflux pump inhibitors in the clinic—A vision for applied use. Biochem. Pharmacol. 2006, 71, 910–918. [Google Scholar] [CrossRef]
- Lomovskaya, O.; Zgurskaya, H.I.; Totrov, M.; Watkins, W.J. Waltzing transporters and ‘the dance macabre’ between humans and bacteria. Nat. Rev. Drug Discov. 2007, 6, 56–65. [Google Scholar] [CrossRef]
- Kuppens, I.E.L.M.; Witteveen, E.O.; Jewell, R.C.; Radema, S.A.; Paul, E.M.; Mangum, S.G.; Beijnen, J.H.; Voest, E.E.; Schellens, J.H.M. A phase I, randomized, open-label, parallel-cohort, dose-finding study of elacridar (GF120918) and oral topotecan in cancer patients. Clin. Cancer Res. 2007, 13, 3276–3285. [Google Scholar] [CrossRef]
- Pusztai, L.; Wagner, P.; Ibrahim, N.; Rivera, E.; Theriault, R.; Booser, D.; Symmans, F.W.; Wong, F.; Blumenschein, G.; Fleming, D.R.; et al. Phase II study of tariquidar, a selective P-glycoprotein inhibitor, in patients with chemotherapy-resistant, advanced breast carcinoma. Cancer 2005, 104, 682–691. [Google Scholar] [CrossRef]
- Malingré, M.M.; Beijnen, J.H.; Rosing, H.; Koopman, F.J.; Jewell, R.C.; Paul, E.M.; Ten Bokkel Huinink, W.W.; Schellens, J.H.M. Co-administration of GF120918 significantly increases the systemic exposure to oral paclitaxel in cancer patients. Br. J. Cancer. 2001, 84, 42–47. [Google Scholar] [CrossRef]
- Krishna, R.; Mayer, L.D. Multidrug resistance (MDR) in cancer: Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur. J. Pharm. Sci. 2000, 11, 265–283. [Google Scholar] [CrossRef]
- Varma, M.V.S.; Ashokraj, Y.; Dey, C.S.; Panchagnula, R. P-glycoprotein inhibitors and their screening: A perspective from bioavailability enhancement. Pharmacol. Res. 2003, 48, 347–359. [Google Scholar] [CrossRef]
- Watanabe, T.; Kokubu, N.; Charnick, S.B.; Naito, M.; Tsuruo, T.; Cohen, D. Interaction of cyclosporin derivatives with the ATPase activity of human P-glycoprotein. Br. J. Pharmacol. 1997, 122, 241–248. [Google Scholar] [CrossRef]
- Fox, E.; Bates, S.E. Tariquidar (XR9576): A P-glycoprotein drug efflux pump inhibitor. Expert Rev. Anticancer Ther. 2007, 7, 447–459. [Google Scholar] [CrossRef]
- Rapposelli, S.; Coi, A.; Imbriani, M.; Bianucci, A.M. Development of classification models for identifying “True” P-glycoprotein (P-gp) inhibitors through inhibition, ATPase activation and monolayer efflux assays. Int. J. Mol. Sci. 2012, 13, 6924–6943. [Google Scholar] [CrossRef]
- Newman, M.J.; Rodarte, J.C.; Benbatoul, K.D.; Romano, S.J.; Zhang, C.; Krane, S.; Moran, E.J.; Uyeda, R.T.; Dixon, R.; Guns, E.S.; et al. Discovery and characterization of OC144–093, a novel inhibitor of P-glycoprotein-mediated multidrug resistance. Cancer Res. 2000, 60, 2964–2972. [Google Scholar]
- Sharom, F.J.; Yu, X.; Chu, J.W.K.; Doige, C.A. Characterization of the ATPase activity of P-glycoprotein from multidrug-resistant Chinese hamster ovary cells. Biochem. J. 1995, 308, 381–390. [Google Scholar]
- Matsunaga, T.; Kose, E.; Yasuda, S.; Ise, H.; Ikeda, U.; Ohmori, S. Determination of P-glycoprotein ATPase activity using luciferase. Biol. Pharm. Bull. 2006, 29, 560–564. [Google Scholar] [CrossRef]
- He, L.; Liu, G.Q. Interaction of multidrug resistance reversal agents with P-glycoprotein ATPase activity on blood-brain barrier. Acta Pharmacol. Sin. 2002, 23, 423–429. [Google Scholar]
