Synchronized Regulation of Different Zwitterionic Metabolites in the Osmoadaption of Phytoplankton
Abstract
:1. Introduction
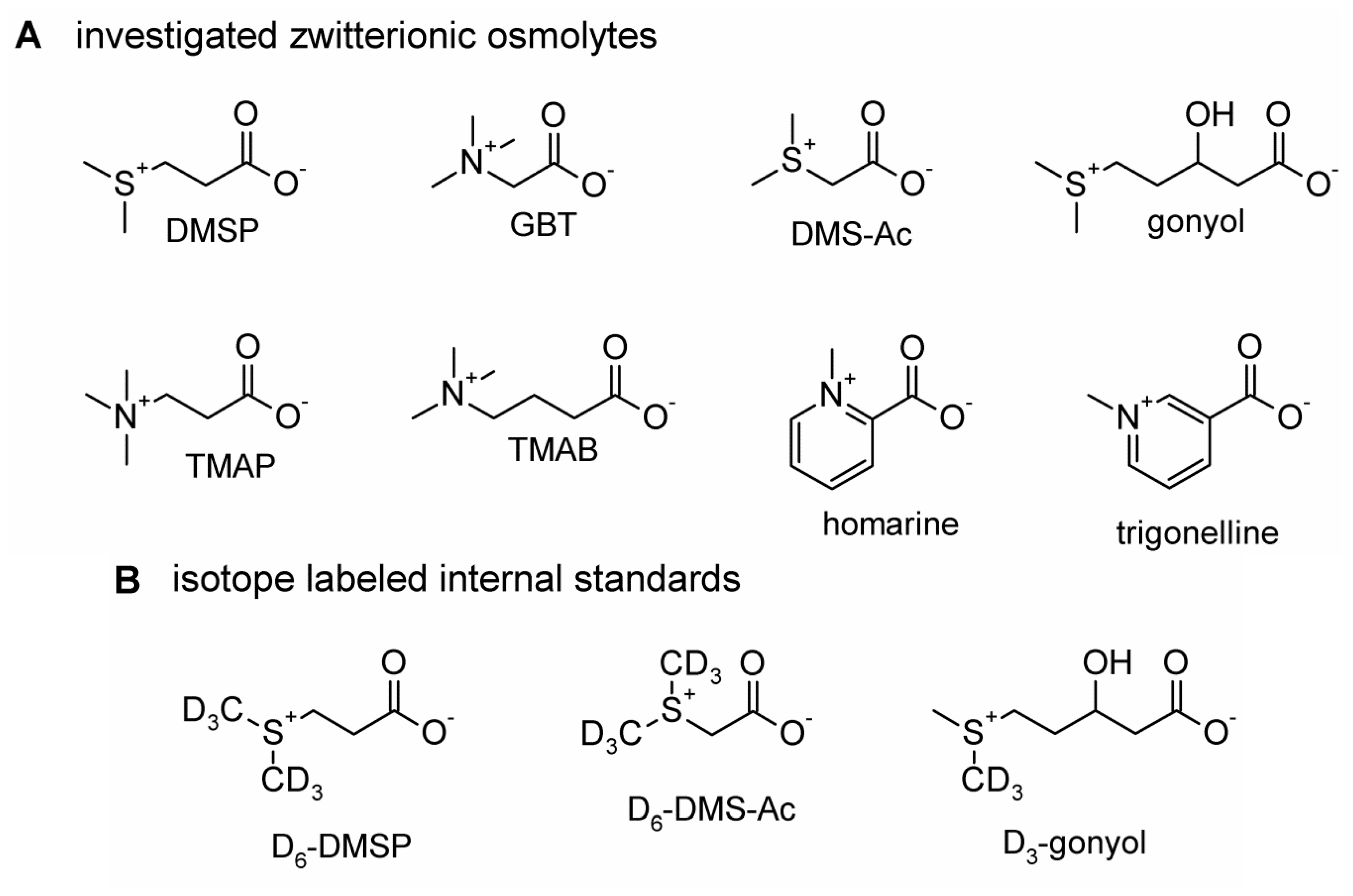
2. Results and Discussion
 ), glycine betaine (GBT, [M + 1] m/z = 118
), glycine betaine (GBT, [M + 1] m/z = 118  ), trimethylammonium butyrate (TMAB, [M + 1] m/z = 146
), trimethylammonium butyrate (TMAB, [M + 1] m/z = 146  ), gonyol ([M + 1] m/z = 179
), gonyol ([M + 1] m/z = 179  ), homarine and trigonelline ([M + 1] m/z = 138
), homarine and trigonelline ([M + 1] m/z = 138  ). Ion traces of GBT, gonyol, TMAB and trigonelline are 10-times amplified.
