Haloperoxidase Mediated Quorum Quenching by Nitzschia cf pellucida: Study of the Metabolization of N-Acyl Homoserine Lactones by a Benthic Diatom
Abstract
:1. Introduction
2. Results and Discussion
2.1. Nitzschia cf pellucida Deactivation of β-Keto-AHLs
| Sample | 0 min | 30 min | 60 min | 120 min | 180 min |
|---|---|---|---|---|---|
| Synthetic seawater + H2O2 + OHHL | + + + | + + + | + + + | + + | + |
| N. cf pellucida | − | − | − | − | − |
| N. cf pellucida + OHHL | + + + | + + + | + + + | + + | + |
| N. cf pellucida + H2O2 + OHHL | + + + | + + | + | − | − |
| N. cf pellucida + HHL | + + + | + + + | + + + | + + | + + |
| N. cf pellucida + H2O2 + HHL | + + + | + + + | + + + | + + | + + |
| N. cf pellucida + H2O2 + OHHL + Catalase | + + + | + + + | + + + | + + | + |
| Sample | Activity |
|---|---|
| 2 μM BrCN | − |
| 4 μM BrCN | − |
| 2 μM BrCN + HHL | + + + |
| 4 μM BrCN + HHL | + + + |
| 2 μM BrCN + OHHL | + + |
| 4 μM BrCN + OHHL | + + |
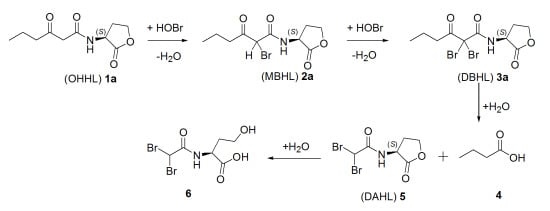
2.2. Degradation Pathway of β-Keto-AHLs

| Compound | (M + H)+ | R.T. (min) a | Relative Abundance (%) at 220 nm | |||
|---|---|---|---|---|---|---|
| 30 min | 60 min | 120 min | 180 min | |||
| (OHHL) 1a | 214 | 5.2 | 37 | 29 | 25 | <3 |
| (MBHL) 2a | 292 | 15.7 | 21 | 24 | 27 | 27 |
| (DBHL) 3a | 370 | 19.3 | 42 | 47 | 48 | 63 |
| (DAHL) 5 | 300 | 4.1 | 0 | 0 | 0 | 7 |
2.3. Synthesis and Biological Evaluation of Halogenated AHL Analogues
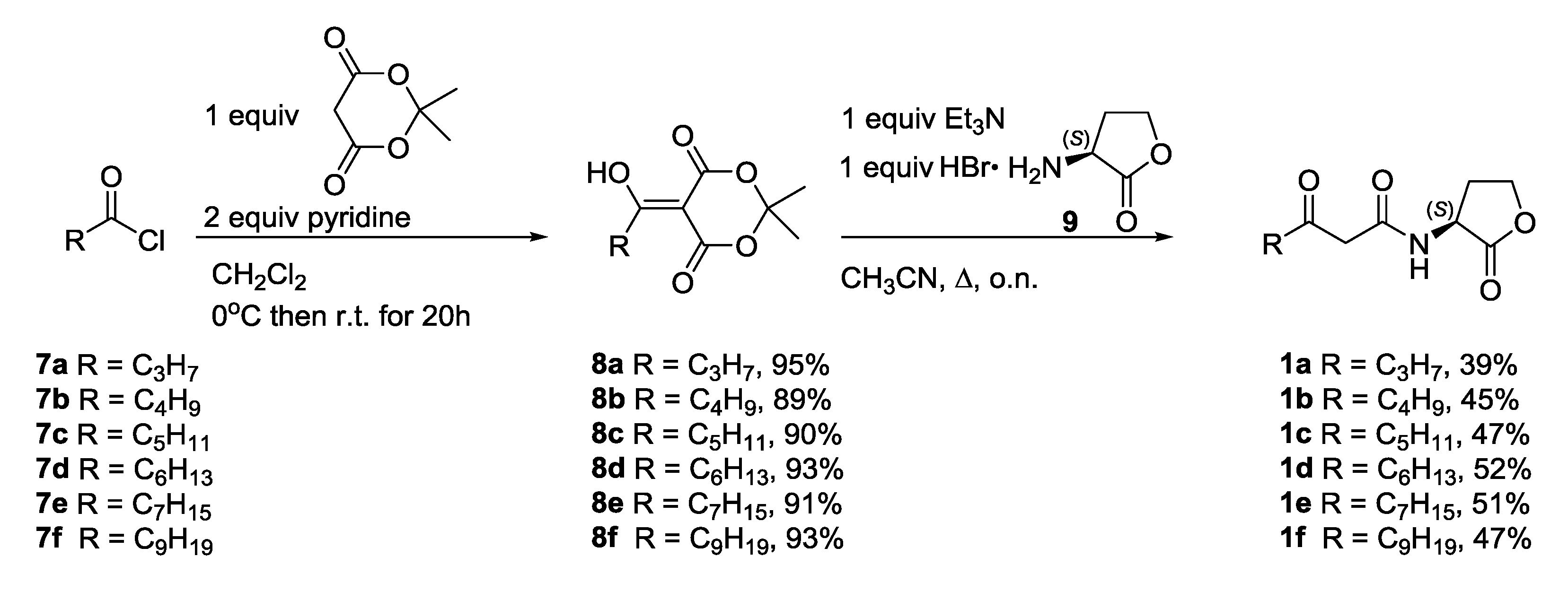
2.3.1. Synthesis of N-3-Oxoacylated Homoserine Lactones
2.3.2. Synthesis of Halogenated N-3-Oxoacylated Homoserine Lactone Analogues

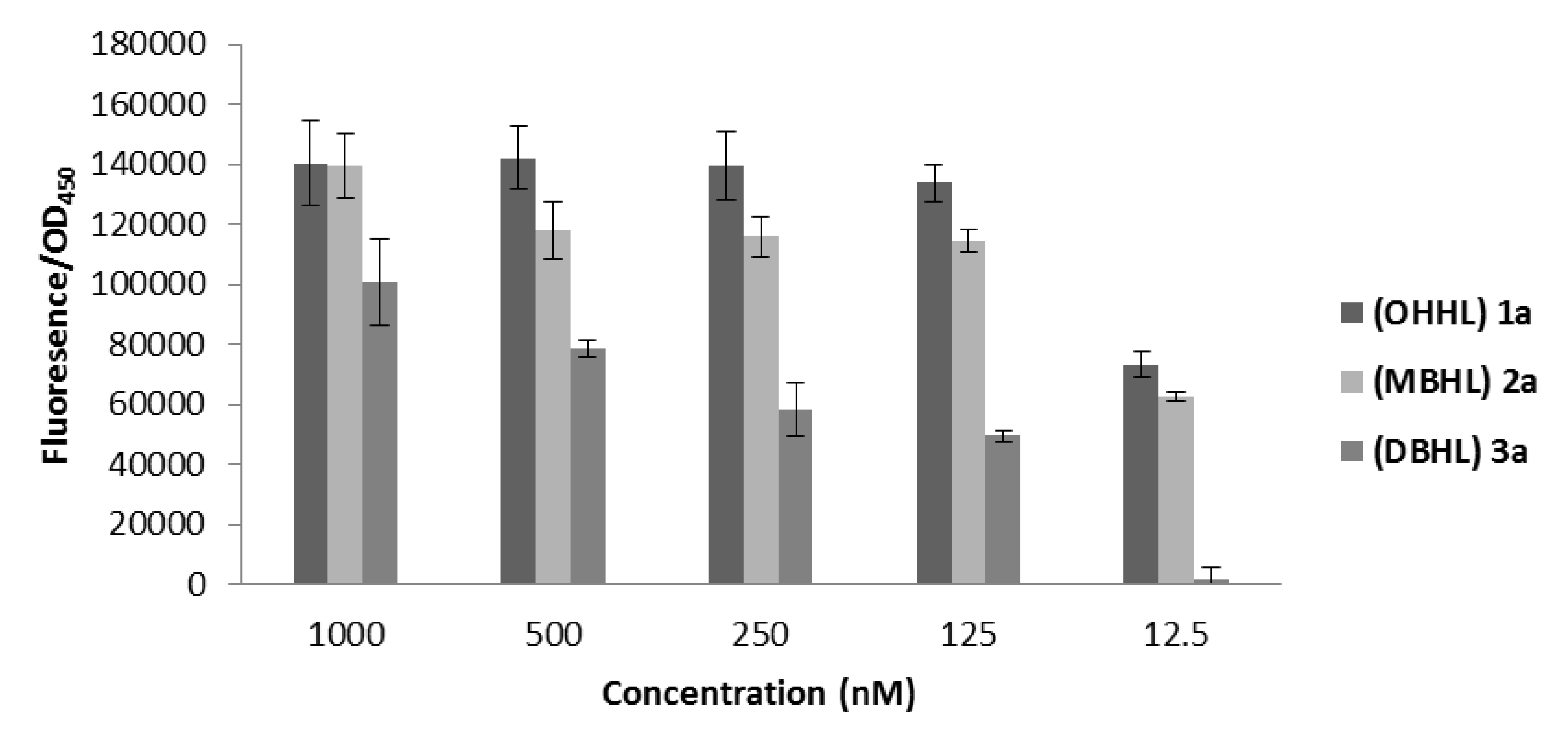
2.3.3. Biological Evaluation
3. Experimental Section
3.1. Deactivation of N-Acylated Homoserine Lactones
3.1.1. N. cf pellucida Culture
3.1.2. Phenol Red Assay
