1. Introduction
The phylum Cnidaria is an ancient group of predominantly marine simple animals that comprise over 11,000 extant species [
1], which share a common diagnostic feature: the cnida [
2]. The cnida is a subcellular organelle-like capsular with eversible tubules [
3,
4] that contains the cnidocysts cells (also called cnidae). Of the three categories of cnidae (nematocysts, ptychocysts, and spirocysts), only nematocysts are found in all cnidarians [
2]. This type of cnida may be associated with the production, discharge and inoculation of venoms in all cnidarians [
5], whereby cnidarians are considered as the largest phylum of generally toxic animals [
6].
Currently around 250 compounds from cnidarians have been identified including peptides, proteins, enzymes, protease inhibitors and non-proteinaceous substances [
7]. Most cnidarians toxins have been successfully isolated from class Anthozoa, particularly from sea anemones, which is partly due by the stability of their toxins compared to jellyfish toxins [
8]. To date, at least 191 proteins from sea anemones are recognized without ambiguities, considering the complete peptide sequences (or the information obtained by the translation of coding sequence submitted “CDSs” to GenBank database) and more than 80% deduced amino acid sequences for proteins over 10 kDa [
9].
Most of these toxins correspond to peptides that act on voltage-gated sodium (Na
v) and potassium channels (K
v), whose molecular weights are between 3.5–6.5 kDa and 3–5 kDa, respectively [
10]. These toxins also seem to have an universal distribution within the group, since all species tested have been found to contain toxins that are lethal or paralytic to crabs [
10]. This claim is well supported by the number of toxins (62 for Na
v and 28 for K
v) characterized [
7]. Moreover, the new Na
v toxins from
Aiptasia diaphana [
11] and the two novel type 1 sea anemone K
v toxins from
Bunodosoma caissarum [
12] should be added to this list.
Despite sea anemones being the best studied in the phylum, the order Ceriantharia, Corallimorpharia and Zoanthiniaria, which are closely related to the order Actiniaria, have stayed unexplored regarding the presence of low molecular weight toxins. There are only few reports on
Zoanthus soociatus, an organism belonging to the order Zoanthiniaria, which is known to present organic compounds such as palytoxin [
13] and some alkaloids affecting platelet aggregation [
14]. Other biological properties have been described for
Z. sociatus preparations, for example, an antifilarial activity by a chloroform-methanol extract [