). Ion traces of GBT, gonyol, TMAB and trigonelline are 10-times amplified.
 ), glycine betaine (GBT, [M + 1] m/z = 118
), glycine betaine (GBT, [M + 1] m/z = 118  ), trimethylammonium butyrate (TMAB, [M + 1] m/z = 146
), trimethylammonium butyrate (TMAB, [M + 1] m/z = 146  ), gonyol ([M + 1] m/z = 179
), gonyol ([M + 1] m/z = 179  ), homarine and trigonelline ([M + 1] m/z = 138
), homarine and trigonelline ([M + 1] m/z = 138  ). Ion traces of GBT, gonyol, TMAB and trigonelline are 10-times amplified.
). Ion traces of GBT, gonyol, TMAB and trigonelline are 10-times amplified.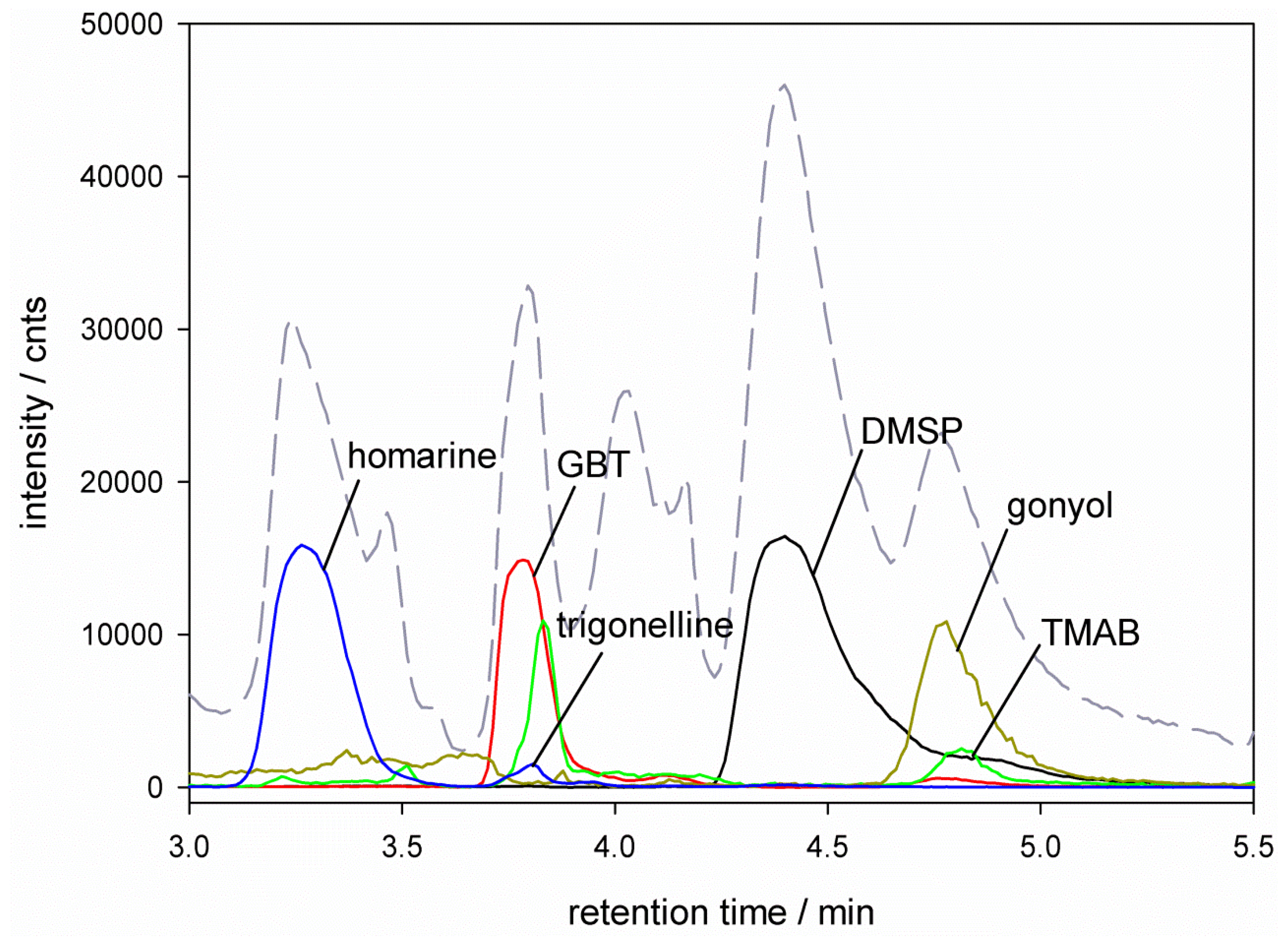
 ), glycine betaine (GBT) ([M + 1] m/z = 118
), glycine betaine (GBT) ([M + 1] m/z = 118  ), dimethylsulfonioacetate (DMS-Ac, [M + 1] m/z = 121
), dimethylsulfonioacetate (DMS-Ac, [M + 1] m/z = 121  ), gonyol ([M + 1] m/z = 179
), gonyol ([M + 1] m/z = 179  ), trigonelline ([M + 1] m/z = 138
), trigonelline ([M + 1] m/z = 138  ) and trimethylammonium propionate (TMAP, [M + 1] m/z = 132
) and trimethylammonium propionate (TMAP, [M + 1] m/z = 132  ). Ion traces of GBT, DMS-Ac, gonyol, TMAP, and trigonelline are 10-times amplified.
