2.1. Effects of Domoic Acid on Algal Monocultures
This study benefitted from the use of a strain of
P. delicatissima reported to produce domoic acid by the Provasoli-Guillard National Center for Marine Algae and Microbiota (NCMA) but which did not produce domoic acid over the course of our experiment, ensuring that the concentration of domoic acid in culture could be directly manipulated. Because this strain was previously reported to produce domoic acid, it is likely that it retained the ability to utilize domoic acid. It is unclear why
P. delicatissima produced no domoic acid in our study, however low irradiance levels used in this experiment have been reported to reduce domoic acid production [
22].
Experiment 1 determined the effect of domoic acid on algal monocultures.
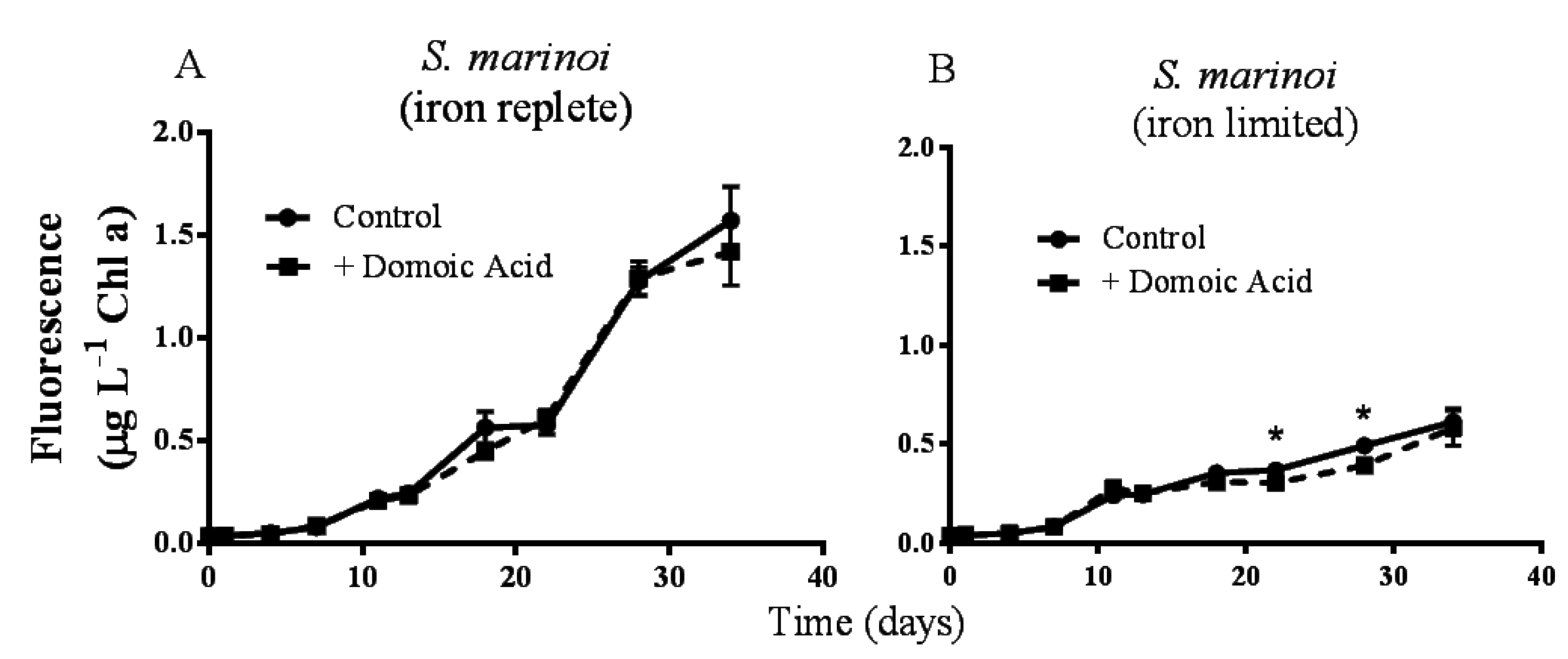
Pseudo-nitzschia delicatissima chlorophyll a fluorescence was not significantly different between control cultures and cultures to which domoic acid had been added, either under iron replete (
p = 0.272) or under low iron culture conditions (
p = 0.603; data not shown). Similarly, the fluorescence of
Skeletonema marinoi cultures was unaffected by domoic acid under iron replete (
p = 0.141) conditions (
Figure 1A). However, under low iron conditions a slight, but significant effect of domoic acid on
S. marinoi cultures was detected (
p < 0.001). Subsequent post-hoc tests determined that the fluorescence of low iron
S. marinoi cultures was significantly less in the presence of domoic acid on day 22 (17.7 ± 4.74% reduction;
p = 0.010) and on day 28 (20.1 ± 4.23% reduction;
p < 0.001) (
Figure 1B). This effect was no longer observable by day 34 (
p = 0.224).