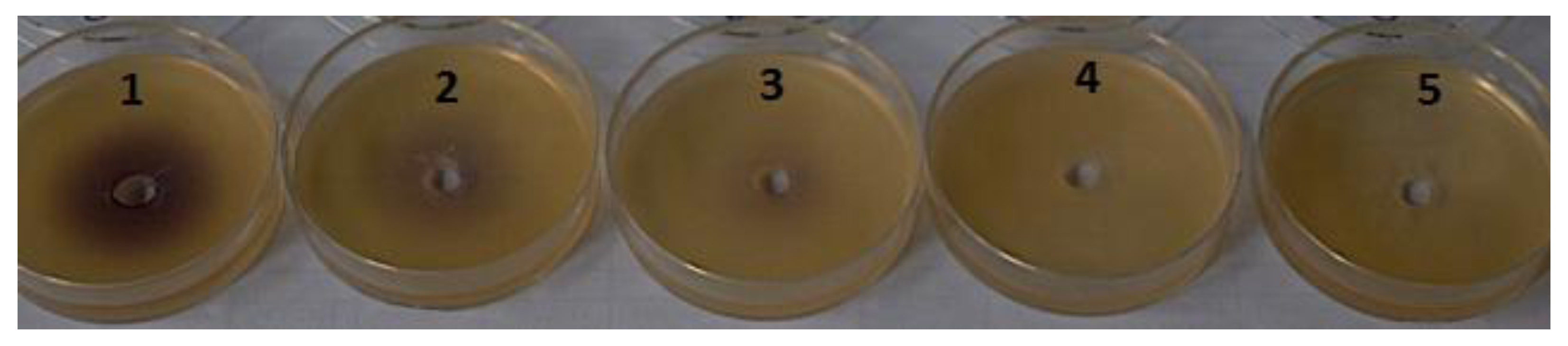
3.1.3. Chromobacterium violaceum Bioassay
3.1.4. Catalase Experiment
3.2. Degradation Pathway
3.2.1. Liquid-Liquid Extraction of OHHL 1a and Degradation Products
3.2.2. HPLC-MS Method
3.3. Chemical Synthesis
3.3.1. Synthesis of Homoserine Lactone Hydrobromide 9
3.3.2. Synthesis of β-Keto-AHLs 1a–f
3.3.3. Synthesis of α-Bromo-β-keto-AHLs 2a–f
3.3.4. Synthesis of α,α-Dichloro-β-keto and α,α-Dibromo-β-keto-AHLs 11a–f and 3a–f
3.3.5. Synthesis of α-Iodo-β-keto-AHLs 10a–f
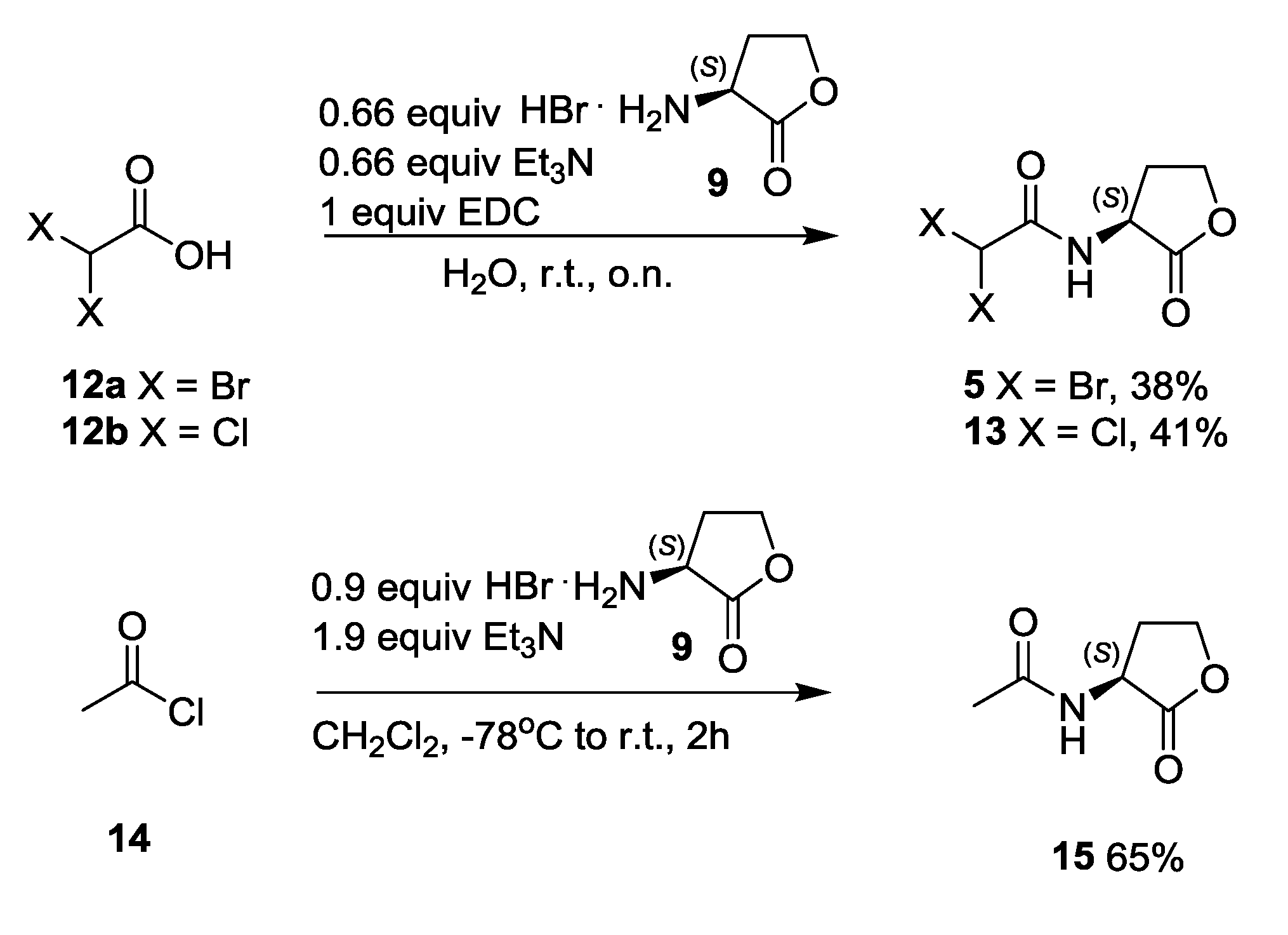
3.3.6. Synthesis of N-α,α-Dibromo- and N-α,α-Dichloroacetyl Homoserine Lactones 5 and 13
3.3.7. Synthesis of N-Acetyl Homoserine Lactone 15
3.4. Biological Activity of Novel Halogenated AHL Analogues
Escherichia coli JB523 Green Fluorescent Protein (GFP) Microplate Assay
4. Conclusions
Acknowledgements
Conflicts of Interest
References
- Cardozo, K.H.M.; Guaratini, T.; Barros, M.P.; Falcão, V.R.; Tonon, A.P.; Lopes, N.P.; Campos, S.; Torres, M.A.; Souza, A.O.; Colepicolo, P.; et al. Metabolites from algae with economical impact. Comp. Biochem. Physiol. C Pharmacol. Toxicol. 2007, 146, 60–78. [Google Scholar] [CrossRef]
- Cabrita, M.T.; Vale, C.; Rauter, A.P. Halogenated Compounds from Marine Algae. Mar. Drugs 2010, 8, 2301–2317. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Hu, W.P.; Munro, M.H.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2009, 26, 170–244. [Google Scholar] [CrossRef]
- Butler, A.; Carter-Franklin, J.N. The role of vanadium bromoperoxidase in the biosynthesis of halogenated marine natural products. Nat. Prod. Rep. 2004, 21, 180–188. [Google Scholar] [CrossRef]
- Butler, A.; Walker, J.V. Marine haloperoxidases. Chem. Rev. 1993, 93, 1937–1944. [Google Scholar] [CrossRef]