). Ion traces of GBT, DMS-Ac, gonyol, TMAP, and trigonelline are 10-times amplified.
 ), glycine betaine (GBT) ([M + 1] m/z = 118
), glycine betaine (GBT) ([M + 1] m/z = 118  ), dimethylsulfonioacetate (DMS-Ac, [M + 1] m/z = 121
), dimethylsulfonioacetate (DMS-Ac, [M + 1] m/z = 121  ), gonyol ([M + 1] m/z = 179
), gonyol ([M + 1] m/z = 179  ), trigonelline ([M + 1] m/z = 138
), trigonelline ([M + 1] m/z = 138  ) and trimethylammonium propionate (TMAP, [M + 1] m/z = 132
) and trimethylammonium propionate (TMAP, [M + 1] m/z = 132  ). Ion traces of GBT, DMS-Ac, gonyol, TMAP, and trigonelline are 10-times amplified.
). Ion traces of GBT, DMS-Ac, gonyol, TMAP, and trigonelline are 10-times amplified.
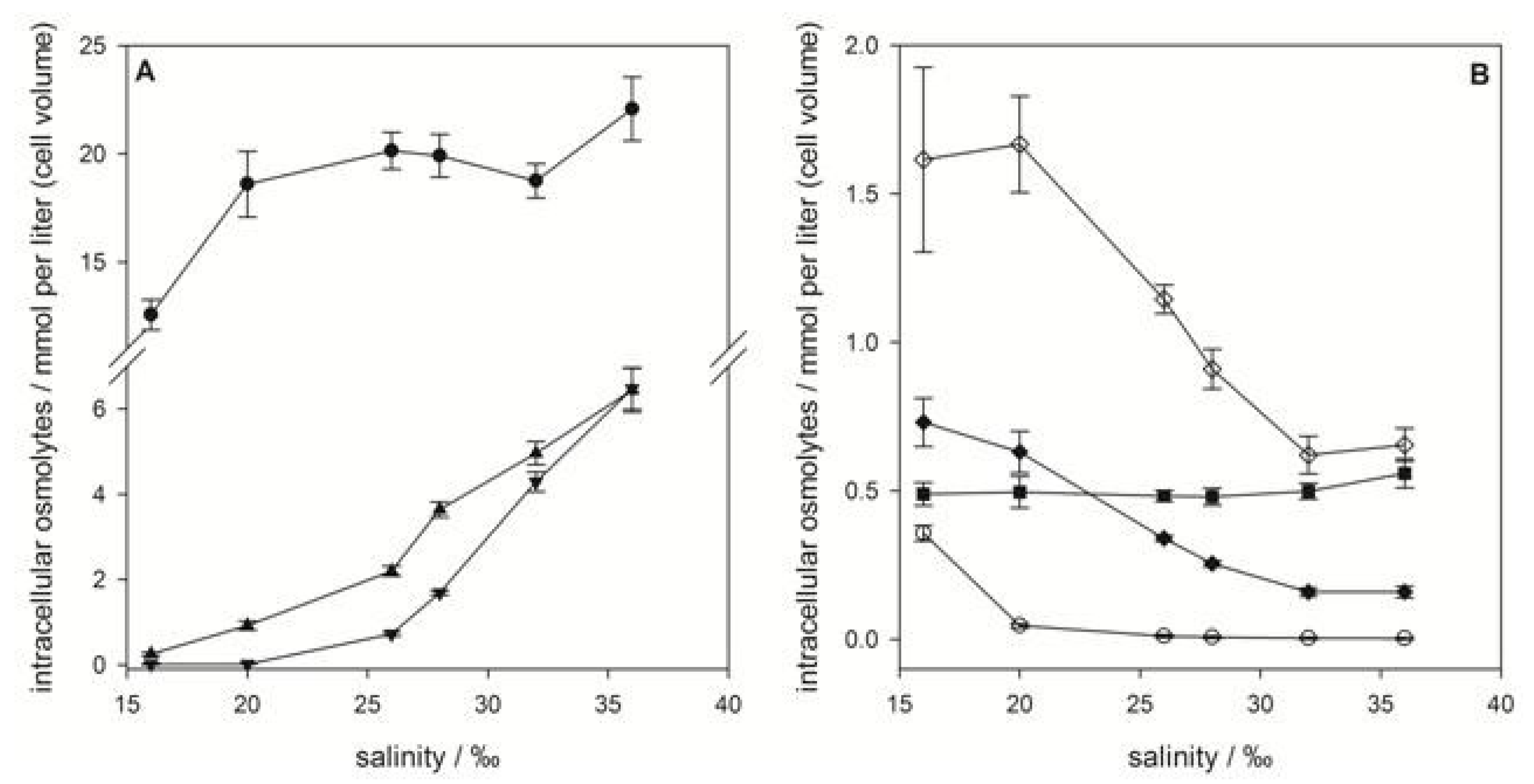
2.1. Emiliania huxleyi
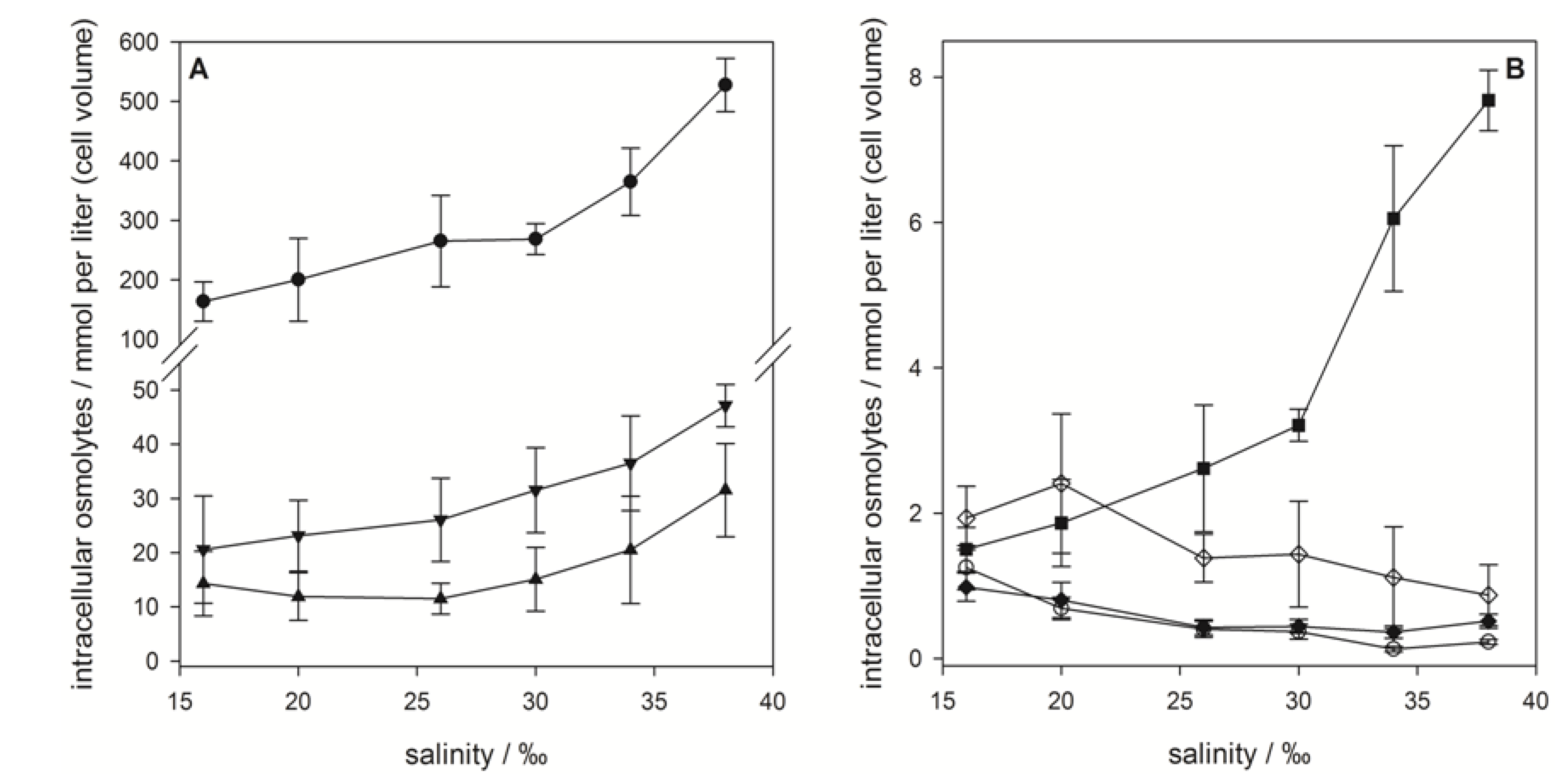
 ), gonyol (
), gonyol (  ), glycine betaine GBT (
), glycine betaine GBT (  ), homarine (
), homarine (  ), trimethylammonium propionate (TMAP) (
), trimethylammonium propionate (TMAP) (  ), trimethylammonium butyrate (TMAB) (
), trimethylammonium butyrate (TMAB) (  ) and trigonelline (
) and trigonelline (  ). Osmolyte concentrations are normalized per cell, error bars represent standard deviation (biological replicates, N = 5).
