Evidence of Anti-Proliferative Activities in Blue Mussel (Mytilus edulis) By-Products
Abstract
:1. Introduction
2. Results and Discussion
2.1. Processing of Blue Mussels
| Fractions | Wet weight (kg) | Dry weight (kg) | Mass balance A (%) |
|---|---|---|---|
| Crude extract (1:1) | 150 | 82.85 | 100 |
| Solid after sieving | 73.50 | 58.87 | 71.06 |
| Liquid after sieving | 190 | 10.05 | 12.13 |
| Solid after centrifugation | 21.75 | 4.33 | 5.22 |
| Liquid phase | 170 | 5.80 | 7.00 |
| 50-kDa ultra-filtration retentate | 25.20 | 1.15 | 1.39 |
| 1-kDa ultra-filtration retentate | 26.50 | 0.64 | 0.77 |
| Nano-filtration retentate | 31.80 | 2.33 | 2.81 |
| Cumulative recovery B | 81.25 |
| Fractions | Dry matter A (%) | Proteins A (%) | Lipids A (%) | Minerals A (%) |
|---|---|---|---|---|
| Crude extract (1:1) | 55.23 ± 1.64 | 10.74 ± 0.37 | 0.97 ± 0.07 | 79.92 ± 1.52 |
| Solid after sieving | 80.10 ± 1.39 | 5.84 ± 0.04 | 0.27 ± 0.02 | 91.29 ± 1.15 |
| Liquid after sieving | 5.29 ± 0.04 | 56.22 ± 4.76 | 8.03 ± 0.60 | 28.75 ± 3.97 |
| Solid after centrifugation | 19.91 ± 0.08 | 45.73 ± 0.20 | 11.08 ± 0.44 | 24.13 ± 1.57 |
| Liquid phase | 3.41 ± 0.00 | 57.77 ± 2.07 | 4.55 ± 1.45 | 21.49 ± 0.00 |
| 50-kDa ultra-filtration retentate | 4.56 ± 0.01 | 56.42 ± 0.09 | 3.18 ± 0.15 | 5.71 ± 0.32 |
| 1-kDa ultra-filtration retentate | 2.42 ± 0.00 | 70.45 ± 0.29 | 4.55 ± 1.75 | 21.49 ± 0.00 |
| Nano-filtration retentate | 7.32 ± 0.00 | 67.55 ± 0.29 | 0.55 ± 0.39 | 15.78 ± 0.29 |
2.2. Chemical Composition of Fractions
2.3. Amino Acid Analyses
| Amino acid A | Centrifugation aqueous phase | 50-kDa ultra-filtration | 1-kDa ultra-filtration | Nano-filtration |
|---|---|---|---|---|
| Essential | ||||
| Histidine | 0.79 ± 0.04 | 1.09 ± 0.04 | 0.91 ± 0.07 | 1.07 ± 0.18 |
| Isoleucine | 1.30 ± 0.04 | 1.41 ± 0.01 | 1.37 ± 0.13 | 1.82 ± 0.03 |