- de Kievit, T.R. Quorum sensing in Pseudomonas aeruginosa biofilms. Environ. Microbiol. 2009, 11, 279–288. [Google Scholar] [CrossRef]
- Whitehead, N.A.; Barnard, A.M.L.; Slater, H.; Simpson, N.J.L.; Salmond, G.P.C. Quorum-sensing in gram-negative bacteria. FEMS Microbiol. Rev. 2001, 25, 365–404. [Google Scholar] [CrossRef]
- Williams, P. Quorum sensing, communication and cross-kingdom signalling in the bacterial world. Microbiology 2007, 153, 3923–3938. [Google Scholar] [CrossRef]
- Decho, A.W.; Frey, R.L.; Ferry, J.L. Chemical Challenges to Bacterial AHL Signaling in the Environment. Chem. Rev. 2011, 111, 86–99. [Google Scholar] [CrossRef]
- Tait, K.; Williamson, H.; Atkinson, S.; Williams, P.; Camara, M.; Joint, I. Turnover of quorum sensing signal molecules modulates cross-kingdom signalling. Environ. Microbiol. 2009, 11, 1792–1802. [Google Scholar] [CrossRef]
- Decho, A.W.; Visscher, P.T.; Ferry, J.; Kawaguchi, T.; He, L.; Przekop, K.M.; Norman, R.S.; Reid, R.P. Autoinducers extracted from microbial mats reveal a surprising diversity of N-acylhomoserine lactones (AHLs) and abundance changes that may relate to diel pH. Environ. Microbiol. 2009, 11, 409–420. [Google Scholar] [CrossRef]
- Wheeler, G.L.; Tait, K.; Taylor, A.; Brownlee, C.; Joint, I. Acyl-homoserine lactones modulate the settlement rate of zoospores of the marine alga Ulva intestinalis via a novel chemokinetic mechanism. Plant Cell Environ. 2006, 29, 608–618. [Google Scholar] [CrossRef]
- Bruckner, C.G.; Bahulikar, R.; Rahalkar, M.; Schink, B.; Kroth, P.G. Bacteria associated with benthic diatoms from Lake Constance: Phylogeny and influences on diatom growth and secretion of extracellular polymeric substances. Appl. Environ. Microbiol. 2008, 74, 7740–7749. [Google Scholar]
- Amin, S.A.; Parker, M.S.; Armbrust, E.V. Interactions between diatoms and bacteria. Microbiol. Mol. Biol. Rev. 2012, 76, 667–684. [Google Scholar] [CrossRef]