). Osmolyte concentrations are normalized per cell, error bars represent standard deviation (biological replicates, N = 5).
 ), gonyol (
), gonyol (  ), glycine betaine GBT (
), glycine betaine GBT (  ), homarine (
), homarine (  ), trimethylammonium propionate (TMAP) (
), trimethylammonium propionate (TMAP) (  ), trimethylammonium butyrate (TMAB) (
), trimethylammonium butyrate (TMAB) (  ) and trigonelline (
) and trigonelline (  ). Osmolyte concentrations are normalized per cell, error bars represent standard deviation (biological replicates, N = 5).
). Osmolyte concentrations are normalized per cell, error bars represent standard deviation (biological replicates, N = 5).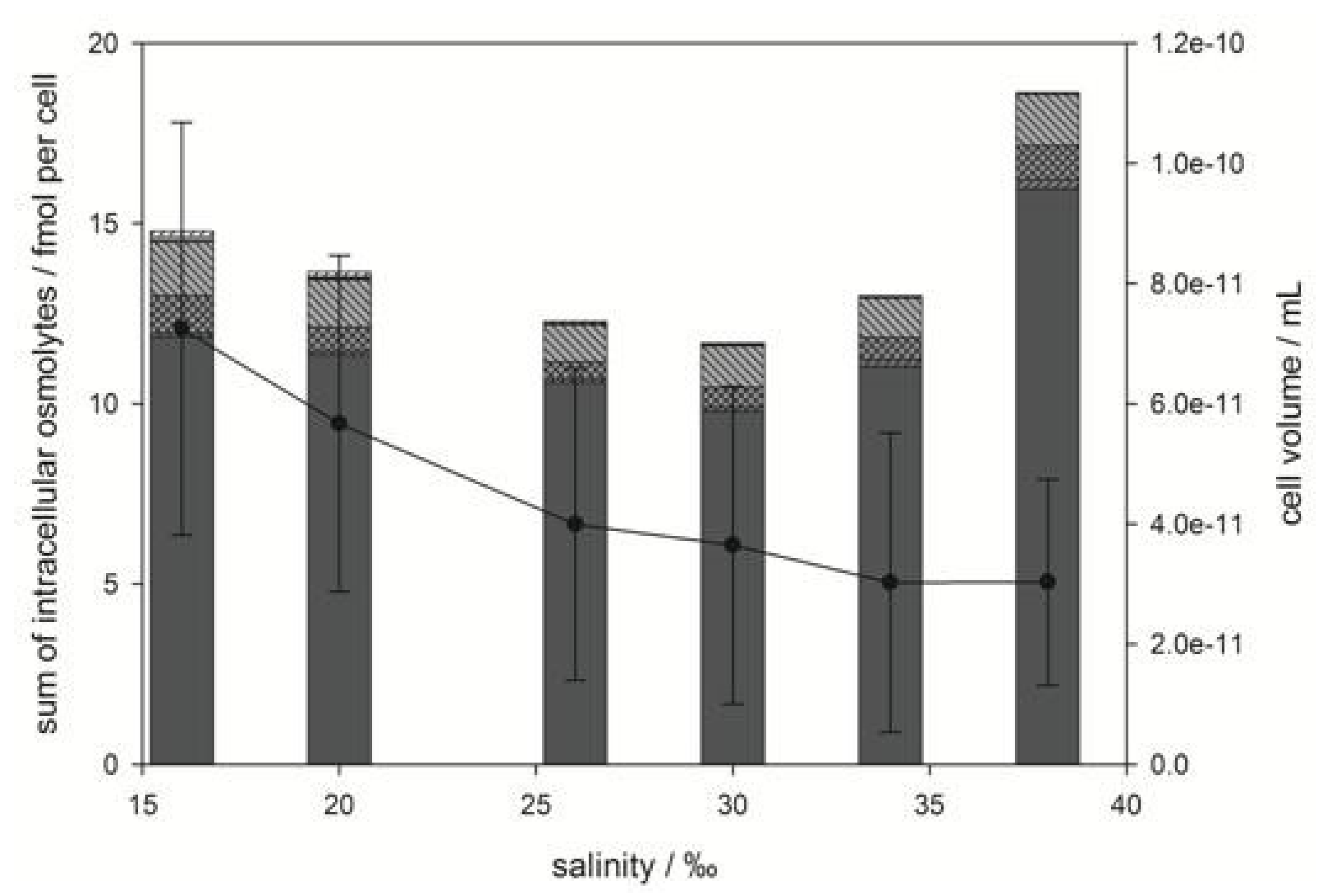
2.2. Prorocentrum minimum
 ), GBT (
), GBT (  ), DMS-Ac (
), DMS-Ac (  ), gonyol (
), gonyol (  ), TMAP (
), TMAP (  ), TMAB (
), TMAB (  ) and trigonelline (
) and trigonelline (  ). Osmolyte concentrations are normalized per cell, error bars represent standard deviation (biological replicates, N = 5).
). Osmolyte concentrations are normalized per cell, error bars represent standard deviation (biological replicates, N = 5).
 ), GBT (
), GBT (  ), DMS-Ac (
), DMS-Ac (  ), gonyol (
), gonyol (  ), TMAP (
), TMAP (  ), TMAB (
), TMAB (  ) and trigonelline (
) and trigonelline (  ). Osmolyte concentrations are normalized per cell, error bars represent standard deviation (biological replicates, N = 5).
). Osmolyte concentrations are normalized per cell, error bars represent standard deviation (biological replicates, N = 5).
3. Experimental Section
3.1. Cultivation of Microalgae
3.2. Cell Counting and Size Measurement
3.3. Sample Preparation
3.4. Equipment
3.5. Osmolyte Analysis
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Kinne, R.K.H. The role of organic osmolytes in osmoregulation—From bacteria to mammals. J. Exp. Zool. 1993, 265, 346–355. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, J.G. Osmotic responses of Dunaliella to the changes of salinity. J. Cell. Physiol. 2009, 219, 251–258. [Google Scholar] [CrossRef]
- Garza-Sanchez, F.; Chapman, D.J.; Cooper, J.B. Nitzschia ovalis (Bacillariophyceae) Mono Lake strain accumulates 1,4/2,5 cyclohexanetetrol in response to increased salinity. J. Phycol. 2009, 45, 395–403. [Google Scholar] [CrossRef]
- Fujii, S.; Nishimoto, N.; Notoya, A.; Hellebust, J.A. Growth and osmoregulation of Chaetoceros muelleri in relation to salinity. Plant Cell Physiol. 1995, 36, 759–764. [Google Scholar]
- Liu, C.-H.; Shih, M.-C.; Lee, T.-M. Free proline levels in Ulva (Chlorophyta) in response to hypersalinity: Elevated NaCl in seawater versus concentrated seawater (note). J. Phycol. 2000, 36, 118–119. [Google Scholar] [CrossRef]
