Pachydictyols B and C: New Diterpenes from Dictyota dichotoma Hudson
Abstract
:1. Introduction
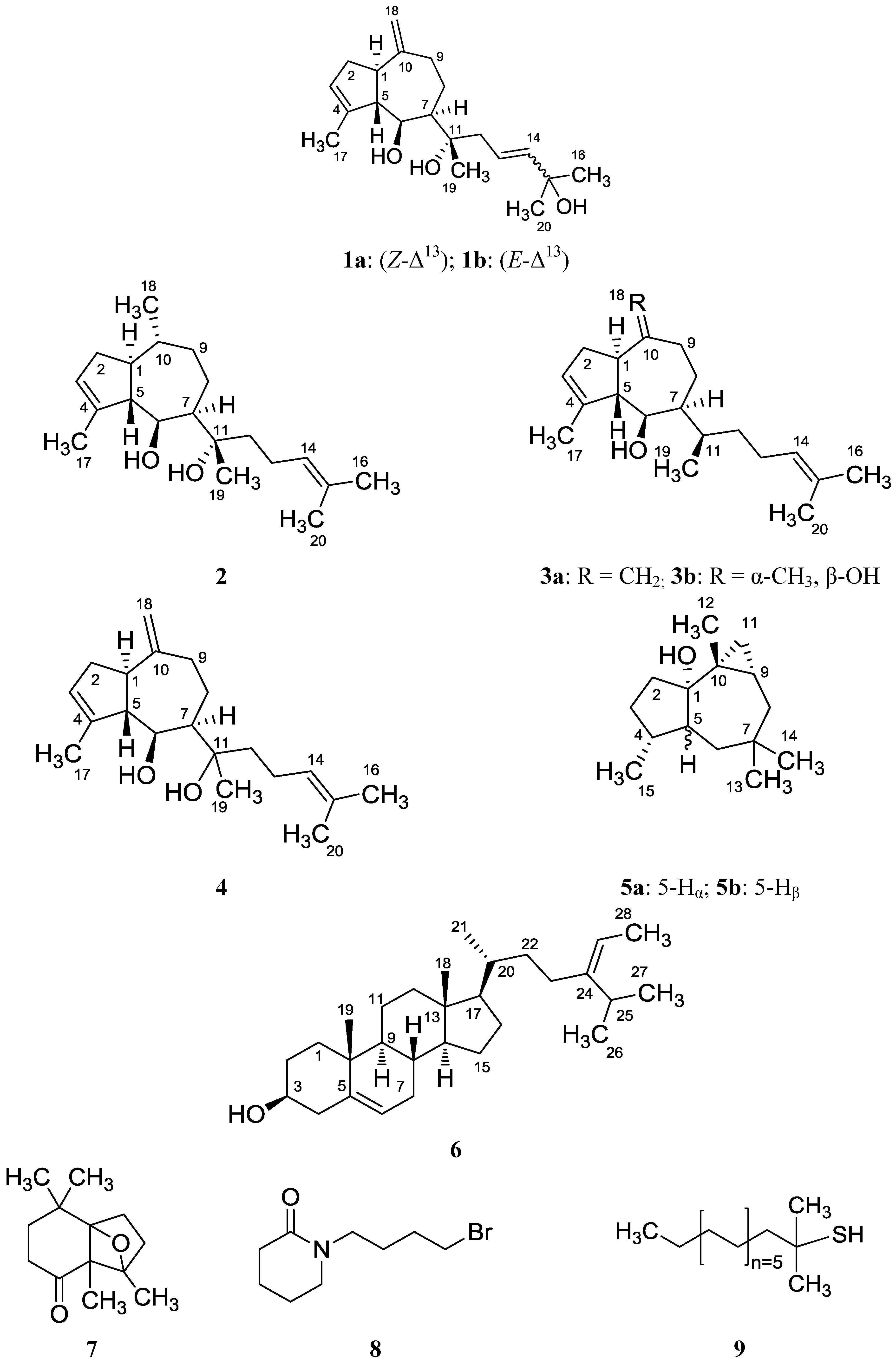
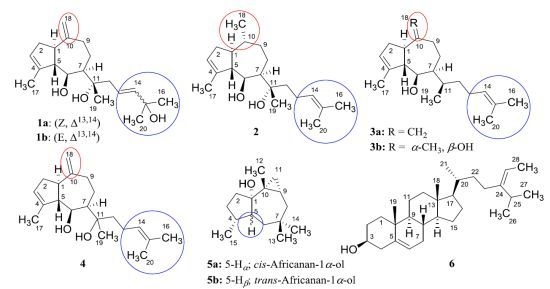
2. Results and Discussion
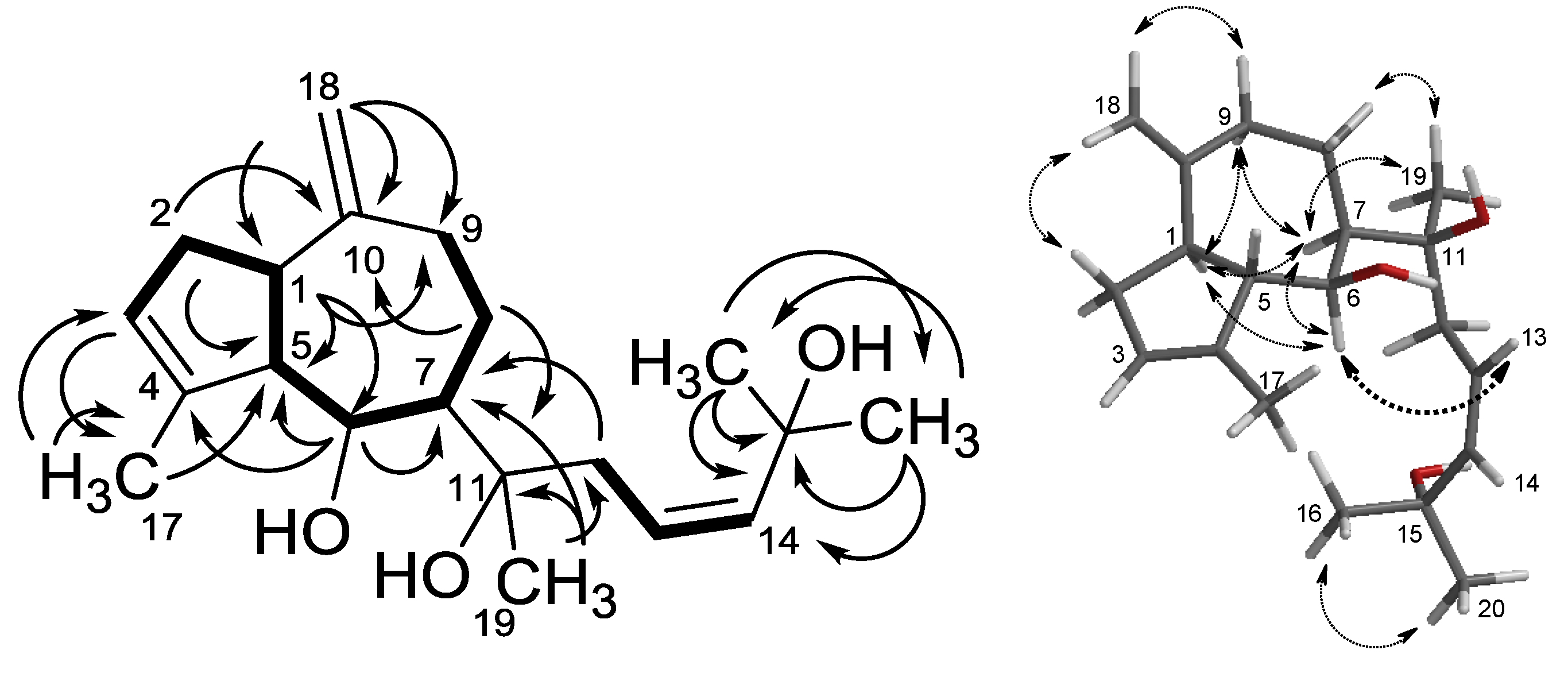
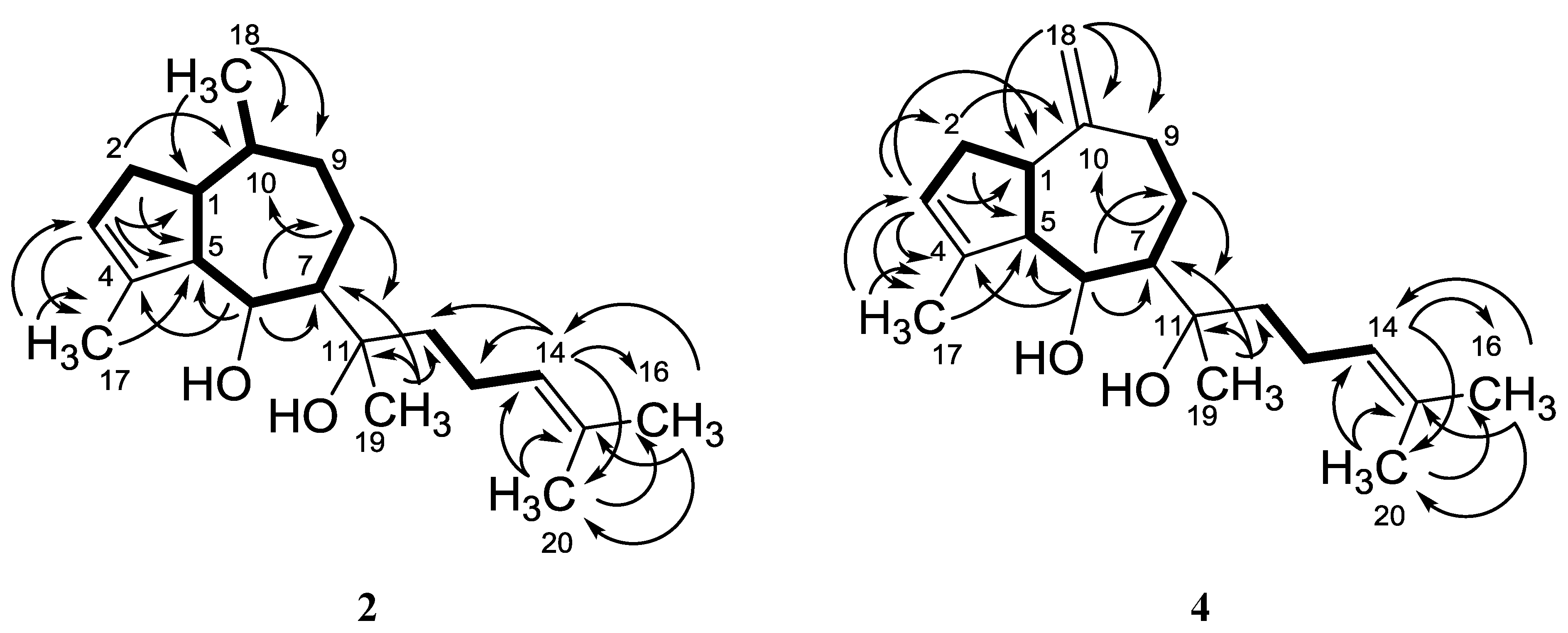
2.1. Structure Analysis and Characterization of Isolated Compounds
| position | cis-Pachydictyol B (1a) | trans-Pachydictyol B (1b) | Pachydictyol A (3a) | |||
|---|---|---|---|---|---|---|
| δC (a) | δH (b) | δC (a) | δH (c) | δC (a) | δH (b) | |
| 1 | 46.0 | 2.50 (m) | 46.1 | 2.52 (m) | 46.1 | 2.67 (m) |
| 2 | 33.6 | 2.43 (m), 2.13 (m) | 33.7 | 2.46 (m), 2.16 (m) | 33.9 | 2.50 (m), 2.22 (m) |
| 3 | 123.9 | 5.28 (m) | 124.2 | 5.30 (m) | 123.9 | 5.33 (m) |
| 4 | 140.8 | - | 140.7 | - | 141.3 | - |
| 5 | 59.8 | 2.33 (m) | 59.9 | 2.36 (m) | 60.4 | 2.30 (m) |
| 6 | 73.9 | 4.18 (dm, 7.6) | 74.1 | 4.18 (m) | 75.1 | 3.92 (d, 7.8) |
| 7 | 48.6 | 1.56 (m) | 49.0 | 1.58 (m) | 47.8 | 1.55 (m) |
| 8 | 21.6 | 1.69 (m) | 21.6 | 1.73 (m), 1.65 (m) | 23.6 | 1.50 (m) |
| 9 | 40.3 | 2.60 (m), 2.04 (m) | 40.3 | 2.62 (dm, 15.7 Hz), 2.06 (m) | 40.6 | 2.62 (m), 2.10 (m) |
| 10 | 151.6 | - | 151.5 | - | 152.4 | - |
| 11 | 75.9 | - | 76.0 | - | 34.8 | 1.21 (m) |
| 12 | 43.8 | 2.42 (m), 2.33 (m) | 44.0 | 2.47 (m), 2.37 (m) | 35.1 | 2.25 (m), 1.53 (m) |
| 13 | 122.1 | 5.63 (br m) | 126.4 | 5.68 (dt, 15.6, 8.0) | 25.7 | 2.04 (m), 1.95 (m) |