- Goecke, F.; Labes, A.; Wiese, J.; Imhoff, J.F. Chemical interactions between marine macroalgae and bacteria. Mar. Ecol. Prog. Ser. 2010, 409, 267–299. [Google Scholar] [CrossRef]
- de Carvalho, C.C.C.R.; Fernandes, P. Production of Metabolites as Bacterial Responses to the Marine Environment. Mar. Drugs 2010, 8, 705–727. [Google Scholar] [CrossRef]
- Dobretsov, S.; Teplitski, M.; Bayer, M.; Gunasekera, S.; Proksch, P.; Paul, V.J. Inhibition of marine biofouling by bacterial quorum sensing inhibitors. Biofouling 2011, 27, 893–905. [Google Scholar] [CrossRef]
- Natrah, F.M.I.; Defoirdt, T.; Sorgeloos, P.; Bossier, P. Disruption of Bacterial Cell-to-Cell Communication by Marine Organisms and its Relevance to Aquaculture. Mar. Biotechnol. 2011, 13, 109–126. [Google Scholar] [CrossRef]
- Manefield, M.; de Nys, R.; Kumar, N.; Read, R.; Givskov, M.; Steinberg, P.; Kjelleberg, S.A. Evidence that halogenated furanones from Delisea pulchra inhibit acylated homoserine lactone (AHL)-mediated gene expression by displacing the AHL signal from its receptor protein. Microbiology 1999, 145, 283–291. [Google Scholar] [CrossRef]
- Manefield, M.; Rasmussen, T.B.; Henzter, M.; Andersen, J.B.; Steinberg, P.; Kjelleberg, S.; Givskov, M. Halogenated furanones inhibit quorum sensing through accelerated LuxR turnover. Microbiology 2002, 148, 1119–1127. [Google Scholar]
- Skindersoe, M.E.; Ettinger-Epstein, P.; Rasmussen, T.B.; Bjarnsholt, T.; de Nys, R.; Givskov, M. Quorum sensing antagonism from marine organisms. Mar. Biotechnol. 2008, 10, 56–63. [Google Scholar] [CrossRef]
- Jha, B.; Kavita, K.; Westphal, J.; Hartmann, A.; Schmitt-Kopplin, P. Quorum Sensing Inhibition by Asparagopsis taxiformis, a Marine Macro Alga: Separation of the Compound that Interrupts Bacterial Communication. Mar. Drugs 2013, 11, 253–265. [Google Scholar] [CrossRef]