- Germann, U.A. Baculovirus-mediated expression of human multidrug resistance cDNA in insect cells and functional analysis of recombinant P-glycoprotein. Methods Enzymol. 1998, 292, 427–441. [Google Scholar]
- Rao, U.S.; Fine, R.L.; Scarborough, G.A. Antiestrogens and steroid hormones: Substrates of the human P-glycoprotein. Biochem. Pharmacol. 1994, 48, 287–292. [Google Scholar] [CrossRef]
- Germann, U.A.; Shlyakhter, D.; Mason, V.S.; Zelle, R.E.; Duffy, J.P.; Galullo, V.; Armistead, D.M.; Saunders, J.O.; Boger, J.; Harding, M.W. Cellular and biochemical characterization of VX-710 as a chemosensitizer: Reversal of P-glycoprotein-mediated multidrug resistance in vitro. Anticancer Drugs 1997, 8, 125–140. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Zhao, L.; Fan, S.; Yang, Z.; Gao, F.; Chen, L.; Xiao, G.G.; Molnár, J.; Wang, Q. Down-regulation of P-glycoprotein is associated with resistance to cisplatin and VP-16 in human lung cancer cell lines. Anticancer Res. 2010, 30, 3593–3598. [Google Scholar]
- Chen, B.A.; Guo, J.J.; Cheng, J. Biomolecular mechanisms of cyclosporine A, tetrandrine and their combination on the reversion of multidrug resistance in human leukemia cell line. Zhongguo Zhong Xi Yi Jie He Za Zhi 2008, 28, 1010–1013. [Google Scholar] [CrossRef]
- Gréen, H.; Lotfi, K.; Zackrisson, A.L.; Peterson, C. Spontaneous reversal of P-Glycoprotein expression in multidrug resistant cell lines. Pharmacol. Toxicol. 2003, 93, 297–304. [Google Scholar] [CrossRef]
- El-Masry, E.M.; Abou-Donia, M.B. Interaction of pyridostigmine bromide and N,N-diethyl-m-toluamide alone and in combination with P-glycoprotein expressed in Escherichia coli leaky mutant. J. Toxicol. Environ. Health A 2006, 69, 919–933. [Google Scholar] [CrossRef]
- Zhao, Q.X.; Chen, B.A.; Cheng, J.; Ding, J.H.; Gao, F.; Gao, C.; Sun, Y.Y.; Wang, J.; Zhao, G.; Bao, W.; et al. Effect of tetrandrine, toremifene and their combination on the reversion of multidrug resistance of K562/A02 cell line. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2008, 16, 61–64. [Google Scholar]
- Shin, S.Y.; Choi, B.H.; Kim, J.R.; Kim, J.H.; Lee, Y.H. Suppression of P-glycoprotein expression by antipsychotics trifluoperazine in adriamycin-resistant L1210 mouse leukemia cells. Eur. J. Pharm. Sci. 2006, 28, 300–306. [Google Scholar] [CrossRef]
- Mickisch, G.H.; Noordzij, M.A.; Gaast, A.V.d.; Gebreamlack, P.; Köhrmann, K.U.; Mogler-Drautz, E.; Kupper, H.; Schröder, F.H. Dexverapamil to modulate vinblastine resistance in metastatic renal cell carcinoma. J. Cancer Res. Clin. Oncol. 1995, 121, R11–R16. [Google Scholar] [CrossRef]
- Bark, H.; Choi, C.H. PSC833, cyclosporine analogue, downregulates MDR1 expression by activating JNK/c-Jun/AP-1 and suppressing NF-kappaB. Cancer Chemother. Pharmacol. 2010, 65, 1131–1136. [Google Scholar] [CrossRef]
- Cornwell, M.M.; Safa, A.R.; Felsted, R.L.; Gottesman, M.M.; Pastan, I. Membrane vesicles from multidrug-resistant human cancer cells contain a specific 150- to 170-kDa protein detected by photoaffinity labeling. Proc. Natl. Acad. Sci. USA 1986, 83, 3847–3850. [Google Scholar] [CrossRef]