| 14 | 141.6 | 5.64 (br m) | 137.4 | 5.60 (d, 15.6) | 124.6 | 5.13 (tq, 8.6, 1.3) |
| 15 | 70.4 | - | 81.6 | - | 131.4 | - |
| 16 | 29.4 | 1.24 (s) | 24.7 | 1.25 (s) | 25.8 | 1.68 (s) |
| 17 | 15.8 | 1.77 (s) | 15.8 | 1.77 (s) | 15.9 | 1.81 (d, 1.3) |
| 18 | 107.3 | 4.72 (br s), 4.69 (br s) | 107.4 | 4.74 (s), 4.70 (s) | 107.0 | 4.74 (br s) |
| 19 | 25.4 | 1.15 (s) | 25.5 | 1.17 (s) | 17.6 | 0.99 (d, 6.0) |
| 20 | 29.8 | 1.25 (s) | 24.0 | 1.28 (s) | 17.7 | 1.61 (s) |
| position | Pachydictyol C (2) | Dictyol C (3b) [14] | Dictyol E (4) | |||
|---|---|---|---|---|---|---|
| δC (a) | δH | δC (a) | δH (b) | δC (a) | δH | |
| 1 | 49.1 | 1.25 (m) | 49.1 | 2.21 | 46.1 | 2.53 (q, 9.8) |
| 2 | 33.0 | 2.21 (m) | 32.9 | n.r. | 33.7 | 2.44 (m), 2.16 (m) |
| 3 | 123.2 | 5.26 (br m) | 123.4 | 5.26 (br s) | 123.9 | 5.28 (br m) |
| 4 | 142.4 | - | 142.5 | - | 140.8 | - |
| 5 | 52.7 | 2.75 (m) | 52.7 | 2.74 (dd,7.8, 6.0) | 60.0 | 2.34 (m) |
| 6 | 74.5 | 3.86 (dd, 8.2, 3.4) | 74.4 | 3.87 (dd,7.8, 3.6) | 74.1 | 4.14 (dd, 7.9, 2.7) |
| 7 | 50.0 | 2.15 (m) | 49.9 | n.r. | 48.3 | 1.60 (m) |
| 8 | 19.8 | 1.27 (m), 1.22 (m) | 19.7 | n.r. | 21.5 | 1.71, 1.61 (2 m) |
| 9 | 34.5 | 1.51 (m) | 46.6 | n.r. | 40.4 | 2.63 (dm, 14.5), 2.06 (m) |
| 10 | 34.9 | 1.19 (m) | 72.4 | - | 151.7 | - |
| 11 | 72.6 | - | 34.4 | n.r. | 76.1 | - |
| 12 | 46.6 | 1.40 (m), 1.88 (m) | 34.7 | n.r. | 40.9 | 1.67 (m) |