Figure 1.
Effects of 482 nM domoic acid on monocultures of S. marinoi under iron replete (A) and low iron (B) conditions (Experiment 1). Graphs show the growth of S. marinoi control cultures (solid line, filled circles) and cultures to which domoic acid was added (dashed line, filled squares). Error bars indicate standard error. Asterisks (*) indicate a significant difference between treatments (p < 0.05) (n = 5 for all).
Figure 1.
Effects of 482 nM domoic acid on monocultures of S. marinoi under iron replete (A) and low iron (B) conditions (Experiment 1). Graphs show the growth of S. marinoi control cultures (solid line, filled circles) and cultures to which domoic acid was added (dashed line, filled squares). Error bars indicate standard error. Asterisks (*) indicate a significant difference between treatments (p < 0.05) (n = 5 for all).
Analysis of domoic acid from the P. delicatissima and S. marinoi cultures in experiment 1 indicated that the concentration in the samples did not decrease over the course of the experiment (p = 0.471). Additionally, the concentration of domoic acid measured in the cultures was never significantly different between any of the treatments (P. delicatissima or S. marinoi, with or without iron) (p = 0.083) (data not shown).
In contrast to previous reports that demonstrate no allelopathic effect of domoic acid [
20], our results suggest that a domoic acid at high concentrations may very slightly inhibit the growth of
S. marinoi. However, the previous study considered phytoplankton grown under nutrient replete conditions. The low iron treatment may have increased the susceptibility of
S. marinoi to other stressors, in this case, domoic acid. Previous studies have shown that nutrient limitation increases the susceptibility of phytoplankton to radiation [
23,
24] and to unidentified allelopathic compounds produced by other phytoplankton [
25]. From this experiment, it is not clear whether domoic acid directly inhibits the growth of
S. marinoi or if domoic acid binds iron, and reduces iron availability to the already iron limited
S. marinoi cells. If domoic acid reduces iron availability for
S. marinoi, it may explain why the effects of domoic acid disappear by day 34. The exudation of many
S. marinoi metabolites increases in the declining culture phase [
26]; these metabolites may include iron containing compounds and lysis of cells might release iron as well. If this iron is available to
S. marinoi the growth of iron limited cultures exposed to domoic acid may recover by the end of the experiment.
It should be also noted that the concentrations of domoic acid used in experiment 1 were quite high (approximately 482 nmol L
−1), and much higher than the concentrations used in subsequent experiments (from 6.34 to 63.4 nmol L
−1). In order to determine whether domoic acid ever has an effect on
S. marionoi monocultures, we chose to test its effects with concentrations higher than those reported in
Pseudo-nitzschia spp. blooms. Although concentrations of >100 nmol L
−1 have been reported in blooms [
16] these values are not commonly reached in the field. In contrast, domoic acid can reach concentrations more than an order of magnitude higher than those used here [
19,
27] in cultures of
Pseudo-nitzschia spp. However, even in situations where the concentration of domoic acid in water samples is low, local maxima might occur. Because domoic acid exuded by
Pseudo-nitzschia spp. will exist in patches of high concentrations surrounding the producing cells [
28,
29], it is possible that
S. marinoi may encounter domoic acid at concentrations used in this study. However, suppression of growth is modest, and whether such effects really provide an advantage under natural conditions remains to be established.