- Dey, S.; Ramachandra, M.; Pastan, I.; Gottesman, M.M.; Ambudkar, S.V. Evidence for two nonidentical drug-interaction sites in the human P-glycoprotein. Proc. Natl. Acad. Sci. USA 1997, 94, 10594–10599. [Google Scholar] [CrossRef]
- Friche, E.; Demant, E.J.; Sehested, M.; Nissen, N.I. Effect of anthracycline analogs on photolabelling of p-glycoprotein by [125I]iodomycin and [3H]azidopine: Relation to lipophilicity and inhibition of daunorubicin transport in multidrug resistant cells. Br. J. Cancer 1993, 67, 226–231. [Google Scholar] [CrossRef]
- Akiyama, S.; Cornwell, M.M.; Kuwano, M.; Pastan, I.; Gottesman, M.M. Most drugs that reverse multidrug resistance also inhibit photoaffinity labeling of P-glycoprotein by a vinblastine analog. Mol. Pharmacol. 1988, 33, 144–147. [Google Scholar]
- Jetté, L.; Murphy, G.F.; Leclerc, J.M.; Béliveau, R. Interaction of drugs with P-glycoprotein in brain capillaries. Biochem. Pharmacol. 1995, 50, 1701–1709. [Google Scholar] [CrossRef]
- Hofmann, J.; Gekeler, V.; Ise, W.; Noller, A.; Mitterdorfer, J.; Hofer, S.; Utz, I.; Gotwald, M.; Boer, R.; Glossmann, H.; et al. Mechanism of action of dexniguldipine-HCl (B8509-035), a new potent modulator of multidrug resistance. Biochem. Pharmacol. 1995, 49, 603–609. [Google Scholar] [CrossRef]
- Hyafil, F.; Vergely, C.; Du Vignaud, P.; Grand-Perret, T. In vitro and in vivo reversal of multidrug resistance by GF120918, an acridonecarboxamide derivative. Cancer Res. 1993, 53, 4595–4602. [Google Scholar]
- Saeki, T.; Tsuruo, T.; Sato, W.; Nishikawsa, K. Drug resistance in chemotherapy for breast cancer. Cancer Chemother. Pharmacol. 2005, 56, 84–89. [Google Scholar] [CrossRef]
- Mistry, P.; Stewart, A.J.; Dangerfield, W.; Okiji, S.; Liddle, C.; Bootle, D.; Plumb, J.A.; Templeton, D.; Charlton, P. In vitro and in vivo reversal of P-glycoprotein-mediated multidrug resistance by a novel potent modulator, XR9576. Cancer Res. 2001, 61, 749–758. [Google Scholar]
- Dantzig, A.H.; Shepard, R.L.; Cao, J.; Law, K.L.; Ehlhardt, W.J.; Baughman, T.M.; Bumol, T.F.; Starling, J.J. Reversal of P-glycoprotein-mediated multidrug resistance by a potent cyclopropyldibenzosuberane modulator, LY335979. Cancer Res. 1996, 56, 4171–4179. [Google Scholar]
- Ekins, S.; Kim, R.B.; Leake, B.F.; Dantzig, A.H.; Schuetz, E.G.; Lan, L.B.; Yasuda, K.; Shepard, R.L.; Winter, M.A.; Schuetz, J.D.; et al. Application of three-dimensional quantitative structure-activity relationships of P-glycoprotein inhibitors and substrates. Mol. Pharmacol. 2002, 61, 974–981. [Google Scholar] [CrossRef]
- Ieiri, I. Functional significance of genetic polymorphisms in P-glycoprotein (MDR1, ABCB1) and breast cancer resistance protein (BCRP, ABCG2). Drug Metab. Pharmacokinet. 2012, 27, 85–105. [Google Scholar] [CrossRef]
- Mayer, A.M.S.; Glaser, K.B.; Cuevas, C.; Jacobs, R.S.; Kem, W.; Little, R.D.; McIntosh, J.M.; Newman, D.J.; Potts, B.C.; Shuster, D.E. The odyssey of marine pharmaceuticals: A current pipeline perspective. Trends Pharmacol. Sci. 2010, 31, 255–265. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2012, 29, 144–222. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2013, 30, 237–323. [Google Scholar] [CrossRef]
- Rinehart, K.L.; Holt, T.G.; Fregeau, N.L.; Stroh, J.G.; Keifer, P.A.; Sun, F.; Li, L.H.; Martin, D.G. Ecteinascidins 729, 743, 745, 759A, 759B, and 770: Potent antitumor agents from the Caribbean tunicate Ecteinascidia turbinata. J. Org. Chem. 1990, 55, 4512–4515. [Google Scholar] [CrossRef]