| 13 | 25.6 | 2.02 (m), 1.94 (m) | 25.5 | n.r. | 23.2 | 1.99 (m) |
| 14 | 124.7 | 5.14 (m) | 124.7 | 5.14 (br t, 7.1) | 124.2 | 5.10 (t, 7.1) |
| 15 | 131.3 | - | 131.6 | - | 131.3 | - |
| 16 | 25.8 | 1.68 (s) | 25.7 | 1.62 (d, 0.9) | 25.6 | 1.64 (s) |
| 17 | 16.3 | 1.82 (s) | 16.3 | 1.85 (dd, 2.0, 1.2) | 15.7 | 1.77 (s) |
| 18 | 17.5 | 0.97 (d, 6.4) | 30.0 | 1.22 (s) | 107.3 | 4.73 (s), 4.70 (br d, 1.3) |
| 19 | 30.0 | 1.19 (s) | 17.5 | 1.00 (d, 6.6) | 25.1 | 1.18 (s) |
| 20 | 17.7 | 1.60 (s) | 17.7 | 1.70 (s) | 17.6 | 1.57 (s) |
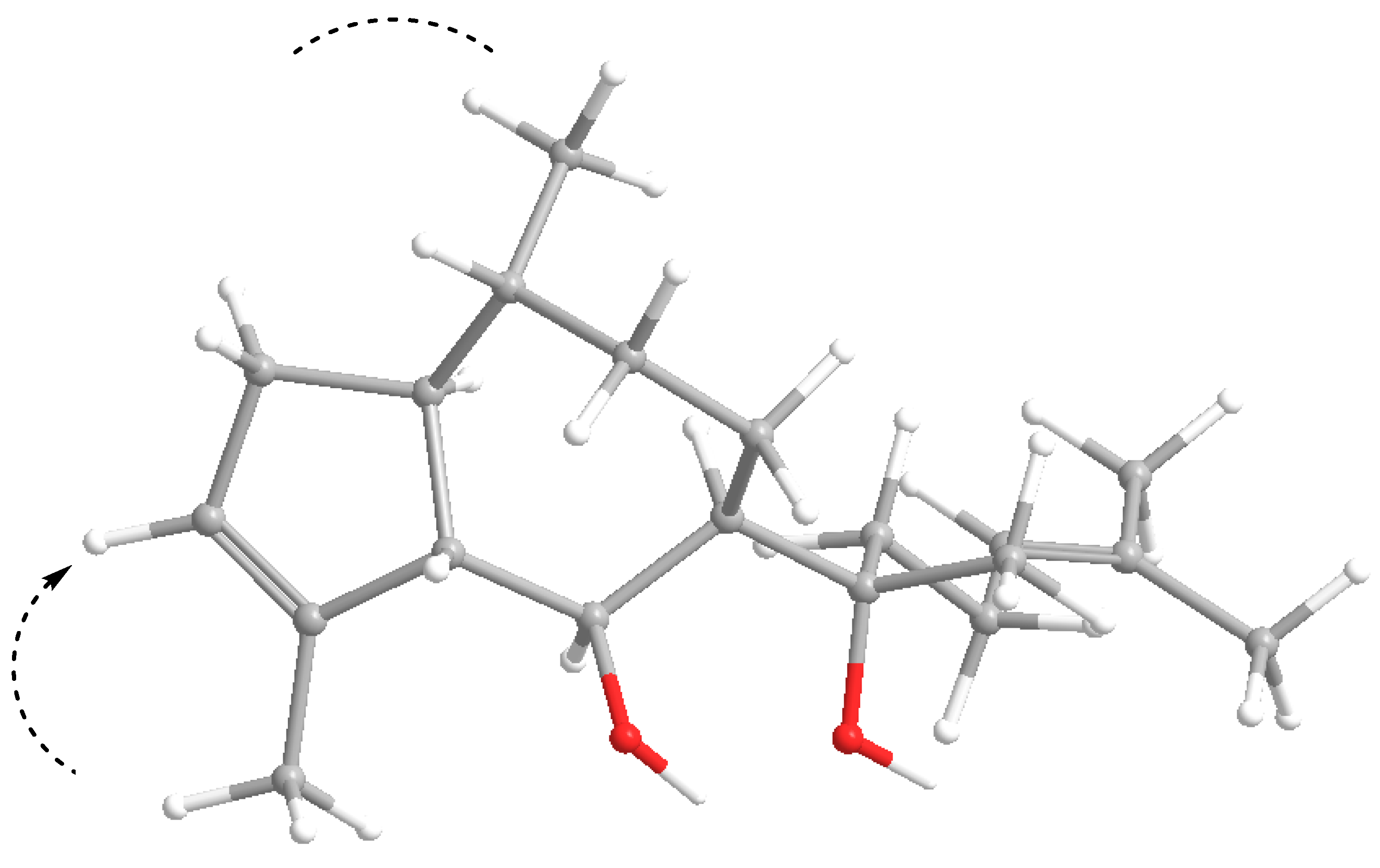
| Position | cis-african-1α-ol (5a) Isolated | cis-africanan-1α-ol (5a) [16] | trans-africanan-1α-ol (5b) [18] | |||
|---|---|---|---|---|---|---|
| δC (a) | δH (b) | δC | δH | δC | δH | |
| 1 | 83.2 | - | 85.3 | - | 85.9 | - |
| 2 | 41.3 | 1.97 (m), 1.52 (m) | 38.9* | 1.47 (m), 1.97 (m) | 38.1 | 1.88 (m), 1.92 (m) |
| 3 | 32.7 | 1.67 (m), 1.38 (m) | 32.7 | 1.68 (m), 1.35 (m) | 30.1 | 1.96 (m), 1.17 (m) |
| 4 | 43.3 | 1.32 (m) | 43.2 | 1.31 (m) | 38.1 | 1.74 (m) |
| 5 | 55.0 | 1.20 (m) | 54.9 | 1.09 (m) | 49.5 | 1.05 (ddd, 11.7, 10.5, 2.7) |
| 6 | 41.8 | 1.06 (m), 1.00 (m) | 41.7 | 0.99 (m), 1.38 (m) | 39.8 | 1.19 (ddd, 14.4, 2.7, 2.1), 1.28 (dd, 14.4, 11.7) |
| 7 | 33.3 | - | 33.3 | - | 33.0 | - |
| 8 | 38.9 | 1.04 (m), 1.49 (m) | 41.2 * | 1.05, 1.47 | 39.7 | 1.89 (dd, 15.0, 11.8), 1.73 (ddd, 15.0, 5.5, 2.1) |
| 9 | 22.3 | 0.81 (m) | 22.2 | 0.79 (m) | 25.7 | 0.74 (m) |