| Leucine | 1.90 ± 0.16 | 1.99 ± 0.03 | 1.99 ± 0.12 | 2.63 ± 0.00 |
| Lysine | 2.20 ± 0.34 | 2.48 ± 0.11 | 2.68 ± 0.08 | 2.80 ± 0.24 |
| Methionine | 0.65 ± 0.15 | 0.37 ± 0.04 | 0.71 ± 0.04 | 0.79 ± 0.01 |
| Phenylalanine | 0.99 ± 0.09 | 1.18 ± 0.06 | 0.92 ± 0.07 | 1.59 ± 0.33 |
| Threonine | 1.45 ± 0.02 | 2.24 ± 0.07 | 1.78 ± 0.08 | 1.80 ± 0.32 |
| Tryptophan | n.a.C | n.a. C | n.a. C | n.a. C |
| Valine | 1.46 ± 0.05 | 1.75 ± 0.04 | 1.61 ± 0.16 | 1.95 ± 0.03 |
| Total (a) | 10.74 | 12.51 | 11.97 | 14.45 |
| Nonessential | ||||
| Alanine | 2.00 ± 0.12 | 1.94 ± 0.01 | 2.32 ± 0.10 | 2.12 ± 0.11 |
| Arginine | 2.53 ± 0.05 | 2.27 ± 0.10 | 2.80 ± 0.24 | 3.86 ± 0.17 |
| Aspartic acid | 3.59 ± 0.41 | 5.08 ± 0.11 | 5.65 ± 0.08 | 4.04 ± 0.05 |
| Cysteine | 0.38 ± 0.02 | 0.67 ± 0.10 | 0.88 ± 0.13 | 0.25 ± 0.03 |
| Glutamic acid | 4.96 ± 0.32 | 6.81 ± 0.09 | 7.52 ± 0.18 | 5.37 ± 0.08 |
| Glycine | 3.63 ± 0.04 | 5.00 ± 0.17 | 4.21 ± 0.24 | 3.06 ± 0.34 |
| Proline | 1.60 ± 0.01 | 2.95 ± 0.11 | 2.36 ± 0.16 | 1.58 ± 0.07 |
| Serine | 1.51 ± 0.07 | 1.94 ± 0.01 | 1.90 ± 0.04 | 1.67 ± 0.16 |
| Taurine B | 3.39 ± 0.05 | 0.58 ± 0.03 | 1.94 ± 0.07 | 4.27 ± 0.53 |
| Tyrosine | 0.91 ± 0.01 | 0.59 ± 0.03 | 1.10 ± 0.03 | 1.51 ± 0.32 |
| Total (b) | 24.50 | 27.83 | 30.68 | 27.73 |
| Grand total (a + b) | 35.24 | 40.34 | 42.65 | 42.18 |
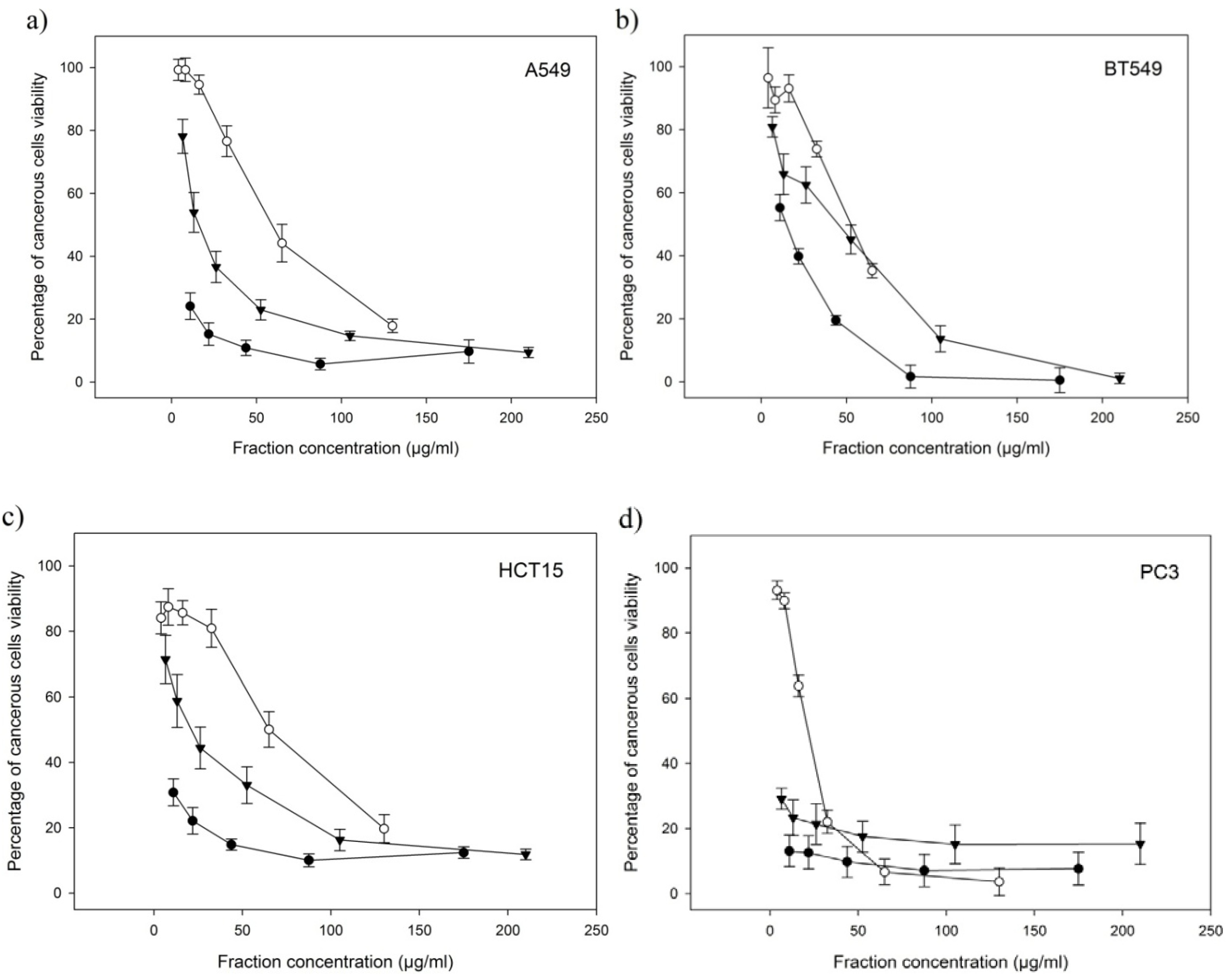
2.4. Anti-Proliferative Activities
3. Experimental Section
3.1. Processing of Blue Mussels
3.2. Fraction Chemical Composition Determination
3.3. Amino Acid Analyses
3.4. Anti-Proliferative Activities
3.4.1. Cell Culture
3.4.2. Sulforhodamine B (SRB) Assay
3.5. Purification of Tested Fractions
4. Conclusions
Acknowledgments
References