15] and an inhibitory effect on Ca
2+ influx in rat β-cells by a low molecular weight fraction [
16]. The latter suggest that there are unraveled biological activities in
Z. sociatus that could account for its toxicity
in vivo. In the present study we obtained a low molecular weight fraction from
Z. sociatus and assessed its toxicological properties in OF-1 mice. A dose-mortality curve was constructed and conspicuous toxic symptoms were monitored and discussed.
2. Results and Discussion
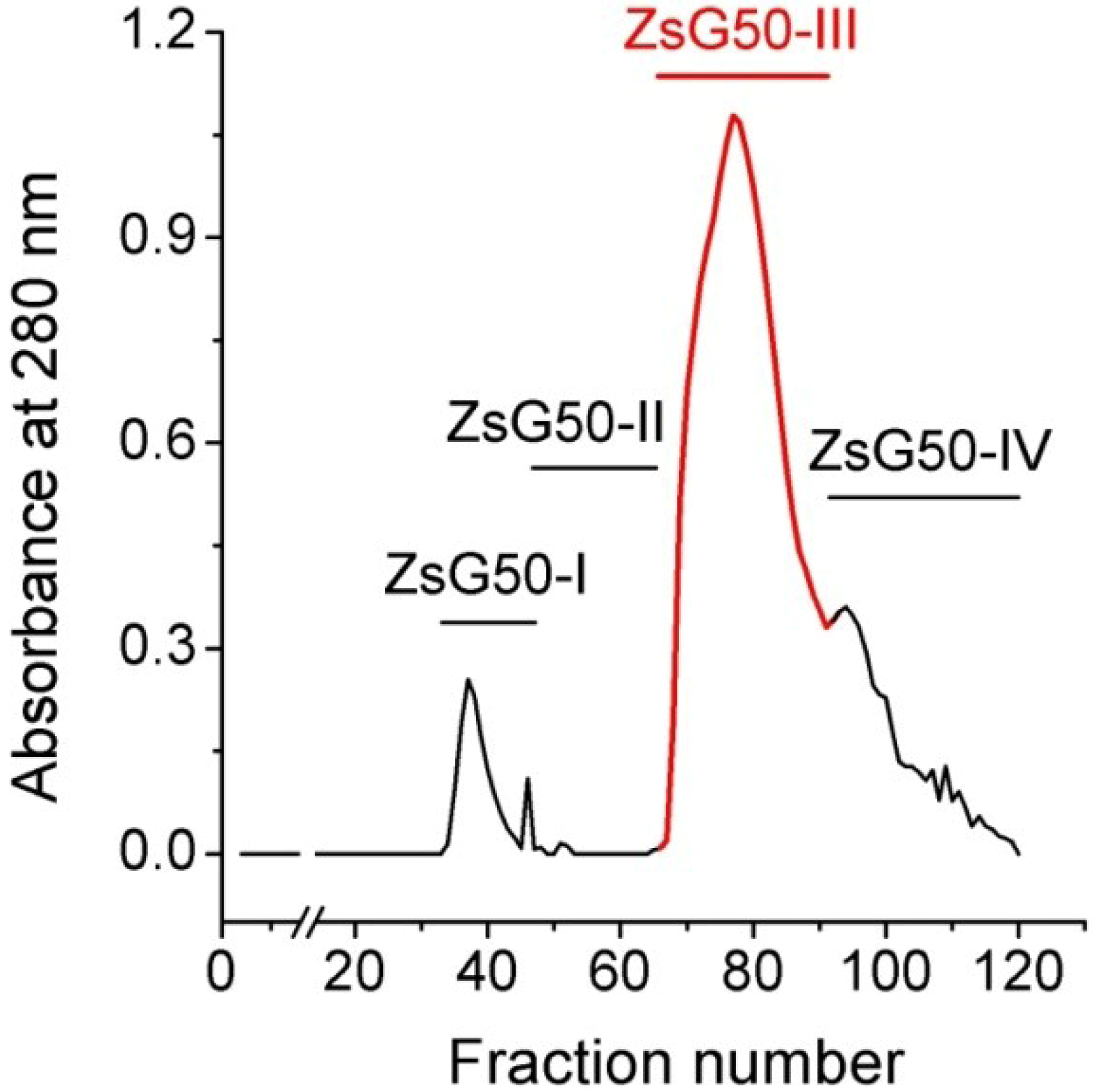
Z. sociatus crude extract was subjected to a Sephadex G50 gel chromatography and the elution of its components was monitored by absorbance at 280 nm. The chromatogram exhibited various peaks that were pooled in four major fractions (
Figure 1). The fraction ZsG50-III contained the most prominent peak and was selected for further studies because it has been demonstrated to contained low molecular weight toxins acting on ion channels in other studies on sea anemones using a similar methodology [
17].
Figure 1.
Sephadex G50 gel filtration chromatogram of Z. sociatus crude extract. Fractions in the chromatogram at 280 nm, obtained by Sephadex G50 gel filtration, were pooled in four major fractions. The fraction comprising the most prominent peak was named ZsG50-III and used for further toxicological studies.
Figure 1.
Sephadex G50 gel filtration chromatogram of Z. sociatus crude extract. Fractions in the chromatogram at 280 nm, obtained by Sephadex G50 gel filtration, were pooled in four major fractions. The fraction comprising the most prominent peak was named ZsG50-III and used for further toxicological studies.
The presence of signals detected by matrix assisted laser desorption/ionization time-of-fly/time-of-fly MALDI-TOF/TOF mass spectra (MS) corresponding to m/z ratio from 700 to 6000 Da. The highest relative abundance corresponds to compounds below 1000 Da (Supplemental Figure S1) that do not seem to be peptides (Supplemental Figures S2 and S3). However, some minor peaks between 2000 and 4000 Da captured and analyzed by MS/MS analysis in reflector positive mode showed typical fragmentation of peptides (Supplemental Figure S4). Molecular weights of these peptides are in the range reported for various toxins; however blast analysis showed no significant similarity with any toxins from the UniProt database. Further procedures in the isolation of pure peaks is required in order to eliminate possible interferences in the detection of peptide signals by the main metabolites in the fraction.