| 10 | 23.6 | - | 23.5 | - | 26.9 | - |
| 11 | 15.3 | 0.66 (dd, 6.4, 5.2), 0.28 (dd, 8.6, 4.1) | 15.2 | 0.66 (m), 0.27 (m) | 16.3 | 0.74 (m), 0.31 (m) |
| 12 | 26.8 * | 1.03 * (s) | 18.9 | 1.03 (s) | 23.5 | 1.12 (s) |
| 13 | 28.3 * | 0.84 * (s) | 29.1 | 0.98 (s) | 35.1 | 0.96 (s) |
| 14 | 29.2 * | 0.98 * (s) | 28.2 | 0.84 (s) | 28.0 | 0.94 (s) |
| 15 | 18.9 * | 1.02 * (d, 6.5) | 26.7 | 1.02 (d) | 19.7 | 0.93 (d, 6.5) |
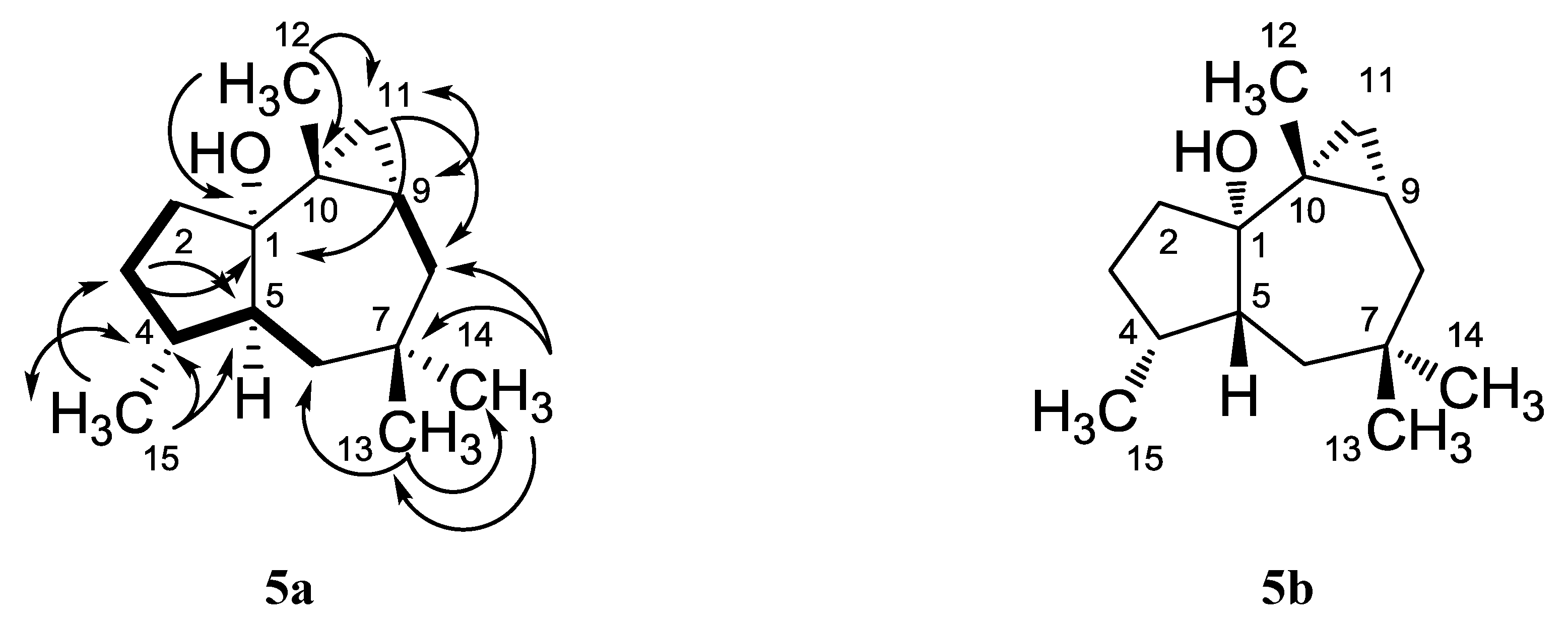
2.2. Biological Activities
| Compound | Antitumor Potency a | Tumor Selectivity b | ||
|---|---|---|---|---|
| Mean IC50 (µM) | Mean IC70 (µM) | n/total | % | |
| cis-pachydictyol B (1a) | >30.0 | >30.0 | 0/12 | 0 |
| pachydictyol C (2) | >30.0 | >30.0 | 0/12 | 0 |
| pachydictyol A (3a) | 23.6 | >30.0 | 0/12 | 0 |
| dictyol E (4) | >30.0 | >30.0 | 0/12 | 0 |
| cis-africanan-1α-ol (5a) | >10.0 | >10.0 | 0/12 | 0 |
| fucosterol (6) | 19.5 | >30.0 | 0/12 | 0 |
3. Experimental Section
3.1. General Experimental Procedures
3.2. Collection and Taxonomy of the Marine Alga
3.3. Extraction and Isolation of the Bioactive Constituents
3.4. Estimation of Phytosterols and Hydrocarbons
3.5. Biological Activity Study
4. Conclusions
Abbreviations
| AM1 | Austin Model 1 (a model used in quantum physics) |
| CI MS | Chemical Ionization Mass Spectra/Mass Spectrometry |
| COSY | Correlation Spectroscopy |
| DCI MS | Desorption Chemical Ionization |