- Teplitski, M.; Chen, H.C.; Rajamani, S.; Gao, M.S.; Merighi, M.; Sayre, R.T.; Robinson, J.B.; Rolfe, B.G.; Bauer, W.D. Chlamydomonas reinhardtii secretes compounds that mimic bacterial signals and interfere with quorum sensing regulation in bacteria. Plant Physiol. 2004, 134, 137–146. [Google Scholar] [CrossRef]
- Natrah, F.M.I.; Kenmegne, M.M.; Wiyoto, W.; Sorgeloos, P.; Bossier, P.; Defoirdt, T. Effects of micro-algae commonly used in aquaculture on acyl-homoserine lactone quorum sensing. Aquaculture 2011, 317, 53–57. [Google Scholar] [CrossRef]
- Hunt, L.R.; Smith, S.M.; Downum, K.R.; Mydlarz, L.D. Microbial Regulation in Gorgonian Corals. Mar. Drugs 2012, 10, 1225–1243. [Google Scholar] [CrossRef]
- Motti, C.A.; Ettinger-Epstein, P.; Willis, R.H.; Tapiolas, D.M. ESI FTICR-MS Analysis of Larvae from the Marine Sponge Luffariella variabilis. Mar. Drugs 2010, 8, 190–199. [Google Scholar] [CrossRef]
- Ettinger-Epstein, P.; Tapiolas, D.M.; Motti, C.A.; Wright, A.D.; Battershill, C.N.; de Nys, R. Production of manoalide and its analogues by the sponge Luffariella variabilis is hardwired. Mar. Biotechnol. 2008, 10, 64–74. [Google Scholar] [CrossRef]
- Defoirdt, T.; Boon, N.; Bossier, P.; Verstraete, W. Disruption of bacterial quorum sensing: An unexplored strategy to fight infections in aquaculture. Aquaculture 2004, 240, 69–88. [Google Scholar] [CrossRef]
- Sandy, M.; Carter-Franklin, J.N.; Martin, J.D.; Butler, A. Vanadium bromoperoxidase from Delisea pulchra: enzyme-catalyzed formation of bromofuranone and attendant disruption of quorum sensing. Chem. Commun. 2011, 47, 12086–12088. [Google Scholar] [CrossRef]