- Wright, A.E.; Forleo, D.A.; Gunawardana, G.P.; Gunasekera, S.P.; Koehn, F.E.; McConnell, O.J. Antitumor tetrahydroisoquinoline alkaloids from the colonial ascidian Ecteinascidia turbinata. J. Org. Chem. 1990, 55, 4508–4512. [Google Scholar] [CrossRef]
- Kanzaki, A.; Takebayashi, Y.; Ren, X.Q.; Miyashita, H.; Mori, S.; Akiyama, S.I.; Pommier, Y. Overcoming multidrug drug resistance in P-glycoprotein/MDR1-overexpressing cell lines by ecteinascidin 743. Mol. Cancer Ther. 2002, 1, 1327–1334. [Google Scholar]
- Carter, N.J.; Keam, S.J. Trabectedin: A review of its use in the management of soft tissue sarcoma and ovarian cancer. Drugs 2007, 67, 2257–2276. [Google Scholar] [CrossRef]
- Andersen, R.J.; Faulkner, D.J.; He, C.H.; van Duyne, G.D.; Clardy, J. Metabolites of the marine prosobranch mollusk Lamellaria sp. J. Am. Chem. Soc. 1985, 107, 5492–5495. [Google Scholar] [CrossRef]
- Lindquist, N.; Fenical, W.; van Duyne, G.D.; Clardy, J. New alkaloids of the lamellarin class from the marine ascidian Didemnum chartaceum (Sluiter, 1909). J. Org. Chem. 1988, 53, 4570–4574. [Google Scholar] [CrossRef]
- Davis, R.A.; Carroll, A.R.; Pierens, G.K.; Quinn, R.J. New lamellarin alkaloids from the australian ascidian, Didemnum chartaceum. J. Nat. Prod. 1999, 62, 419–424. [Google Scholar] [CrossRef]
- Urban, S.; Butler, M.S.; Capon, R.J. Lamellarins O and P: New aromatic metabolites from the australian marine sponge Dendrilla cactos. Aust. J. Chem. 1994, 47, 1919–1924. [Google Scholar] [CrossRef]
- Urban, S.; Hobbs, L.; Hooper, J.N.A.; Capon, R.J. Lamellarins Q and R: New aromatic metabolites from an australian marine sponge, Dendrilla cactos. Aust. J. Chem. 1995, 48, 1491–1494. [Google Scholar] [CrossRef]
- Carroll, A.R.; Bowden, B.F.; Coll, J.C. Studies of Australian ascidians. I. Six new lamellarin-class alkaloids from a colonial ascidian, Didemnum sp. Austr. J. Chem. 1993, 46, 489–501. [Google Scholar] [CrossRef]
- Urban, S.; Capon, R.J. Lamellarin-S: A new aromatic metabolite from an Australian Tunicate, Didemnum sp. Aust. J. Chem. 1996, 49, 711–713. [Google Scholar]
- Reddy, M.V.R.; Faulkner, D.J.; Venkateswarlu, Y.; Rao, M.R. New lamellarin alkaloids from an unidentified ascidian from the Arabian Sea. Tetrahedron 1997, 53, 3457–3466. [Google Scholar] [CrossRef]
- Reddy, M.V.R.; Rao, M.R.; Rhodes, D.; Hansen, M.S.T.; Rubins, K.; Bushman, F.D.; Venkateswarlu, Y.; Faulkner, D.J. Lamellarin alpha 20-sulfate, an inhibitor of HIV-1 integrase active against HIV1 virus in cell culture. J. Med. Chem. 1999, 42, 1901–1907. [Google Scholar] [CrossRef]
- Quesada, A.R.; García Grávalos, M.D.; Fernández Puentes, J.L. Polyaromatic alkaloids from marine invertebrates as cytotoxic compounds and inhibitors of multidrug resistance caused by P-glycoprotein. Br. J. Cancer 1996, 74, 677–682. [Google Scholar] [CrossRef]
- Degnan, B.M.; Hawkins, C.J.; Lavin, M.F.; McCaffrey, E.J.; Parry, D.L.; van den Brenk, A.L.; Watters, D.J. New cyclic peptides with cytotoxic activity from the ascidian Lissoclinum patella. J. Med. Chem. 1989, 32, 1349–1354. [Google Scholar] [CrossRef]