| EI MS | Electron Impact Mass Spectra/Mass Spectrometry |
| GC-MS analysis | Gas Chromatographic-Mass Spectrometric analysis |
| HMBC | Heteronuclear Multiple-Bond Correlation |
| HMQC | Heteronuclear Multiple-Quantum Correlation |
| HREI MS | High Resolution Electron Impact Mass Spectra/Mass Spectrometry |
| HRESI MS | High Resolution Electrospray Mass Spectra/Mass Spectrometry |
| HSQC | Heteronuclear Single Quantum Correlation |
| NMR | Nuclear Magnetic Resonance |
| NOE | Nuclear Overhauser Effect |
| NOESY | Nuclear Overhauser Effect Spectroscopy |
| PTLC | Preparative Thin-layer Chromatography |
| TLC | Thin Layer Chromatography |
Acknowledgments
Conflicts of Interest
References
- Vashishta, B.R. Botany for Degree Students, Part 1, Algae, 7th ed.; S. Chand & Company Ltd.: New Delhi, India, 1984; p. 5. [Google Scholar]
- Duran, R.; Zubia, E.; Ortega, M.J.; Salva, J. New diterpenoids from the alga Dictyota dichotoma. Tetrahedron 1997, 53, 8675–8688. [Google Scholar] [CrossRef]
- Gedaraa, S.R.; Abdel-Halim, O.B.; El-Sharkawya, S.H.; Salama, O.M.; Shier, T.W.; Halim, A.F. Cytotoxic hydroazulene diterpenes from the brown alga Dictyota dichotoma. Z. Naturforsch. 2003, 58b, 17–22. [Google Scholar]
- Freitas, O.S.P.; Santos de Oliveira, A.; de-Paula, J.C.; Pereira, R.C.; Cavalcanti, D.N.; Teixeira, V.L. Chemical variation in the diterpenes from the Brazilian brown alga Dictyota mertensii (Dictyotaceae, Phaeophyta). Nat. Prod. Commun. 2007, 2, 13–15. [Google Scholar]