- Borchardt, S.A.; Allain, E.J.; Michels, J.J.; Stearns, G.W.; Kelly, R.F.; McCoy, W.F. Reaction of acylated homoserine lactone bacterial signaling molecules with oxidized halogen antimicrobials. Appl. Environ. Microbiol. 2001, 67, 3174–3179. [Google Scholar] [CrossRef]
- Hill, V.L.; Manley, S.L. Release of reactive bromine and iodine from diatoms and its possible role in halogen transfer in polar and tropical oceans. Limnol. Oceanogr. 2009, 54, 812–822. [Google Scholar] [CrossRef]
- Wever, R.; van der Horst, M.A. The role of vanadium haloperoxidases in the formation of volatile brominated compounds and their impact on the environment. Dalton Trans. 2013, 42, 11778–11786. [Google Scholar] [CrossRef]
- Vanelslander, B.; Paul, C.; Grueneberg, J.; Prince, E.K.; Gillard, J.; Sabbe, K.; Pohnert, G.; Vyverman, W. Daily bursts of biogenic cyanogen bromide (BrCN) control biofilm formation around a marine benthic diatom. Proc. Natl. Acad. Sci. USA 2012, 109, 2412–2417. [Google Scholar] [CrossRef]
- Moore, R.M.; Webb, M.; Tokarczyk, R.; Wever, R. Bromoperoxidase and iodoperoxidase enzymes and production of halogenated methanes in marine diatom cultures. J. Geophys. Res. 1996, 101, 20899–20908. [Google Scholar] [CrossRef]
- Yates, E.A.; Philipp, B.; Buckley, C.; Atkinson, S.; Chhabra, S.R.; Sockett, R.E.; Goldner, M.; Dessaux, Y.; Camara, M.; Smith, H.; Williams, P. N-acylhomoserine lactones undergo lactonolysis in a pH-, temperature-, and acyl chain length-dependent manner during growth of Yersinia pseudotuberculosis and Pseudomonas aeruginosa. Infect. Immun. 2002, 70, 5635–5646. [Google Scholar] [CrossRef]
- Collen, J.; Delrio, M.J.; Garciareina, G.; Pedersen, M. Photosynthetic production of hydrogen peroxide by Ulva rigida C. Ag. (Chlorophyta). Planta 1995, 196, 225–230. [Google Scholar]