- Dickson, D.M.J.; Kirst, G.O. The role of β-dimethylsulphoniopropionate, glycine betaine and homarine in the osmoacclimation of Platymonas subcordiformis. Planta 1986, 167, 536–543. [Google Scholar] [CrossRef]
- Dickson, D.M.J.; Kirst, G.O. Osmotic adjustment in marine eukaryotic algae—The role of inorganic ions, quaternary ammonium, tertiary sulfonium and carbohydrate solutes. 1. Diatoms and a rhodophyte. New Phytol. 1987, 106, 645–655. [Google Scholar] [CrossRef]
- Dickson, D.M.J.; Kirst, G.O. Osmotic adjustment in marine eukaryotic algae—The role of inorganic ions, quaternary ammonium, tertiary sulfonium and carbohydrate solutes. 2. Prasinophytes and haptophytes. New Phytol. 1987, 106, 657–666. [Google Scholar] [CrossRef]
- Kirst, G.O.; Thiel, C.; Wolff, H.; Nothnagel, J.; Wanzek, M.; Ulmke, R. Dimethylsulfoniopropionate (DMSP) in icealgae and its possible biological role. Mar. Chem. 1991, 35, 381–388. [Google Scholar] [CrossRef]
- Sunda, W.; Kieber, D.J.; Kiene, R.P.; Huntsman, S. An antioxidant function for DMSP and DMS in marine algae. Nature 2002, 418, 317–320. [Google Scholar] [CrossRef]
- Karsten, U.; Wiencke, C.; Kirst, G.O. The effect of salinity changes upon the physiology of eulittoral green macroalgae from antarctica and southern chile. 2. Intracellular inorganic ions and organic compounds. J. Exp. Bot. 1991, 42, 1533–1539. [Google Scholar] [CrossRef]
- Cantoni, G.L.; Anderson, D.G. Enzymatic cleavage of dimethylpropiothetin by Polysiphonia lanosa. J. Biol. Chem. 1956, 222, 171–177. [Google Scholar]
- Turner, S.M.; Malin, G.; Liss, P.S.; Harbour, D.S.; Holligan, P.M. The seasonal variation of dimethyl sulfide and dimethylsulfoniopropionate concentrations in nearshore waters. Limnol. Oceanogr. 1988, 33, 364–375. [Google Scholar] [CrossRef]
- Kiene, R.P.; Linn, L.J.; Bruton, J.A. New and important roles for DMSP in marine microbial communities. J. Sea Res. 2000, 43, 209–224. [Google Scholar] [CrossRef]
- Kettle, A.J.; Andreae, M.O. Flux of dimethylsulfide from the oceans: A comparison of updated data seas and flux models. J. Geophys. Res. Atmos. 2000, 105, 26793–26808. [Google Scholar] [CrossRef]
- Keller, M.D.; Kiene, R.P.; Matrai, P.A.; Bellows, W.K. Production of glycine betaine and dimethylsulfoniopropionate in marine phytoplankton. I. Batch cultures. Mar. Biol. 1999, 135, 237–248. [Google Scholar] [CrossRef]
- Nakamura, H.; Fujimaki, K.; Sampei, O.; Murai, A. Gonyol: Methionine-induced sulfonium accumulation in a dinoflagellate Gonyaulax polyedra. Tetrahedron Lett. 1993, 34, 8481–8484. [Google Scholar] [CrossRef]
- Van Bergeijk, S.A.; van der Zee, C.; Stal, L.J. Uptake and excretion of dimethylsulphoniopropionate is driven by salinity changes in the marine benthic diatom Cylindrotheca closterium. Eur. J. Phycol. 2003, 38, 341–349. [Google Scholar] [CrossRef]