- Teixeira, V.L.; Almeida, S.A.; Kelecom, A. Chemsystematic and biogeographic studies of the diterpenes. Biochem. Syst. Ecol. 1990, 18, 87–92. [Google Scholar] [CrossRef]
- De-Paula, J.C.; Bueno, L.B.; Cavalcanti, D.N.; Yoneshigue-Valentin, Y.; Teixeira, V.L. Diterpenes from the brown alga Dictyota crenulata. Molecules 2008, 13, 1253–1262. [Google Scholar] [CrossRef]
- Suzuki, M.; Yamada, H.; Kurata, K. Dictyterpenoids A and B, two novel diterpenoids with feeding-deterrent activity from the brown alga Dilophus okamurai. J. Nat. Prod. 2002, 65, 121–125. [Google Scholar] [CrossRef]
- Schmitt, T.M.; Lindquist, N.; Hay, M.E. Seaweed secondary metabolites as antifoulants. Effects of Dictyota spp. diterpenes on survivorship, settlement, and development of marine invertebrate larvae. Chemoecology 1998, 8, 125–131. [Google Scholar] [CrossRef]
- Soto, R.H.; Rovirosa, R.J.; San Martin, A.; Argandona, V. Secondary metabolites of Dictyota crenulata. Bol. Soc. Chil. Quim. 1994, 39, 173–178. [Google Scholar]
- Patil, A.D.; Berry, D.; Brooks, D.P.; Hemling, M.E.; Kumar, N.V.; Mitchell, M.P.; Ohlstein, E.H.; Westley, J.W. A diterpene epoxide from the marine brown alga Dictyota sp.: Possible vasopressin V1 receptor antagonist. Phytochemistry 1993, 33, 1061–1064. [Google Scholar] [CrossRef]
