Brominated Skeletal Components of the Marine Demosponges, Aplysina cavernicola and Ianthella basta: Analytical and Biochemical Investigations
Abstract
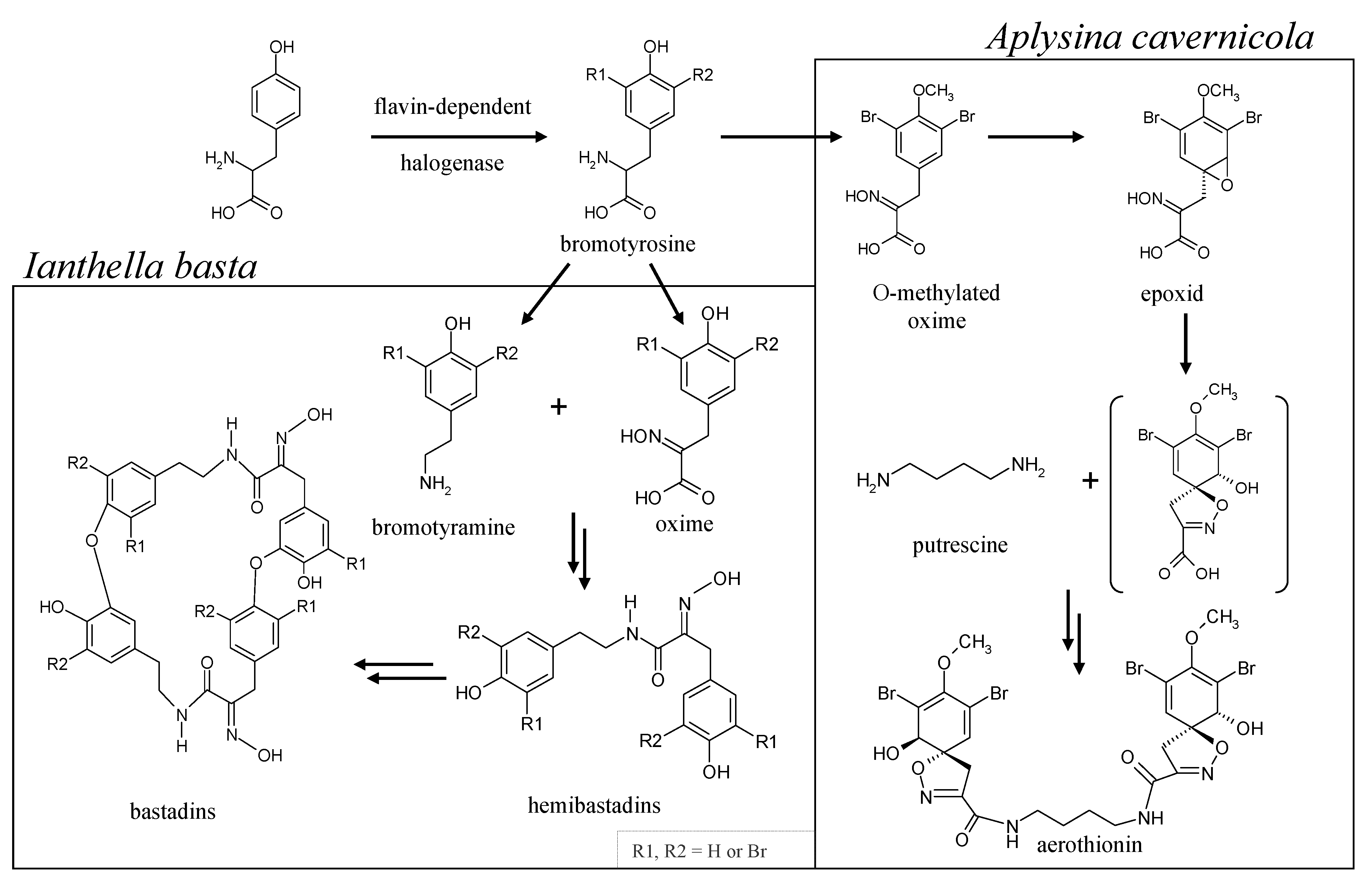
:1. Introduction
2. Results and Discussion
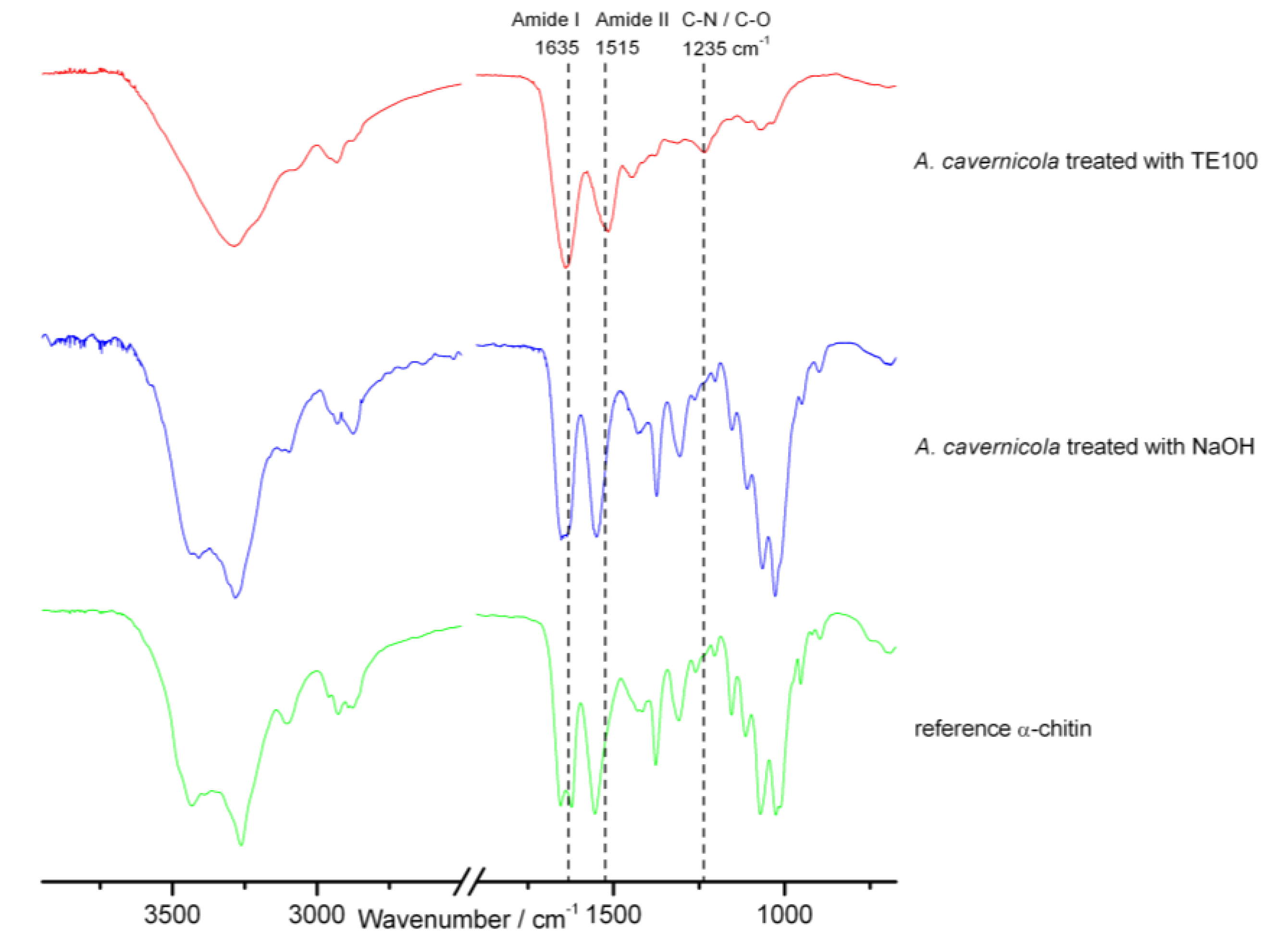