- Kim, S.-K.; Wijesekara, I. Development and biological activities of marine-derived bioactive peptides: A review. J. Funct. Foods 2010, 2, 1–9. [Google Scholar] [CrossRef]
- Simmons, T.L.; Andrianasolo, E.; McPhail, K.; Flatt, P.; Gerwick, W.H. Marine natural products as anticancer drugs. Mol. Cancer Ther. 2005, 4, 333–342. [Google Scholar]
- Charlet, M.; Chernysh, S.; Philippe, H.; Hetru, C.; Hoffmann, J.A.; Bulet, P. Innate Immunity: Isolation of several cysteine-rich antimicrobial peptides from the blood of a mollusc, Mytilus edulis. J. Biol. Chem. 1996, 271, 21808–21813. [Google Scholar]
- Mitta, G.; Vandenbulckeb, F.; Rocha, P. Minireview: Original involvement of antimicrobial peptides in mussel innate immunity. FEBS Lett. 2000, 486, 185–190. [Google Scholar] [CrossRef]
- Benkendorff, K. Molluscan biological and chemical diversity: Secondary metabolites and medicinal resources produced by marine molluscs. Biol. Rev. 2010, 85, 757–775. [Google Scholar]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive peptides from marine processing waste and shellfish: A review. J. Funct. Foods 2012, 4, 6–24. [Google Scholar] [CrossRef]
- Picot, L.; Bordenave, S.; Didelot, S.; Fruitier-Arnaudin, I.; Sannier, F.; Thorkelsson, G.; Bergé, J.P.; Guérard, F.; Chabeaud, A.; Piot, J.M. Antiproliferative activity of fish protein hydrolysates on human breast cancer cell lines. Process Biochem. 2006, 41, 1217–1222. [Google Scholar] [CrossRef]
- Benkendorff, K.; McIver, C.; Abbott, C. Bioactivity of the Murex homeopathic remedy and of extracts from an australian muricid mollusc against human cancer cells. Evid. Based Compl. Altern. Med. 2011, 2011, 879585:1–879585:12. [Google Scholar]
- Fisheries and Oceans Canada (DFO). Aquaculture Canada: Facts and Figures. Available online: http://www.dfo-mpo.gc.ca/aquaculture/ref/stats/aqua-ff-fc-2009-eng.htm#ch421 (accessed on 12 May 2012).