- Cavalcanti, D.N.; Rezende, C.M.; Pinto, A.C.; Teixeira, V.L. Diterpenoid constituents from the brown alga Dictyota menstrualis (Dictyotaceae, Phaeophyta). Nat. Prod. Commun. 2006, 1, 609–611. [Google Scholar]
- Teixeira, V.L.; Cavalcanti, D.N.; Pereira, R.C. Chemotaxonomic study of the diterpenes from the brown alga Dictyota menstrualis. Biochem. Syst. Ecol. 2001, 29, 313–316. [Google Scholar] [CrossRef]
- Pereira, R.C.; Teixeira, V.L.; Kelecom, A. Chemical defenses against herbivores in marine algae. 1. The brown alga Dictyota dichotoma (Hudson) Lamouroux from Brazil. An. Acad. Bras. Cienc. 1994, 66, 229–235. [Google Scholar]
- Danise, B.; Minale, L.; Riccio, R.; Amico, V.; Oriente, G.; Piattelli, M.; Tringali, C.; Fattorusso, E.; Magno, S.; Mayol, L. Further perhydroazulene diterpenes from marine organisms. Experientia 1977, 33, 413–415. [Google Scholar] [CrossRef]
- Ovenden, S.P.B.; Nielson, J.L.; Liptrot, C.H.; Willis, R.H.; Tapiolas, D.M.; Wright, A.D.; Motti, C.A. Update of spectroscopic data for 4-hydroxydictyolactone and dictyol E isolated from a Halimeda stuposa-Dictyota sp. Assemblage. Molecules 2012, 17, 2929–2938. [Google Scholar] [CrossRef]
- Fricke, C. Terpenoide Inhaltsstoffe von Lebermosen und Heilpflanzen. Ph.D. Thesis, University Hamburg, Germany, 1999. Available online: http://www.sub.uni-hamburg.de/opus/volltexte/1999/189/ (accessed on 22 September 2012). [Google Scholar]
- SPARTAN'08; Wavefunction, Inc.: Irvine, CA, USA, 2009.