2.2. Effects of Domoic Acid on Algal Co-Cultures
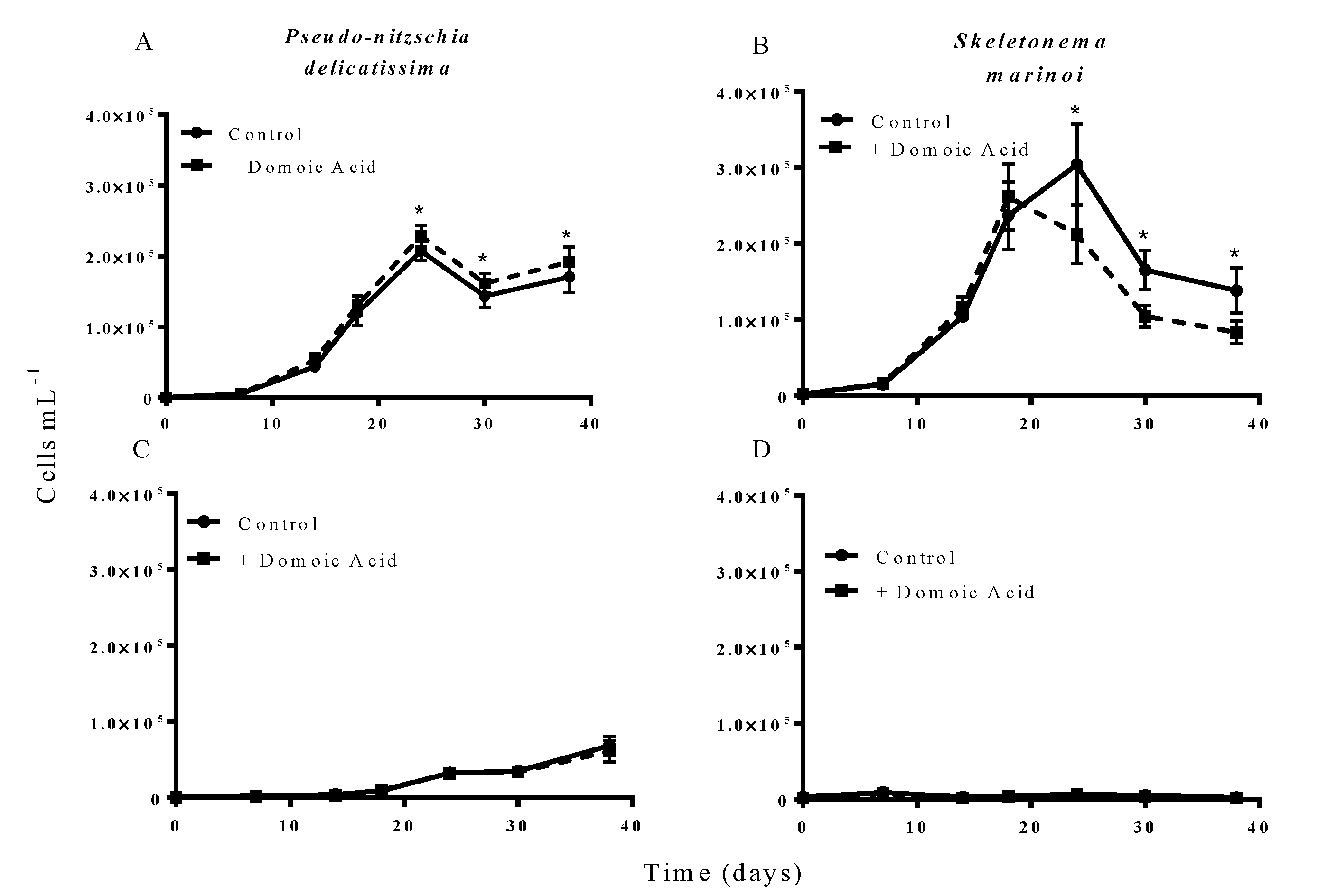
The results from experiment 2, testing the effect of domoic acid in situations where the two phytoplankton species directly interacted, indicated that domoic acid has the potential to increase the abundance of
P. delicatissima and to decrease the abundance of
S. marinoi in co-cultures. When co-cultures were grown under iron replete conditions, the addition of “high” (64 nM) domoic acid concentrations marginally but significantly increased growth of
P. delicatissima (
p = 0.016) (
Figure 2A). From day 22 until the end of the experiment on day 38 the number of
P. delicatissima cells in co-cultures with domoic acid was between 10.6% and 17.4% higher than in cultures with no additional domoic acid (
p = 0.004–0.012). There was no significant effect of the addition of “high” concentrations of domoic acid on the growth of
S. marinoi in co-cultures under iron replete conditions (
p = 0.087). However, we did detect a significant interaction of time and treatment (
p = 0.003), suggesting that
S. marinoi growth curves were different between co-cultures with and without domoic acid. Closer inspection revealed that between day 22 and day 38, the concentration of
S. marinoi cells was between 23.9% and 37.6% lower in the treatment that received domoic acid when compared to the treatment that did not receive domoic acid (
p = 0.024 to <0.001) (
Figure 2B). In contrast, there was no significant effect of domoic acid addition on the growth of either
P. delicatissima (
p = 0.465) (
Figure 2C) or
S. marinoi (
p = 0.089) (
Figure 2D) in low iron co-cultures. Similarly, there was no significant interaction for
S. marinoi or
P. delicatissima cells between the low iron treatments with and without domoic acid and time (
p = 0.972, and
p = 0.855, respectively).