To estimate the range of toxicity of the ZsG50-III fraction was started at a dose (150 μg/kg) and monitored the symptoms increasing in three-fold steps, until lethality was observed. Mice inoculated intraperitoneally with 150 μg/kg of the fraction of interest showed the same typical grooming activity of controls after injection. In the 450 μg/kg dose, animals decrease their exploratory activity 10 min after injection and remained near the walls of the cage. This symptom was accompanied with disordered breathing. At 1350 μg/kg these toxic effects were observed in less than a minute after inoculation, causing spasms, palpitations, convulsions and dead after 2 min. These effects were observed before sudden dead, which was preceded by dyspnea and reduced motile activity, suggesting cardiac arrest as the main cause of lethality. Certain drugs have been reported to cause respiratory and cardiovascular complications before cardiac arrest in mammals [
18], including some cnidarian toxins [
19]. It may be possible that the lethal effects were caused mainly by non-peptide toxins abundant in the fraction with molecular weight below 1000 Da. However the presence of peptide toxins that can be acting synergically should not be discarded.
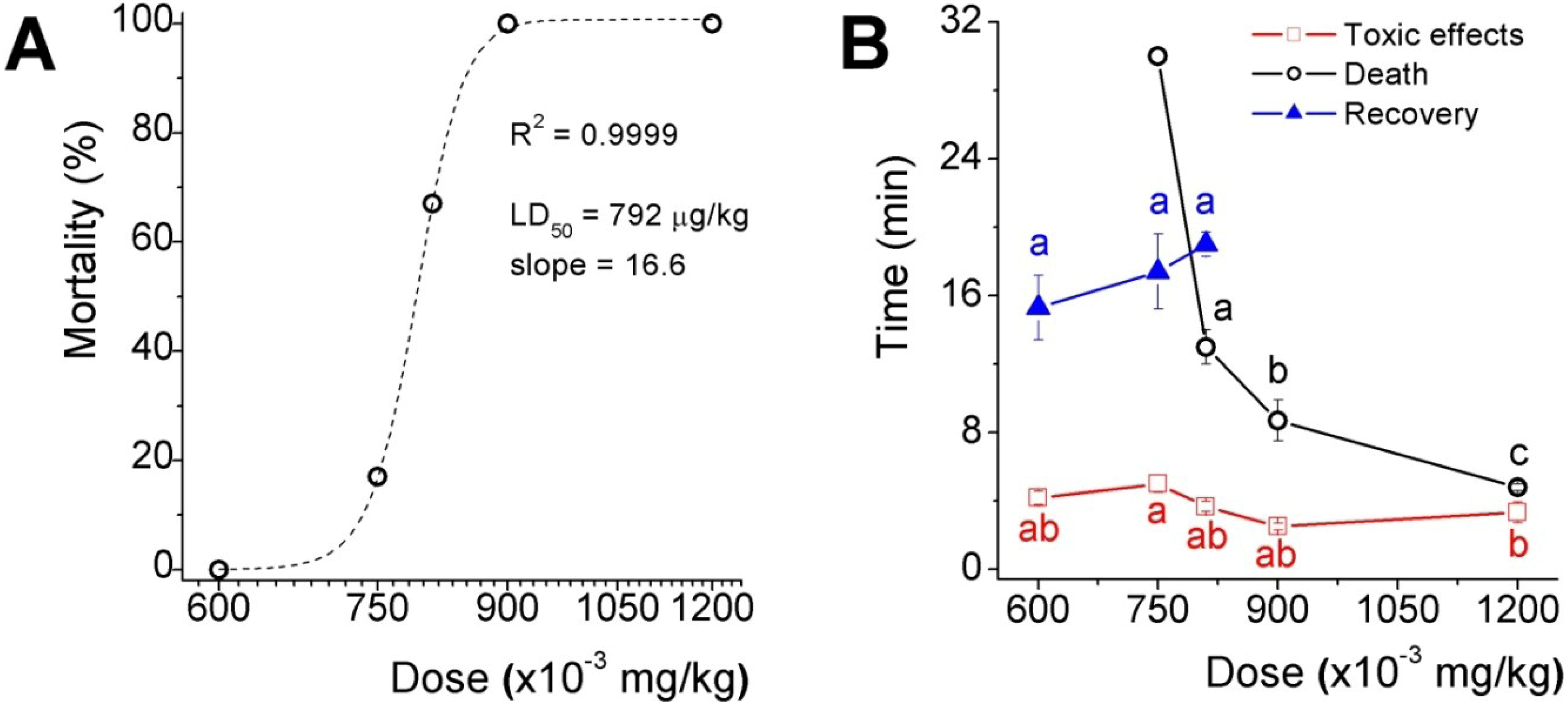
The acute toxicity results of five doses selected in the range between the two higher doses assayed in the preliminary test is shown as the percentage of lethality
versus the dose plot fitted to a dose-response sigmoid curve (
Figure 2A), which LD
50 was 792 μg/kg and the slope factor was 16.6 (expressing the dose as mg/kg). Toxicological effects appeared in less than 5 min after inoculation in mice from all groups, recovery, however, delayed proportionally to dose in those groups where lethality were not absolute and the time to death decreased in an exponential fashion (
Figure 2B). It is worth to mention that 33 and 50% of mice inoculated with the two higher doses, presented fecal and urinary incontinency, perhaps because of relaxation of sphincter smooth muscle.