- Michels, J.J.; Allain, E.J.; Borchardt, S.A.; Hu, P.F.; McCoy, W.F. Degradation pathway of homoserine lactone bacterial signal molecules by halogen antimicrobials identified by liquid chromatography with photodiode array and mass spectrometric detection. J. Chromatogr. A 2000, 898, 153–165. [Google Scholar] [CrossRef]
- Persson, T.; Hansen, T.H.; Rasmussen, T.B.; Skinderso, M.E.; Givskov, M.; Nielsen, J. Rational design and synthesis of new quorum-sensing inhibitors derived from acylated homoserine lactones and natural products from garlic. Org. Biomol. Chem. 2005, 3, 253–262. [Google Scholar] [CrossRef]
- Hodgkinson, J.T.; Galloway, W.R.J.D.; Casoli, M.; Keane, H.; Su, X.; Salmond, G.P.C.; Welch, M.; Spring, D.R. Robust routes for the synthesis of N-acylated-l-homoserine lactone (AHL) quorum sensing molecules with high levels of enantiomeric purity. Tetrahedron Lett. 2011, 52, 3291–3294. [Google Scholar] [CrossRef]
- Khan, A.T.; Goswami, P.; Choudhury, L.H. A mild and environmentally acceptable synthetic protocol for chemoselective alpha-bromination of beta-keto esters and 1,3-diketones. Tetrahedron Lett. 2006, 47, 2751–2754. [Google Scholar]
- Meketa, M.L.; Mahajan, Y.R.; Weinreb, S.M. An efficacious method for the halogenation of beta-dicarbonyl compounds under mildly acidic conditions. Tetrahedron Lett. 2005, 46, 4749–4751. [Google Scholar] [CrossRef]
- Pravst, I.; Zupan, M.; Stavber, S. Halogenation of ketones with N-halosuccinimides under solvent-free reaction conditions. Tetrahedron 2008, 64, 5191–5199. [Google Scholar] [CrossRef]
- Amara, N.; Mashiach, R.; Amar, D.; Krief, P.; Spieser, S.A.H.; Bottomley, M.J.; Aharoni, A.; Meijler, M.M. Covalent Inhibition of Bacterial Quorum Sensing. J. Am. Chem. Soc. 2009, 131, 10610–10619. [Google Scholar] [CrossRef]
- McClean, K.H.; Winson, M.K.; Fish, L.; Taylor, A.; Chhabra, S.R.; Camara, M.; Daykin, M.; Lamb, J.H.; Swift, S.; Bycroft, B.W.; et al. Quorum sensing and Chromobacterium violaceum: Exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 1997, 143, 3703–3711. [Google Scholar] [CrossRef]