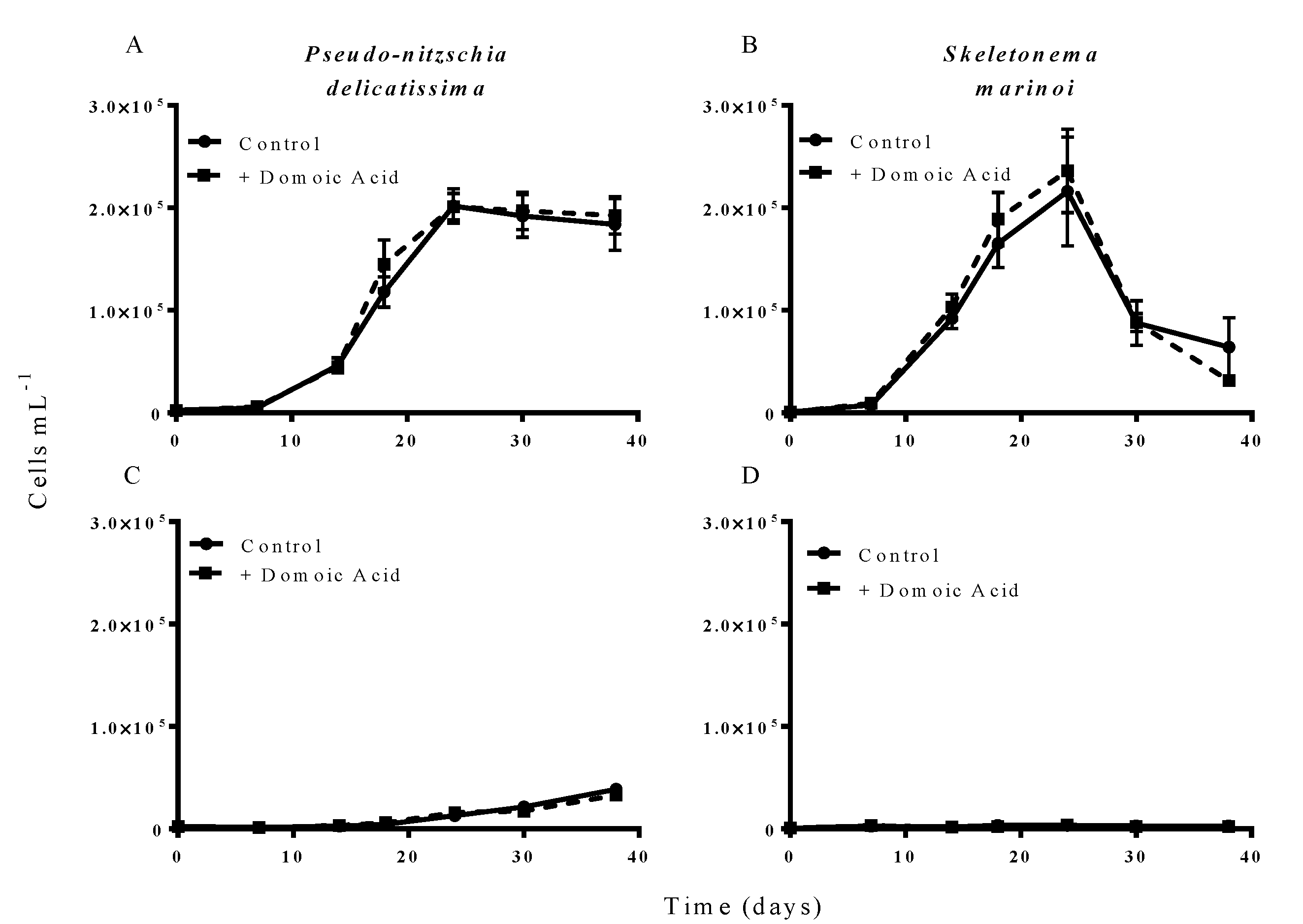
Additions of “low” (6.4 nM) concentrations of domoic acid did not influence the growth of either
P. delicatissima or
S. marinoi under either low iron or iron replete conditions.
P. delicatissima growth under iron replete conditions was not significantly different with or without added low domoic acid concentrations (
p = 0.259) and there was no significant interaction between treatment and time (
p = 0.559) (
Figure 3A). Likewise,
P. delicatissima growth under low iron conditions was not significantly different between cultures with added domoic acid and without (
p = 0.626) and there was no significant interaction between of treatment and time (
p = 0.221) (
Figure 3C). Similarly, the growth of
S. marinoi was not different between treatments with and without domoic acid under iron replete (
p = 0.808) or low iron conditions (
p = 0.158). There was no significant interaction between treatment and time for
S. marinoi cells in either low iron (
p = 0.224) or iron replete cultures (
p = 0.325) (
Figure 3B,D).