Figure 2.
Acute toxicity assay of the low molecular weight fraction (ZsG50-III) from Z. sociatus crude extract. (A) Mortality versus dose curve. The plot shows a sigmoid equation fitting with the LD50 and the slope factor for the low molecular weight fraction from Z. sociatus; (B) The plot shows the time to listed events at each dose. The appearance of cardiovascular symptoms was common to all doses, and around 5 min after inoculation. At the lower dose, there was a time window of 10 min approximately between the onset of these effects and their disappearance. Symbols represent mean ± SEM. Letters represent statistical differences for a p < 0.05.
Figure 2.
Acute toxicity assay of the low molecular weight fraction (ZsG50-III) from Z. sociatus crude extract. (A) Mortality versus dose curve. The plot shows a sigmoid equation fitting with the LD50 and the slope factor for the low molecular weight fraction from Z. sociatus; (B) The plot shows the time to listed events at each dose. The appearance of cardiovascular symptoms was common to all doses, and around 5 min after inoculation. At the lower dose, there was a time window of 10 min approximately between the onset of these effects and their disappearance. Symbols represent mean ± SEM. Letters represent statistical differences for a p < 0.05.
Considering that an impairment of Ca
2+ fluxes has been previously reported for low molecular weight compounds in
Z. sociatus crude extract in excitable cells [
16] and that voltage dependent Ca
2+ channels are relevant to cardiac function [
20], we decided to explore the effect of ZsG50-III fraction on KCl-induced cardiac arrest in mice, as it is known that hyperkalemia can produce cardiac arrest [
21].