- Guérard, F.; Decourcelle, N.; Sbourin, C.; Floch-Laizet, C.; Le Grel, L.; Le Floch, P.; Gourlay, F.; Le Delezir, R.; Jaouen, P.; Bourseau, P. Recent developments of marine ingredients for food and nutraceutical applications: A review. J. Sci. Halieut. Aquat. 2010, 2, 21–27. [Google Scholar]
- Clare, D.A.; Swaisgood, H.E. Bioactive milk peptides: A prospectus. J. Dairy Sci. 2000, 83, 1187–1195. [Google Scholar] [CrossRef]
- Murray, B.A.; FitzGerald, R.J. Angiotensin converting enzyme inhibitory peptides derived from food proteins: Biochemistry, bioactivity and production. Curr. Pharm. Des. 2007, 13, 773–791. [Google Scholar] [CrossRef]
- Elias, R.J.; Kellerby, S.S.; Decker, E.A. Antioxidant activity of proteins and peptides. Crit. Rev. Food Sci. 2008, 48, 430–441. [Google Scholar] [CrossRef]
- Mayer, A.M.S.; Gustafson, K.R. Marine pharmacology in 2005–2006: Antitumour and cytotoxic compounds. Eur. J. Cancer 2008, 44, 2357–2387. [Google Scholar] [CrossRef]
- Korhonen, H.; Pihlanto, A. Food-derived bioactive peptides—opportunities for designing future foods. Curr. Pharm. Des. 2003, 9, 1297–1308. [Google Scholar]
- Cho, S.S.; Lee, H.K.; Yu, C.Y.; Kim, M.J.; Seong, E.S.; Ghimire, B.K.; Son, E.H.; Choung, M.G.; Lim, J.D. Isolation and charaterization of bioactive peptides from hwangtae (yellowish dried alaska pollack) protein hydrolysate. J. Food Sci. Nutr. 2008, 13, 196–203. [Google Scholar] [CrossRef]
- Dumay, J.; Barthomeuf, C.; Berge, J.P. How enzymes may be helpful for upgrading fish by-products: Enhancement of fat extraction. J. Aquat. Food Prod. Technol. 2004, 13, 69–84. [Google Scholar]
- Ruttanapornvareesakul, Y.; Ikeda, M.; Hara, K.; Osatomi, K.; Osako, K.; Kongpun, O.; Nozaki, Y. Concentration-dependent suppressive effect of shrimp head protein hydrolysate on dehydration-induced denaturation of lizardfish myofibrils. Bioresour. Technol. 2006, 97, 762–769. [Google Scholar] [CrossRef]
- Beaulieu, L.; Thibodeau, J.; Bryl, P.; Carbonneau, M.-E. Characterization of enzymatic hydrolyzed snow crab (Chionoecetes opilio) by-product fractions: A source of high-valued biomolecules. Bioresour. Technol. 2009, 100, 3332–3342. [Google Scholar] [CrossRef]
- Liaset, B.; Nortvedt, R.; Lied, E.; Espe, M. Studies on the nitrogen recovery in enzymic hydrolysis of Atlantic salmon (Salmo salar, L.) frames by Protamex™ protease. Process Biochem. 2002, 37, 1263–1269. [Google Scholar]
- Sindayikengera, S.; Xia, W.-S. Nutritional evaluation of caseins and whey proteins and their hydrolysates from Protamex. J. Zhejiang Univ. Sci. B 2006, 7, 90–98. [Google Scholar] [CrossRef]
- Liaset, B.; Julshamn, K.; Espe, M. Chemical composition and theoretical nutritional evaluation of the produced fractions from enzymic hydrolysis of salmon frames with Protamex™. Process Biochem. 2003, 38, 1747–1759. [Google Scholar] [CrossRef]
- Currey, J.D. The design of mineralised hard tissues for their mechanical functions. J. Exp. Biol. 1999, 202, 3285–3294. [Google Scholar]
- Marie, B.; Le Roy, N.; Zanella-Cléon, I.; Becchi, M.; Marin, F. Molecular evolution of mollusc shell proteins: Insights from proteomic analysis of the edible mussel mytilus. J. Mol. Evol. 2011, 72, 531–546. [Google Scholar] [CrossRef]
- Marin, F.; Luquet, G.; Marie, B.; Medakovic, D. Molluscan shell proteins: Primary structure, origin, and evolution. Curr. Top. Dev. Biol. 2007, 80, 209–276. [Google Scholar] [CrossRef]