Figure 2.
Effect of 64 nM (high) domoic acid on co-cultures of S. marinoi and P. delicatissima (Experiment 2). Graphs show cell numbers of P. delicatissima (A) and S. marinoi (B) under iron replete conditions as well as cell numbers of P. delicatissima (C) and S. marinoi under low iron conditions (D). Cell numbers in control cultures (solid line, filled circles) and cultures to which domoic acid was added (dashed line, filled squares) are depicted in each graph. Error bars indicate standard error. Asterisks (*) indicate a significant difference between treatments (p < 0.05) (n = 7 for all).
Figure 2.
Effect of 64 nM (high) domoic acid on co-cultures of S. marinoi and P. delicatissima (Experiment 2). Graphs show cell numbers of P. delicatissima (A) and S. marinoi (B) under iron replete conditions as well as cell numbers of P. delicatissima (C) and S. marinoi under low iron conditions (D). Cell numbers in control cultures (solid line, filled circles) and cultures to which domoic acid was added (dashed line, filled squares) are depicted in each graph. Error bars indicate standard error. Asterisks (*) indicate a significant difference between treatments (p < 0.05) (n = 7 for all).
Figure 3.
Effect of 6.4 nM (low) domoic acid on co-cultures of S. marinoi and P. delicatissima (Experiment 2). Graphs show cell numbers of P. delicatissima (A) and S. marinoi (B) under iron replete conditions as well as cell numbers of P. delicatissima (C) and S. marinoi under low iron conditions (D). Cell numbers in control cultures (solid line, filled circles) and cultures to which domoic acid was added (dashed line, filled squares) are depicted in each graph. Error bars indicate standard error. No significant differences were detected between treatments (n = 7 for all).
Figure 3.
Effect of 6.4 nM (low) domoic acid on co-cultures of S. marinoi and P. delicatissima (Experiment 2). Graphs show cell numbers of P. delicatissima (A) and S. marinoi (B) under iron replete conditions as well as cell numbers of P. delicatissima (C) and S. marinoi under low iron conditions (D). Cell numbers in control cultures (solid line, filled circles) and cultures to which domoic acid was added (dashed line, filled squares) are depicted in each graph. Error bars indicate standard error. No significant differences were detected between treatments (n = 7 for all).
When domoic acid was added to cultures at a concentration of 64 nmol L
−1, the growth of
P. delicatissima was slightly stimulated while the growth of the competitor,
S. marinoi, was more dramatically inhibited, with the final ratio of
P. delicatissima to
S. marinoi to be 2.3 ± 0.4 in the treatment containing iron and domoic acid, and only 1.2 ± 0.3 in the comparable treatment without domoic acid (
Figure 2A,B). The fact that similar effects were not observed under low iron conditions (
Figure 2C,D) might be due to the fact that iron concentrations were too low to support
S. marinoi growth even in the absence of domoic acid, a possibility supported by the weak growth of
S. marinoi monocultures in iron limited media (
Figure 1B). This hypothesis could also explain why no differences in
S. marinoi cell densities were detected until day 22 of experiment 2, after
P. delicatissima reached stationary phase, since iron concentration was unlikely to be limiting, even with the addition of domoic acid, before this point (
Figure 2B). The results from experiment 2 are in contrast to the results from experiment 1, which found domoic acid had an effect on
S. marinoi only in low iron conditions (
Figure 1A). We hypothesize that the difference is caused by the presence of
P. delicatissima in the co-cultures used in experiment 2, which used iron in the media, reducing concentrations in the iron replete media to a level at which domoic acid had an effect. In addition,
P. delicatissima may have reduced the iron concentrations in low iron media below the level required to support
S. marinoi growth, so that the addition of domoic acid did not have any effect.
While our results do establish that domoic acid can improve the competitive ability of
P. delicatissima, it is less clear whether this effect is related to iron availability. We propose two alternate hypotheses. First, domoic acid prevents
S. marinoi from iron acquisition, either by directly binding the metal ions, as we suggested for
S. marinoi monocultures (
Figure 1B), making them inaccessible for
S. marinoi or by facilitating iron uptake by
P. delicatissima. Alternately, domoic acid could improve the competitive ability of
P. delicatissima through a mechanism that does not involve iron and the differences between the effects of domoic acid on low iron and iron replete cultures could be caused by differences in the physiological state of diatom cells. However, this second hypothesis is less likely given previous reports of the interactions between domoic acid and iron in blooms and cultures of
Pseudo-nitzschia spp. [
16,
18,
21].