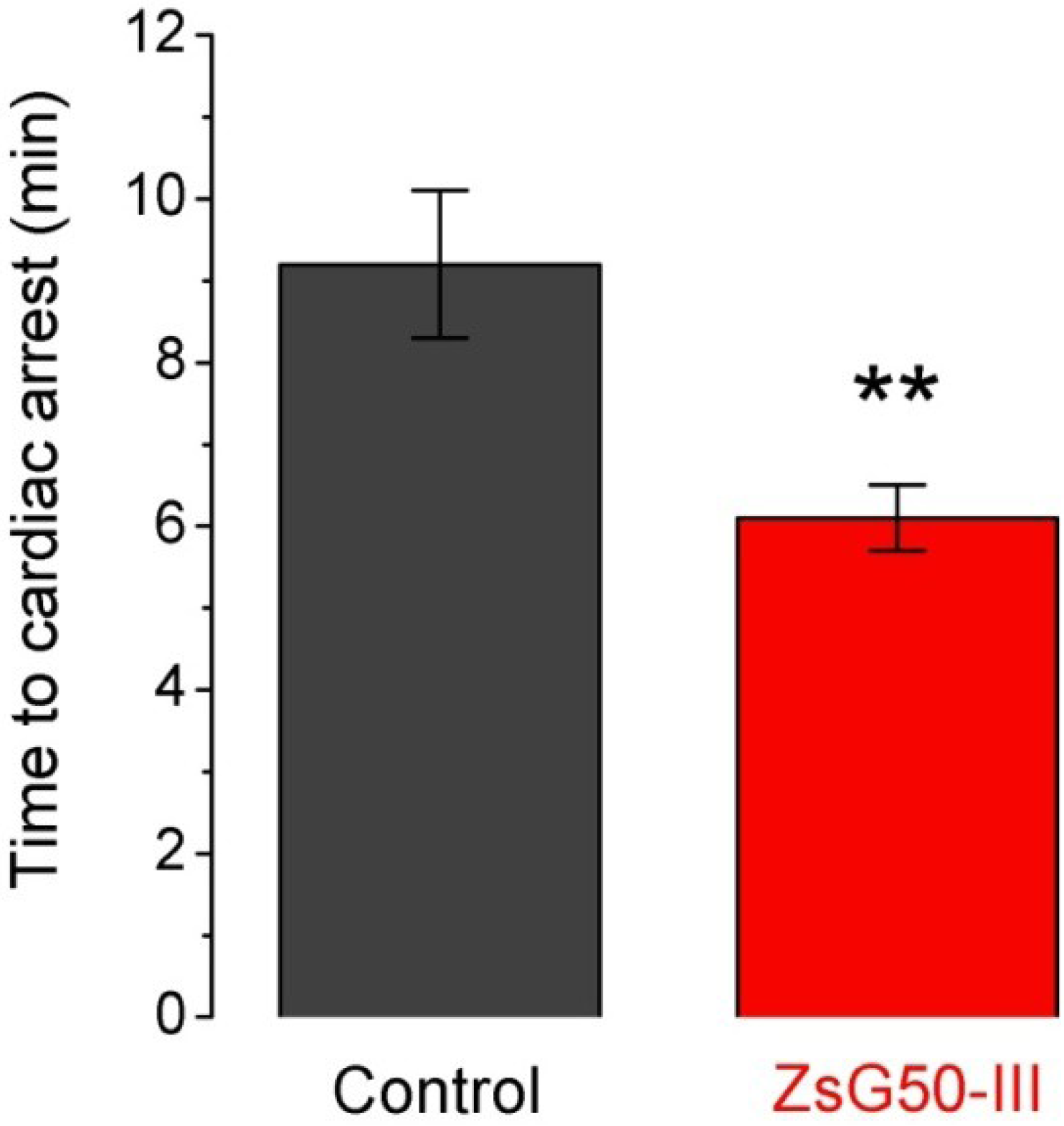
Seven mice were inoculated with KCl (1000 mg/kg) and six of them (86%) presented sudden death after 9.2 min in average (
Figure 3). A similar dose of NaCl was applied to another group of six mice and no lethality was observed suggesting that cardiac arrest was mediated by high K
+ instead an hyperosmotic load. To analyze if the fraction ZsG50-III could modify the lethal effect of KCl, we used a non-lethal dose of the fraction (600 μg/kg) and compared the times to cardiac arrest respect to the KCl only group. All mice treated with KCl and ZsG50-III died in an average time of 6.1 min, which indicated that the fraction of interest accelerated KCl-cardiac arrest. Moreover, the controls with NaCl in this condition (
N = 6) did not presented mortality and the symptoms were similar to the observed in the acute toxicity test for the same dose of the fraction in physiological saline.
Figure 3.
The low molecular weight fraction ZsG50-III accelerated the KCl-induced time to cardiac arrest. Bars represent the time to cardiac arrest after inoculation of a lethal dose of KCl (1000 mg/kg) in controls (N = 6) and simultaneous administration of KCl and 600 μg/kg of the low molecular weight fraction in the ZsG50-III treated group (N = 7). Bars represent mean ± SEM. ** p < 0.01.
Figure 3.
The low molecular weight fraction ZsG50-III accelerated the KCl-induced time to cardiac arrest. Bars represent the time to cardiac arrest after inoculation of a lethal dose of KCl (1000 mg/kg) in controls (N = 6) and simultaneous administration of KCl and 600 μg/kg of the low molecular weight fraction in the ZsG50-III treated group (N = 7). Bars represent mean ± SEM. ** p < 0.01.