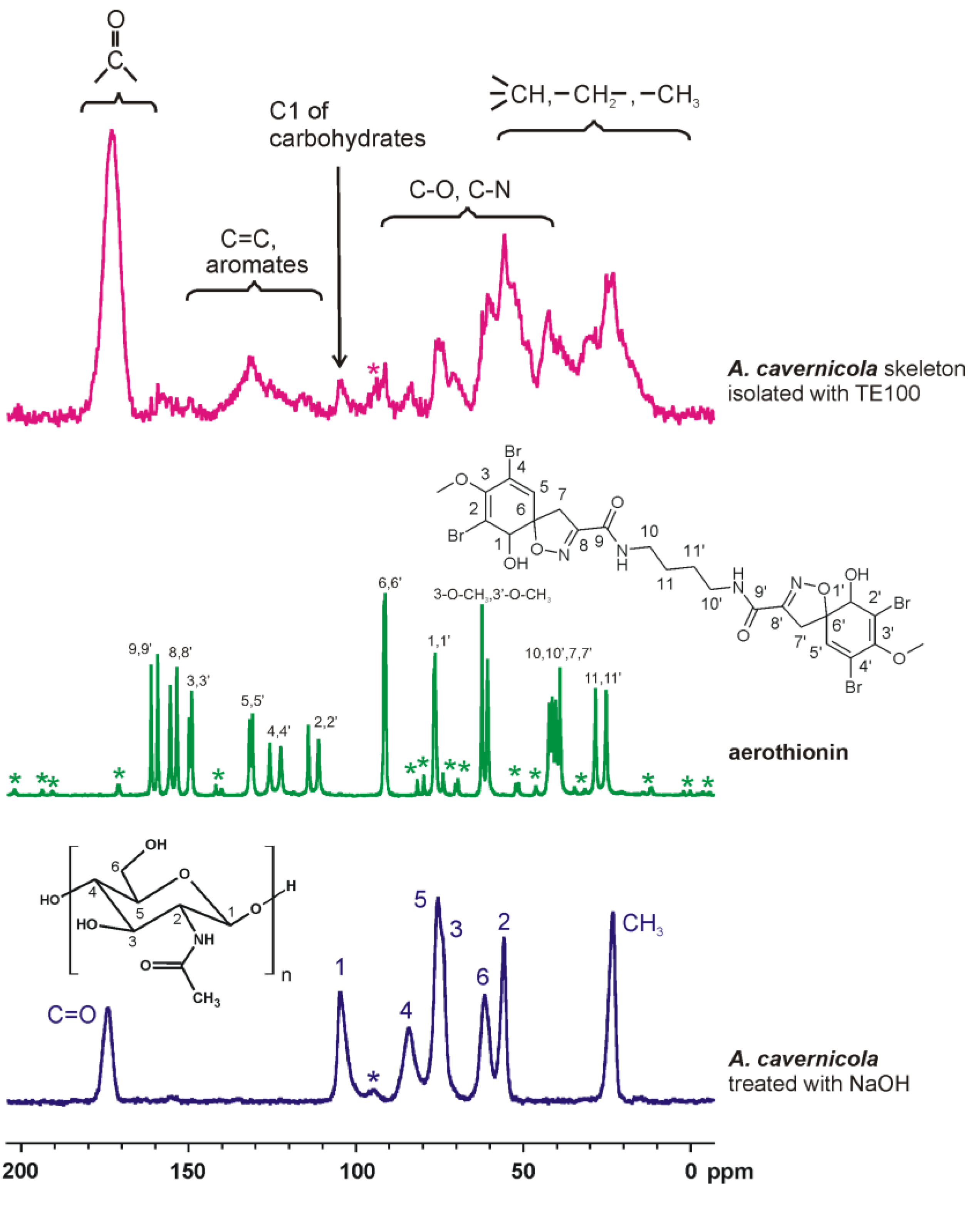
2.1. Isolation and Characterization of the Sponge Skeletons

| A. cavernicola mg Br/g dry weight * | I. basta mg Br/g dry weight * | |
|---|---|---|
| Sponge tissue | 60 ± 5 | 72 ± 10 |
| Skeleton (isolated) | 40 ± 3 | 51 ± 4 |