- Cosquer, A.; Pichereau, V.; Pocard, J.A.; Minet, J.; Cormier, M.; Bernard, T. Nanomolar levels of dimethylsulfoniopropionate, dimethylsulfonioacetate, and glycine betaine are sufficient to confer osmoprotection to Escherichia coli. Appl. Environ. Microbiol. 1999, 65, 3304–3311. [Google Scholar]
- Spielmeyer, A.; Gebser, B.; Pohnert, G. Investigations of the uptake of dimethylsulfoniopropionate by phytoplankton. ChemBioChem 2011, 12, 2276–2279. [Google Scholar] [CrossRef]
- Spielmeyer, A.; Pohnert, G. Influence of temperature and elevated carbon dioxide on the production of dimethylsulfoniopropionate and glycine betaine by marine phytoplankton. Mar. Environ. Res. 2012, 73, 62–69. [Google Scholar]
- Thierstein, H.R.; Young, J.R. Coccolithophores; Springer-Verlag: Berlin, Germany, 2004. [Google Scholar]
- Yallop, M.L. Distribution patterns and biomass estimates of diatoms and autotrophic dinoflagellates in the NE Atlantic during June and July 1996. Deep Sea Res. II 2001, 48, 825–844. [Google Scholar] [CrossRef]
- Tango, P.J.; Magnien, R.; Butler, W.; Luckett, C.; Luckenbach, M.; Lacouture, R.; Poukish, C. Impacts and potential effects due to Prorocentrum minimum blooms in Chesapeake Bay. Harmful Algae 2005, 4, 525–531. [Google Scholar] [CrossRef]
- Hernández-Becerril, D.U.; Cortés Altamirano, R.; Alonso, R.R. The dinoflagellate genus Prorocentrum along the coasts of the Mexican Pacific. Hydrobiologia 2000, 418, 111–121. [Google Scholar] [CrossRef]
- Pertola, S.; Kuosa, H.; Olsonen, R. Is the invasion of Prorocentrum minimum (Dinophyceae) related to the nitrogen enrichment of the Baltic Sea? Harmful Algae 2005, 4, 481–492. [Google Scholar]
- Spielmeyer, A.; Pohnert, G. Daytime, growth phase and nitrate availability dependent variations of dimethylsulfoniopropionate in batch cultures of the diatom Skeletonema marinoi. J. Exp. Mar. Biol. Ecol. 2012, 413, 121–130. [Google Scholar] [CrossRef]
- Maier, I.; Calenberg, M. Effect of extracellular Ca2+ and Ca2+-antagonists on the movement and chemoorientation of male gametes of Ectocarpus siliculosus (Phaeophyceae). Bot. Acta 1994, 107, 451–460. [Google Scholar]
- Spielmeyer, A.; Pohnert, G. Direct quantification of dimethylsulfoniopropionate (DMSP) with hydrophilic interaction liquid chromatography/mass spectrometry. J. Chromatogr. B 2010, 878, 3238–3242. [Google Scholar] [CrossRef]
- Gasteiger, E.L.; Haake, P.C.; Gergen, J.A. An investigation of the distribution and function of homarine (N-methyl picolinic acid). Ann. N. Y. Acad. Sci. 1960, 90, 622–636. [Google Scholar] [CrossRef]
- Bandaranayake, W.M.; Bourne, D.J.; Sim, R.G. Chemical composition during maturing and spawning of the sponge Dysidea herbacea (Porifera: Demospongiae). Comp. Biochem. Phys. B 1997, 118, 851–859. [Google Scholar] [CrossRef]