- Del Coronel, A.; Cerda-García-Rojas, C.M.; Joseph-Nathan, P.; Catalán, C.A.N. Chemical composition, seasonal variation and a new sesquiterpene alcohol from the essential oil of Lippia integrifolia. Flavour Fragr. J. 2006, 21, 839–847. [Google Scholar]
- Abou-ElWafa, G.S.E.; Shaaban, M.; Shaaban, K.A.; El-Naggar, M.E.E.; Laatsch, H. Three new unsaturated fatty acids from the marine green alga Ulva fasciata Delile. Z. Naturforsch. 2009, 64b, 1199–1207. [Google Scholar]
- Maskey, R.P.; Kock, I.; Shaaban, M.; Grün-Wollny, I.; Helmke, E.; Mayer, F.; Wagner-Döbler, I.; Laatsch, H. Low molecular weight oligo-β-hydroxybutyric acids and 3-hydroxy-N-phenethyl-butyramide new products from microorganisms. Polym. Bull. 2002, 49, 87–93. [Google Scholar]
- Anderson, A.J.; Dawes, E.A. Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol. Rev. 1990, 54, 450–472. [Google Scholar]
- Takahashi, A.; Kurasawa, S.; Ikeda, D.; Okami, Y.; Takeuchi, T. Altemicidin, a new acaricidal and antitumor substance. I. Taxonomy, fermentation, isolation and physico-chemical and biological properties. J. Antibiot. 1989, 32, 1556–1561. [Google Scholar]
- Sajid, I.; Fondja Yao, C.B.; Shaaban, K.A.; Hasnain, S.; Laatsch, H. Antifungal and antibacterial activities of indigenous Streptomyces isolates from saline farmlands: Prescreening, ribotyping and metabolic diversity. World J. Microbiol. Biotechnol. 2009, 25, 601–610. [Google Scholar] [CrossRef]
- Burkholder, P.R.; Burkholder, L.M.; Almodovar, L.R. Antibiotic activity of some marine algae of Puerto Rico. Bot. Mar. 1960, 2, 149–156. [Google Scholar]
- Biabani, M.A.F.; Baake, M.; Lovisetto, B.; Laatsch, H.; Helmke, E.; Weyland, H. Anthranilamides: New antimicroalgal active substance from a marine Streptomyces sp. J. Antibiot. 1998, 51, 333–340. [Google Scholar] [CrossRef]
- Zinad, D.S.; Shaaban, K.A.; Abdalla, M.A.; Islam, T.; Schüffler, A.; Laatsch, H. Bioactive isocoumarins from a terrestrial Streptomyces sp. ANK302. Nat. Prod. Commun. 2011, 6, 45–48. [Google Scholar]
- Nasr, A.H. The Marine Algae of Alexandria. 1—A Report on Some Marine Algae Collected from the Vicinity of Alexandria; Notes and Memoirs No. 36; Government Press: Cairo, Egypt, 1940; p. 33. [Google Scholar]
- Abou-El Wafa, G.S.E.; El-Naggar, M.E.E. Studies on the biological activities of some species of Egyptian marine algae. Personal communication, 2005. [Google Scholar]
- Abou-El Wafa, G.S.E. Comparative Studies on Biogenic Compounds in Some Species of Egyptian Marine Algae. Ph.D. Thesis, El-Mansoura University, Egypt, 2011. [Google Scholar]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Dengler, W.A.; Schulte, J.; Berger, D.P.; Mertelsmann, R.; Fiebig, H.H. Development of a propidium iodide fluorescence assay for proliferation and cytotoxicity assays. Anticancer Drugs 1995, 6, 522–532. [Google Scholar] [CrossRef]
Supplementary Files
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Abou-El-Wafa, G.S.E.; Shaaban, M.; Shaaban, K.A.; El-Naggar, M.E.E.; Maier, A.; Fiebig, H.H.; Laatsch, H. Pachydictyols B and C: New Diterpenes from Dictyota dichotoma Hudson. Mar. Drugs 2013, 11, 3109-3123. https://doi.org/10.3390/md11093109
Abou-El-Wafa GSE, Shaaban M, Shaaban KA, El-Naggar MEE, Maier A, Fiebig HH, Laatsch H. Pachydictyols B and C: New Diterpenes from Dictyota dichotoma Hudson. Marine Drugs. 2013; 11(9):3109-3123. https://doi.org/10.3390/md11093109
Chicago/Turabian StyleAbou-El-Wafa, Ghada S. E., Mohamed Shaaban, Khaled A. Shaaban, Mohamed E. E. El-Naggar, Armin Maier, Heinz H. Fiebig, and Hartmut Laatsch. 2013. "Pachydictyols B and C: New Diterpenes from Dictyota dichotoma Hudson" Marine Drugs 11, no. 9: 3109-3123. https://doi.org/10.3390/md11093109
APA StyleAbou-El-Wafa, G. S. E., Shaaban, M., Shaaban, K. A., El-Naggar, M. E. E., Maier, A., Fiebig, H. H., & Laatsch, H. (2013). Pachydictyols B and C: New Diterpenes from Dictyota dichotoma Hudson. Marine Drugs, 11(9), 3109-3123. https://doi.org/10.3390/md11093109