The fact that domoic acid stimulated the growth of
P. delicatissima only modestly under iron replete conditions, but not at all under low iron conditions (
Figure 2A,C) is in contrast to previous results, which have shown a marked increase in
Pseudo-nitzschia cell numbers in response to domoic acid [
18,
21]. However, these studies have considered natural assemblages of the phytoplankton community. Sources of iron in these samples are likely to have been diverse, consisting primarily of iron bound to organic ligands and may be accessible for
Pseudo-nitzschia through domoic acid [
10,
11]. In contrast, iron in our experiment was added to replete cultures as FeCl3 with an equivalent concentration of EDTA. Although additional sources of iron in both replete and limited cultures likely included impurities present in other trace metals and contained in the seawater used to make media, it is unlikely that the medium contained significant amounts of iron bound to the array of bacterial siderophores, porphyrin-complexes, and other organic ligands present in natural phytoplankton communities [
13]. It is likely that the iron in this experiment was already available to
P. delicatissima, limiting the advantage to be gained from the presence of domoic acid. Concentrations of domoic acid of more than 100 nmol L
−1 have been detected during
Pseudo-nitzschia blooms in Monterey Bay, California [
16]. Therefore, the domoic acid concentrations used in this experiment, 64 and 6.4 nmol L
−1 are ecologically relevant. We could reliable quantify approximately 0.6 nmol L
−1 domoic acid, but were able to detect significantly lower concentrations. Although it is not possible to determine whether the strain of
P. delicatissima used in this experiment produced very low levels of domoic acid, it is clear that
P. delicatissima produced domoic acid at concentrations much lower than the low domoic acid treatment used in this experiment. Moreover, we detected no domoic acid at any time point in any treatment unless it was directly added to the cultures.
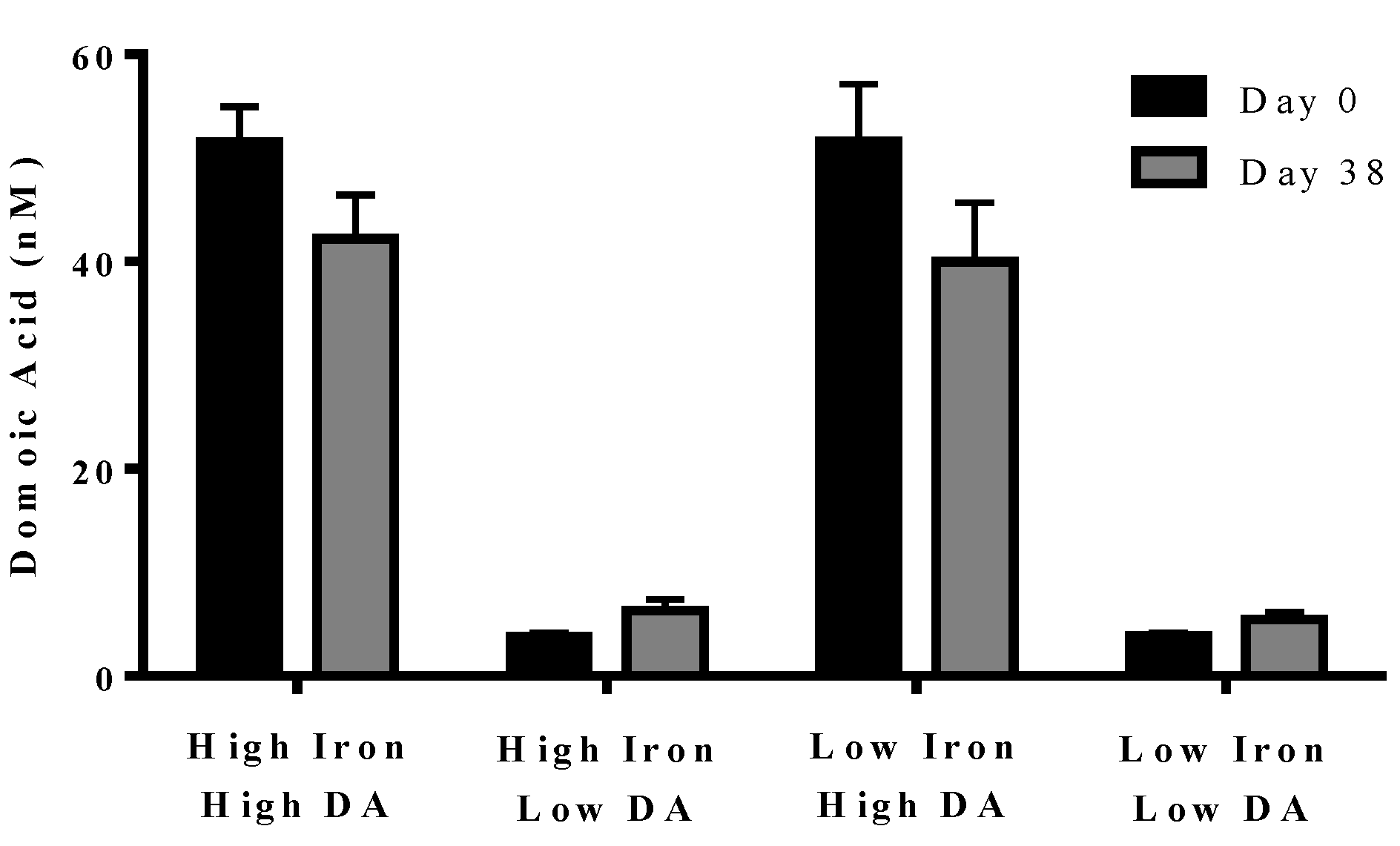
In experiment 2, domoic acid was measured at the beginning (day 0) and end (day 38) of the experiment. No domoic acid was detected in any of the cultures, either with or without iron, unless it was added separately (data not shown). Domoic acid concentration in the treatment did not change over the course of the experiment (