- Roy, R.S.; Gehring, A.M.; Milne, J.C.; Belshaw, P.J.; Walsh, C.T. Thiazole and oxazole peptides: Biosynthesis and molecular machinery. Nat. Prod. Rep. 1999, 16, 249–263. [Google Scholar] [CrossRef]
- Williams, A.B.; Jacobs, R.S. A marine natural product, patellamide D, reverses multidrug resistance in a human leukemic cell line. Cancer Lett. 1993, 71, 97–102. [Google Scholar] [CrossRef]
- Aoki, S.; Yoshioka, Y.; Miyamoto, Y.; Higuchi, K.; Setiawan, A.; Murakami, N.; Chen, Z.S.; Sumizawa, T.; Akiyama, S.I.; Kobayashi, M. Agosterol A, a novel polyhydroxylated sterol acetate reversing multidrug resistance from a marine sponge of Spongia sp. Tetrahedron Lett. 1998, 39, 6303–6306. [Google Scholar]
- Aoki, S.; Chen, Z.S.; Higasiyama, K.; Setiawan, A.; Akiyama, S.; Kobayashi, M. Reversing effect of agosterol A, a spongean sterol acetate, on multidrug resistance in human carcinoma cells. Jpn. J. Cancer Res. 2001, 92, 886–895. [Google Scholar] [CrossRef]
- Chen, Z.S.; Aoki, S.; Komatsu, M.; Ueda, K.; Sumizawa, T.; Furukawa, T.; Okumura, H.; Ren, X.Q.; Belinsky, M.G.; Lee, K.; et al. Reversal of drug resistance mediated by multidrug resistance protein (MRP) 1 by dual effects of agosterol A on MRP1 function. Int. J. Cancer 2001, 93, 107–113. [Google Scholar] [CrossRef]
- Gunasekera, S.P.; Gunasekera, M.; Longley, R.E.; Schulte, G.K. Discodermolide: A new bioactive polyhydroxylated lactone from the marine sponge Discodermia dissoluta. J. Org. Chem. 1990, 55, 4912–4915. [Google Scholar] [CrossRef]
- Longley, R.E.; Gunasekera, S.P.; Faherty, D.; Mclane, J.; Dumont, F. Immunosuppression by discodermolide. Ann. N. Y. Acad. Sci. 1993, 696, 94–107. [Google Scholar]
- Kalesse, M. The chemistry and biology of discodermolide. ChemBioChem 2000, 1, 171–175. [Google Scholar] [CrossRef]
- Kowalski, R.J.; Giannakakou, P.; Gunasekera, S.P.; Longley, R.E.; Day, B.W.; Hamel, E. The microtubule-stabilizing agent discodermolide competitively inhibits the binding of paclitaxel (Taxol) to tubulin polymers, enhances tubulin nucleation reactions more potently than paclitaxel, and inhibits the growth of paclitaxel-resistant cells. Mol. Pharmacol. 1997, 52, 613–622. [Google Scholar]
- Kawakami, A.; Miyamoto, T.; Higuchi, R.; Uchiumi, T.; Kuwano, M.; Van Soest, R.W.M. Structure of a novel multidrug resistance modulator, irciniasulfonic acid, isolated from a marine sponge, Ircinia sp. Tetrahedrom Lett. 2001, 42, 3335–3337. [Google Scholar] [CrossRef]
- Emura, C.; Higuchi, R.; Miyamoto, T. Irciniasulfonic acid B, a novel taurine conjugated fatty acid derivative from a Japanese marine sponge, Ircinia sp. Tetrahedron 2006, 62, 5682–5685. [Google Scholar] [CrossRef]
- Aoki, S.; Cao, L.; Matsui, K.; Rachmat, R.; Akiyama, S.I.; Kobayashi, M. Kendarimide A, a novel peptide reversing P-glycoprotein-mediated multidrug resistance in tumor cells, from a marine sponge of Haliclona sp. Tetrahedron 2004, 60, 7053–7059. [Google Scholar]
- Jain, S.; Laphookhieo, S.; Shi, Z.; Fu, L.W.; Akiyama, S.I.; Chen, Z.S.; Youssef, D.T.A.; van Soest, R.W.M.; El Sayed, K.A. Reversal of P-glycoprotein-mediated multidrug resistance by Sipholane triterpenoids. J. Nat. Prod. 2007, 70, 928–931. [Google Scholar] [CrossRef]