- Taboada, J.; Pereira-Crespo, S.; Bande-Castro, M.J. Use of Limestone from Mussel Shells in Acid Soil of Galicia (NW Spain). In Treatment and Use of Non-Conventional Organic Residues in Agriculture: Challenges and Opportunities towards Sustainable Management, Proceedings of the 14th Ramiran International Conference, Lisboa, Portugal, 12–15 September 2010; FAO European Cooperative Research Network on the Recycling of Agricultural, Municipal and Industrial Residues in Agriculture: Lisboa, Portugal, 2010. [Google Scholar]
- Wang, X.; Song, A.; Li, L.; Li, X.; Zhang, R.; Bao, J. Effect of calcium carbonate in waste office paper on enzymatic hydrolysis efficiency and enhancement procedures. Korean J. Chem. Eng. 2011, 28, 550–556. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Kassaveti, A. Fish industry waste: Treatments, environmental impacts, current and potential uses. Int. J. Food Sci. Technol. 2008, 43, 726–745. [Google Scholar] [CrossRef]
- Barnaby, C. An Investigation into Reuse of Organic Waste Produced by the New Zealand Mussel Industry. Master Thesis, Auckland University, Auckland, New Zealand, June 2004. [Google Scholar]
- MacLeod, J.A.; Kuo, S.; Gallant, T.L.; Grimmett, M. Seafood processing wastes as nutrient sources for crop production. Can. J. Soil Sci. 2006, 86, 631–640. [Google Scholar]
- Agriculture and Agri-Food Canada (AAFC). Fish and Seafood, Fact Sheets, Blue Mussels. Available online: http://www.ats-sea.agr.gc.ca/sea-mer/4797-eng.htm (accessed on 12 June 2012).
- Hellou, J.; Law, R.J. Stress on stress response of wild mussels, Mytilus edulis and Mytilus trossulus, as an indicator of ecosystem health. Environ. Pollut. 2003, 126, 407–416. [Google Scholar] [CrossRef]
- Lubet, P.; Brichon, G.; Besnard, J.Y.; Zwingelstein, G. Composition and metabolism of lipids in some tissues of the mussel Mytilus galloprovincialis L. (Moll. bivalvia)—In vivo and in vitro incorporation of 1(3)-[3H]-glycerol. Comp. Biochem. Phys. B 1985, 82, 425–431. [Google Scholar]
- Beaulieu, L.; Thibodeau, J.; Bryl, P.; Carbonneau, M.-E. Proteolytic processing of Atlantic mackerel (Scomber scombrus) and biochemical characterisation of hydrolysates. Int. J. Food Sci. Technol. 2009, 44, 1609–1618. [Google Scholar] [CrossRef]
- Liaset, B.; Espe, M. Nutritional composition of soluble and insoluble fractions obtained by enzymatic hydrolysis of fish-raw materials. Process Biochem. 2008, 43, 42–48. [Google Scholar] [CrossRef]
- Kristinsson, H.G.; Rasco, B.A. Hydrolysis of salmon muscle proteins by an enzyme mixture extracted from Atlantic Salmon (Salmo salar) pyloric caeca. J. Food Biochem. 2000, 24, 177–187. [Google Scholar]
- Silverman, H.G.; Roberto, F.F. Understanding marine mussel adhesion. Mar. Biotechnol. 2007, 9, 661–681. [Google Scholar] [CrossRef]
- Bardales, J.R.; Hellman, U.; Villamarın, J.A. Identification of multiple isoforms of the cAMP-dependent protein kinase catalytic subunit in the bivalve mollusc Mytilus galloprovincialis. FEBS J. 2008, 275, 4479–4489. [Google Scholar] [CrossRef]
- Boutet, I.; Moraga, D.; Marinovic, L.; Obreque, J.; Chavez-Crooker, P. Characterization of reproduction-specific genes in a marine bivalve mollusc: Influence of maturation stage and sex on mRNA expression. Gene 2008, 47, 130–138. [Google Scholar]
- Tyler, M.I. Amino Acid Analysis Protocols. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2000; Volume 159, p. 280. [Google Scholar]
- Waite, J.H.; Tanzer, M.L. Polyphenolic substance of Mytilus edulis: Novel adhesive containing l-dopa and hydroxyproline. Science 1981, 212, 1038–1040. [Google Scholar]