p = 0.210). The domoic acid concentration was higher in high domoic acid than in low domoic acid treatments (
p < 0.001 in all cases). Domoic acid concentration was not different between low iron and iron replete cultures for high domoic acid treatments (
p = 0.624–0.748) or low domoic acid treatments (
p = 0.850–0.941) (
Figure 4). These results indicate a negligible turnover and degradation of domoic acid in phytoplankton cultures.
Figure 4.
Concentration of domoic acid in “high” and “low” domoic acid treatment co-cultures on day 0 (black bars) and day 38 (grey bars) (Experiment 2). No significant differences were found between iron replete and low iron treatments, nor were difference detected over time. Error bars indicate standard error (n = 7 for all).
Figure 4.
Concentration of domoic acid in “high” and “low” domoic acid treatment co-cultures on day 0 (black bars) and day 38 (grey bars) (Experiment 2). No significant differences were found between iron replete and low iron treatments, nor were difference detected over time. Error bars indicate standard error (n = 7 for all).
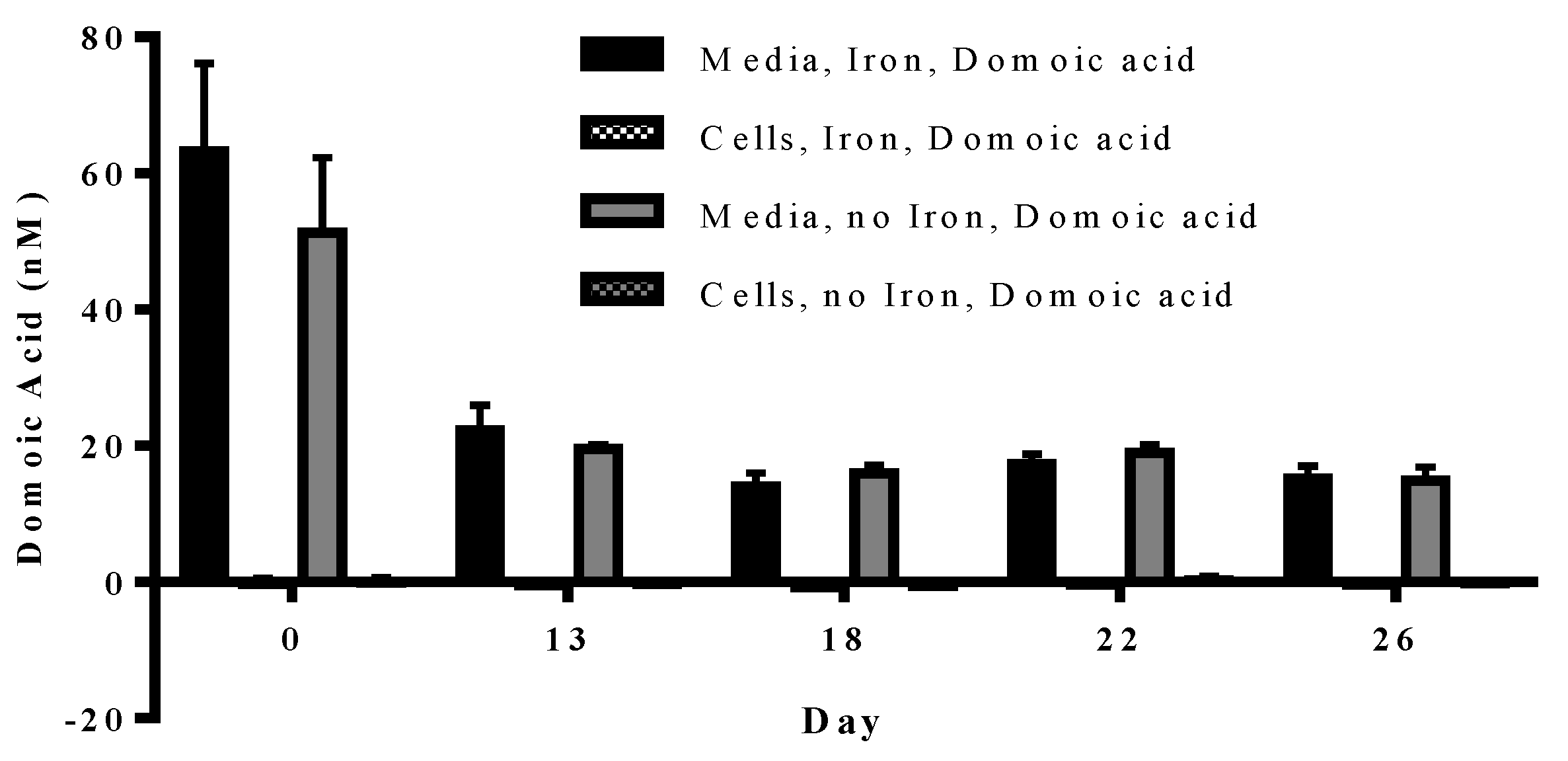
2.3. Domoic Acid Is Not Taken up by P. delicatissima Cells
Experiment 3 tested whether
P. delicatissima takes up domoic acid under low iron or iron replete conditions in the presence of 63.4 nM domoic acid concentrations. We found that, over the course of the
P. delicatissima growth curve, the domoic acid content in cells grown in low iron and iron replete cultures was never significantly different from zero (
p = 0.156–0.938). Although there was no significant difference between the domoic acid concentration within the low iron and iron replete media (
p = 0.355) or cell (
p = 0.827) samples, domoic acid concentration did decrease over time (
p < 0.001) (
Figure 5).
Our results suggest that if domoic acid does chelate iron as a mechanism for iron uptake, complexes are dissociated at the cell surface. These results are consistent with the accepted picture of iron uptake by eukaryotic phytoplankton. While bacteria possess specific siderophore receptors to take up siderophore complexed iron [
30], eukaryotic phytoplankton are believed to use a plasma membrane ferrireductase [