- Jain, S.; Abraham, I.; Carvalho, P.; Kuang, Y.H.; Shaala, L.A.; Youssef, D.T.A.; Avery, M.A.; Chen, Z.S.; El Sayed, K.A. Sipholane triterpenoids: Chemistry, reversal of ABCB1/P-glycoprotein-mediated multidrug resistance, and pharmacophore modeling. J. Nat. Prod. 2009, 72, 1291–1298. [Google Scholar] [CrossRef]
- Shi, Z.; Jain, S.; Kim, I.W.; Peng, X.X.; Abraham, I.; Youssef, D.T.A.; Fu, L.W.; El Sayed, K.; Ambudkar, S.V.; Chen, Z.S. Sipholenol A, a marine-derived sipholane triterpene, potently reverses P-glycoprotein (ABCB1)-mediated multidrug resistance in cancer cells. Cancer Sci. 2007, 98, 1373–1380. [Google Scholar] [CrossRef]
- Abraham, I.; Jain, S.; Wu, C.P.; Khanfar, M.A.; Kuang, Y.; Dai, C.L.; Shi, Z.; Chen, X.; Fu, L.; Ambudkar, S.V.; et al. Marine sponge-derived sipholane triterpenoids reverse P-glycoprotein (ABCB1)-mediated multidrug resistance in cancer cells. Biochem. Pharmacol. 2010, 80, 1497–1506. [Google Scholar] [CrossRef]
- Smith, C.D.; Zilfou, J.T.; Stratmann, K.; Patterson, G.M.; Moore, R.E. Welwitindolinone analogues that reverse P-glycoprotein-mediated multiple drug resistance. Mol. Pharmacol. 1995, 47, 241–247. [Google Scholar]
- Huang, X.C.; Sun, Y.L.; Salim, A.A.; Chen, Z.S.; Capon, R.J. Parguerenes: Marine red alga bromoditerpenes as inhibitors of P-glycoprotein (ABCB1) in multidrug resistant human cancer cells. Biochem. Pharmacol. 2013, 85, 1257–1268. [Google Scholar] [CrossRef]
- Pettit, G.R. The Bryostatins. In Fortschritte der Chemie organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products; Herz, W., Kirby, G.W., Steglich, W., Tamm, Ch., Eds.; Springer: Vienna, Austria, 1991; Volume 57, pp. 153–195. [Google Scholar]
- Mutter, R.; Wills, M. Chemistry and clinical biology of the bryostatins. Bioorg. Med. Chem. 2000, 8, 1841–1860. [Google Scholar] [CrossRef]
- Kraft, A.S.; Smith, J.B.; Berkow, R.L. Bryostatin, an activator of the calcium phospholipid-dependent protein kinase, blocks phorbol ester-induced differentiation of human promyelocytic leukemia cells HL-60. Proc. Natl. Acad. Sci. USA 1986, 83, 1334–1338. [Google Scholar] [CrossRef]
- Jetten, A.M.; George, M.A.; Pettit, G.R.; Rearick, J.I. Effects of bryostatins and retinoic acid on phorbol ester- and diacylglycerol-induced squamous differentiation in human tracheobronchial epithelial cells. Cancer Res. 1989, 49, 3990–3995. [Google Scholar]
- Spitaler, M.; Utz, I.; Hilbe, W.; Hofmann, J.; Grunicke, H.H. PKC-independent modulation of multidrug resistance in cells with mutant (V185) but not wild-type (G185) P-glycoprotein by bryostatin 1. Biochem. Pharmacol. 1998, 56, 861–869. [Google Scholar] [CrossRef]
- Chen, W.H.; Wang, S.K.; Duh, C.Y. Polyhydroxylated steroids from the bamboo coral Isis hippuris. Mar. Drugs 2011, 9, 1829–1839. [Google Scholar]
- Tanaka, J.; Trianto, A.; Musman, M.; Issa, H.H.; Ohtani, I.I.; Ichiba, T.; Higa, T.; Yoshida, W.Y.; Scheuer, P.J. New polyoxygenated steroids exhibiting reversal of multidrug resistance from the gorgonian Isis hippuris. Tetrahedron 2002, 58, 6259–6266. [Google Scholar] [CrossRef]
- Raju, R.; Piggott, A.M.; Huang, X.C.; Capon, R.J. Nocardioazines: A novel bridged diketopiperazine scaffold from a marine-derived bacterium inhibits P-glycoprotein. Org. Lett. 2011, 13, 2770–2773. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).