- Blunden, G.; Guiry, M.D.; Druehl, L.D.; Kogame, K.; Kawai, H. Trigonelline and other betaines in species of laminariales. Nat. Prod. Commun. 2012, 7, 863–865. [Google Scholar]
- Blunden, G.; Morse, P.F.; Mathe, I.; Hohmann, J.; Critchley, A.T.; Morrell, S. Betaine yields from marine algal species utilized in the preparation of seaweed extracts used in agriculture. Nat. Prod. Commun. 2010, 5, 581–585. [Google Scholar]
- Blackwell, J.R.; Gilmour, D.J. Physiological response of the unicellular green alga Chlorococcum submarinum to rapid changes in salinity. Arch. Microbiol. 1991, 157, 86–91. [Google Scholar]
- Veldhuis, M.J.W.; Admiraal, W. Influence of phosphate depletion on the growth and colony formation of Phaeocystis pouchetii. Mar. Biol. 1987, 95, 47–54. [Google Scholar] [CrossRef]
- Van Rijssel, M.; Gieskes, W.W.C. Temperature, light, and the dimethylsulfoniopropionate (DMSP) content of Emiliania huxleyi (Prymnesiophyceae). J. Sea Res. 2002, 48, 17–27. [Google Scholar] [CrossRef]
- Bucciarelli, E.; Sunda, W.G.; Belviso, S.; Sarthou, G. Effect of the diel cycle on production of dimethylsulfoniopropionate in batch cultures of Emiliania huxleyi. Aquat. Microb. Ecol. 2007, 48, 73–81. [Google Scholar] [CrossRef]
- Engström-Öst, J.; Repka, S.; Mikkonen, M. Interactions between plankton and cyanobacterium Anabaena with focus on salinity, growth and toxin production. Harmful Algae 2011, 10, 530–535. [Google Scholar]
- Röder, K.; Hantzsche, F.M.; Gebühr, C.; Miene, C.; Helbig, T.; Krock, B.; Hoppenrath, M.; Luckas, B.; Gerdts, G. Effects of salinity, temperature and nutrients on growth, cellular characteristics and yessotoxin production of Protoceratium reticulatum. Harmful Algae 2012, 15, 59–70. [Google Scholar]
Supplementary Files
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gebser, B.; Pohnert, G. Synchronized Regulation of Different Zwitterionic Metabolites in the Osmoadaption of Phytoplankton. Mar. Drugs 2013, 11, 2168-2182. https://doi.org/10.3390/md11062168
Gebser B, Pohnert G. Synchronized Regulation of Different Zwitterionic Metabolites in the Osmoadaption of Phytoplankton. Marine Drugs. 2013; 11(6):2168-2182. https://doi.org/10.3390/md11062168
Chicago/Turabian StyleGebser, Björn, and Georg Pohnert. 2013. "Synchronized Regulation of Different Zwitterionic Metabolites in the Osmoadaption of Phytoplankton" Marine Drugs 11, no. 6: 2168-2182. https://doi.org/10.3390/md11062168
APA StyleGebser, B., & Pohnert, G. (2013). Synchronized Regulation of Different Zwitterionic Metabolites in the Osmoadaption of Phytoplankton. Marine Drugs, 11(6), 2168-2182. https://doi.org/10.3390/md11062168