31] that reduces organically bound Fe(III) to inorganic Fe(II). Iron may be taken up either as inorganic Fe(II) or following reoxidation to Fe(III) [
13]. If
P. delicatissima uses a ferrireducatase to access iron bound to domoic acid, domoic acid would remain outside of the cell. Similarly, if domoic acid indirectly helps
Pseudo-nitschia spp. access iron (e.g., by making copper available for a high-affinity uptake system [
18]), domoic acid-copper complexes must also be dissociated at the cell surface, potentially through a cupric-reductase system. Such a system has been reported from the 2–20 μm size class of a natural phytoplankton assemblage [
32].
Figure 5.
Concentration of particulate (patterned bars) and dissolved (solid bars) domoic acid in P. delicatissima cultured under iron replete (black bars) and low iron (grey bars) conditions. Particulate domoic acid was not significantly different from 0. Dissolved domoic acid was not significantly different between iron replete and low iron treatments. However, domoic acid concentration significantly decreased over time (p < 0.001). Error bars indicate standard error (n = 7 for all).
Figure 5.
Concentration of particulate (patterned bars) and dissolved (solid bars) domoic acid in P. delicatissima cultured under iron replete (black bars) and low iron (grey bars) conditions. Particulate domoic acid was not significantly different from 0. Dissolved domoic acid was not significantly different between iron replete and low iron treatments. However, domoic acid concentration significantly decreased over time (p < 0.001). Error bars indicate standard error (n = 7 for all).
In contrast to the
P. delicatissima co-cultures, where domoic acid levels were constant, in
P. delicatissima monocultures domoic acid levels decreased over the course of the experiment. It is not clear what factors were responsible for this difference, although many reasons can be envisaged for the finding. Future studies should investigate the possibility of altered physiological or physicochemical properties of the cells and/or medium in co-cultures. In fact, multiple changes in the endo- and exometabolome have been observed in standard co-culturing experiments, indicating the strong and diverse physiological response of algae to the presence of other species [
33].