- Andersen, J.B.; Heydorn, A.; Hentzer, M.; Eberl, L.; Geisenberger, O.; Christensen, B.B.; Molin, S.; Givskov, M. Gfp-based N-acyl homoserine-lactone sensor systems for detection of bacterial communication. Appl. Environ. Microbiol. 2001, 67, 575–585. [Google Scholar] [CrossRef]
Supplementary Files
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Syrpas, M.; Ruysbergh, E.; Blommaert, L.; Vanelslander, B.; Sabbe, K.; Vyverman, W.; De Kimpe, N.; Mangelinckx, S. Haloperoxidase Mediated Quorum Quenching by Nitzschia cf pellucida: Study of the Metabolization of N-Acyl Homoserine Lactones by a Benthic Diatom. Mar. Drugs 2014, 12, 352-367. https://doi.org/10.3390/md12010352
Syrpas M, Ruysbergh E, Blommaert L, Vanelslander B, Sabbe K, Vyverman W, De Kimpe N, Mangelinckx S. Haloperoxidase Mediated Quorum Quenching by Nitzschia cf pellucida: Study of the Metabolization of N-Acyl Homoserine Lactones by a Benthic Diatom. Marine Drugs. 2014; 12(1):352-367. https://doi.org/10.3390/md12010352
Chicago/Turabian StyleSyrpas, Michail, Ewout Ruysbergh, Lander Blommaert, Bart Vanelslander, Koen Sabbe, Wim Vyverman, Norbert De Kimpe, and Sven Mangelinckx. 2014. "Haloperoxidase Mediated Quorum Quenching by Nitzschia cf pellucida: Study of the Metabolization of N-Acyl Homoserine Lactones by a Benthic Diatom" Marine Drugs 12, no. 1: 352-367. https://doi.org/10.3390/md12010352
APA StyleSyrpas, M., Ruysbergh, E., Blommaert, L., Vanelslander, B., Sabbe, K., Vyverman, W., De Kimpe, N., & Mangelinckx, S. (2014). Haloperoxidase Mediated Quorum Quenching by Nitzschia cf pellucida: Study of the Metabolization of N-Acyl Homoserine Lactones by a Benthic Diatom. Marine Drugs, 12(1), 352-367. https://doi.org/10.3390/md12010352