The acceleration of KCl-induced cardiac arrest by ZsG50-III suggests that the fraction could enhance the KCl-mediated cardiac dysfunction. It has been reported that
Z. sociatus crude extract contains low molecular weight compounds that inhibit Ca
2+ influx to pancreatic beta cells and impairs glucose tolerance in rats [
16]. Interestingly it is well known that overdose of Ca
2+ channel blockers cause cardiovascular failure [
22,
23] but also hyperglycemia as a result, in part, of insulin secretion impairment [
24]. In the light of previous studies and our results, a malfunction of Ca
2+ fluxes could account for the observed cardiac toxicity during administration of high doses of ZsG50-III. However, further efforts should be made to unravel the exact mechanisms and to identify the responsible molecules.
3. Experimental Section
3.1. General Procedures
Z. sociatus were provided by the National Aquarium of Cuba, La Habana, Cuba. The zooids were brought to the Laboratory alive and kept in clean seawater until crude extract preparation. Sample collection and crude extract preparation. Briefly, specimens were cut in small pieces after removal of their stolonal bases and blended in distilled water at 4 °C. The whole-bodies homogenate was filtered in a spun glass mesh to remove large pieces of tissue and the filtrate was centrifuged twice in a Beckmann CS-6RK centrifuge at 1376× g during 30 min at 4 °C. Finally, the supernatants were recovered and freeze-dried.
3.2. Gel Filtration
The low molecular weight fraction from Z. sociatus was obtained by gel filtration chromatography of crude extract in Sephadex G50 matrix (Amersham Pharmacia Biotech, Uppsala, Sweden). Two grams (2 g) of crude extract were dissolved in 20 mL of 0.1 M ammonium acetate buffer (pH 6.7), centrifuged as described above and the supernatant was filtered through a 0.22 μm membrane (Merck Millipore, Billerica, MA, USA). The filtrate was applied to a chromatographic column (3.3 × 84 cm), packed with a Sephadex G50 matrix and previously equilibrated with the same ammonium acetate buffer. Chromatography was performed at a constant linear flow rate of 3.9 cm/h collecting fractions of 8.4 mL while monitoring elution through absorbance at 280 nm. Collected fractions were pooled in four major fractions and the third, usually the most prominent and containing low molecular weight compounds, was freeze-dried and used for experiments. The protein concentration was assessed using a bicinchoninic acid kit (Thermo Scientific, Rockford, IL, USA).
3.3. Mass Spectrometry Analysis
To evaluate the complexity of the fraction of interest MALDI-TOF/TOF (4800 Plus MALDI-TOF/TOF Analyzer; AB SCIEX, Framingham, MA, USA) spectra were obtained in positive linear mode from 700 Da to 12,000 Da and reflector positive mode 700–4000 Da using the matrix α-cyano-4-hydroxycinnamic acid (α-CHCA) and also sequencing of peptide/metabolite in MS/MS mode. Samples were previously concentrated and cleaned according to the manufacturer’s instructions on a micro C18 ZipTiP column (Millipore, Bedford, MA, USA). Afterwards, some of these peptides were selected to cleave for partial sequencing.
3.4. Acute Toxicity Test
Five groups of six OF-1 male mice were intraperitoneally (i.p.) administered with three doses of the fraction of interest: 150, 450 and 1350 μg/kg in three 18–22 g OF-1 male mice each. Previous to the inoculation the fraction was dissolved in physiological saline (0.9% NaCl solution) and controls received a similar volume of vehicle alone. The percentage of mortality as a function of the dose was fitted to a Dose-Response curve to determine the mean Lethal Dose (LD50) and the slope factor. Observations were done during the first hour post inoculation and the control and the surviving mice were monitored again after 24 h. Controls received a similar volume of saline and no dead was recorded. OF-1 mice were obtained from National Center for the Production of Laboratory Animals (CENPALAB), Cuba. Animal manipulation was performed according to the “International Guiding Principles for Biomedical Research Involving Animals” (Council for International Organizations of Medical Sciences, Geneva, Switzerland, 2012).
3.5. Data Analysis
Comparisons were performed by the non-parametric Mann-Whitney and Kruskal-Wallis (with a post-hoc Dunn) tests using GraphPad InStat version 3.00 (GraphPad Software, San Diego, CA, USA). Graphics were constructed using Origin version 7 (OriginLab Corporation, Northampton, MA, USA), as well as dose-response curve fitting.