- Babarro, J.M.F.; Fernandez Reiriz, M.J.; Garriso, J.L.; Labarta, U. Free amino acid composition in juveniles of Mytilus galloprovincialis: Spatial variability after Prestige oil spill. Comp. Biochem. Phys. A 2006, 145, 204–213. [Google Scholar]
- Bouckenooghe, T.; Remacle, C.; Reusens, B. Is taurine a functional nutrient? Curr. Opin. Clin. Nutr. 2006, 9, 723–733. [Google Scholar]
- Zandee, D.I.; Kluytmans, J.H.; Zurburg, W. Seasonal variations in biochemical composition of Mytilus edulis with reference to energy metabolism and gametogenesis. Neth. J. Sea Res. 1980, 14, 1–29. [Google Scholar] [CrossRef]
- Wright, S.H.; Secomb, T.W. Epidermal taurine transport in marine mussels. Am. J. Physiol. 1984, 247, 346–355. [Google Scholar]
- Allen, K.; Awapara, J. Metabolism of Sulfur Amino Acids in Mytilus edulis and Rangia cuneata. Biol. Bull. 1960, 118, 173–182. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists, Official Methods of Analysis, 17th ed; AOAC: Washington, DC, USA, 2002.
- Blight, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Phys. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M.; Snader, K.M. Natural products as sources of new drugs over the period 1981–2002. J. Nat. Prod. 2003, 66, 1022–1037. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Marine natural products and related compounds in clinical and advanced preclinical trials. J. Nat. Prod. 2004, 67, 1216–1238. [Google Scholar] [CrossRef]
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A.; et al. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Natl. Cancer Inst. 1991, 93, 757–766. [Google Scholar]
- Rubinstein, L.V.; Shoemaker, R.H.; Paull, K.D.; Simon, R.M.; Tosini, S.; Skehan, P.; Scudiero, D.A.; Monks, A.; Boyd, M.R. Comparison of in vitro anticancer-drug-screening data generated with a tetrazolium assay versus a protein assay against a diverse panel of human tumor cell lines. J. Natl. Cancer Inst. 1990, 82, 1113–1117. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Beaulieu, L.; Thibodeau, J.; Bryl, P.; Carbonneau, M.-E. Proteolytic processing of herring (Clupea harengus) and biochemical characterization of hydrolysates. Int. J. Food Sci. Technol. 2009, 44, 2113–2119. [Google Scholar] [CrossRef]
- Bayne, B.L. Marine Mussels: Their Ecology and Physiology; Cambridge University Press: Cambridge, UK, 1976; p. 523. [Google Scholar]
- Wendel, M.; Heller, A.R. Anticancer actions of omega-3 fatty acids—current state and future perspectives. Anticancer Agents Med. Chem. 2009, 9, 457–470. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Beaulieu, L.; Thibodeau, J.; Bonnet, C.; Bryl, P.; Carbonneau, M.-E. Evidence of Anti-Proliferative Activities in Blue Mussel (Mytilus edulis) By-Products. Mar. Drugs 2013, 11, 975-990. https://doi.org/10.3390/md11040975
Beaulieu L, Thibodeau J, Bonnet C, Bryl P, Carbonneau M-E. Evidence of Anti-Proliferative Activities in Blue Mussel (Mytilus edulis) By-Products. Marine Drugs. 2013; 11(4):975-990. https://doi.org/10.3390/md11040975
Chicago/Turabian StyleBeaulieu, Lucie, Jacinthe Thibodeau, Claudie Bonnet, Piotr Bryl, and Marie-Elise Carbonneau. 2013. "Evidence of Anti-Proliferative Activities in Blue Mussel (Mytilus edulis) By-Products" Marine Drugs 11, no. 4: 975-990. https://doi.org/10.3390/md11040975
APA StyleBeaulieu, L., Thibodeau, J., Bonnet, C., Bryl, P., & Carbonneau, M.-E. (2013). Evidence of Anti-Proliferative Activities in Blue Mussel (Mytilus edulis) By-Products. Marine Drugs, 11(4), 975-990. https://doi.org/10.3390/md11040975