| Skeleton after MeOH extraction | 35 ± 2 | 44 ± 4 |
| Scaffold after NaOH treatment | 0 | 0 |
2.2. The Effect of MeOH Extraction upon the Isolated Sponge Skeletons
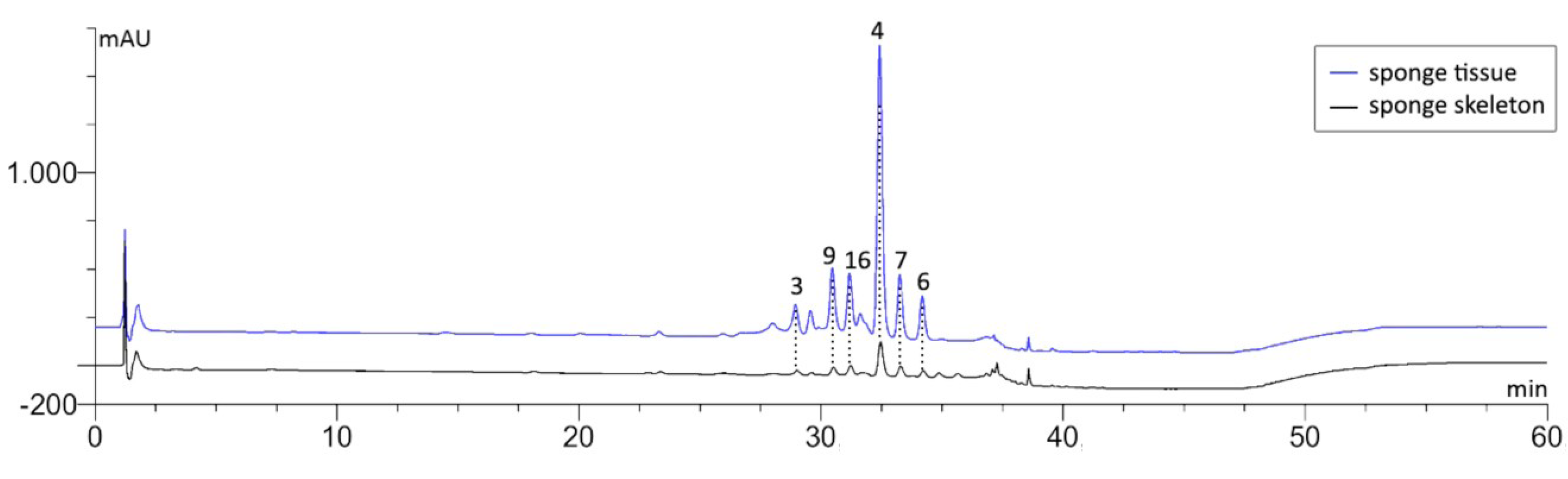
| Bastadin No. | Bastadin Content in Isolated Skeleton/mg g−1 dry weight | Bastadin Content in Sponge Tissue/mg g−1 dry weight |
|---|---|---|
| 3 | 0.02 | 7 |
| 9 | 0.05 | 15 |
| 16 | 0.10 | 14 |
| 4 | 0.21 | 77 |
| 7 | 0.07 | 15 |
| 6 | 0.06 | 10 |
| total | 0.51 | 138 |
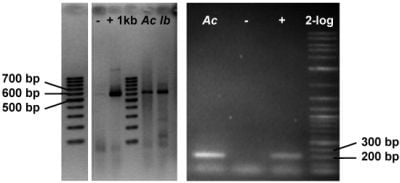
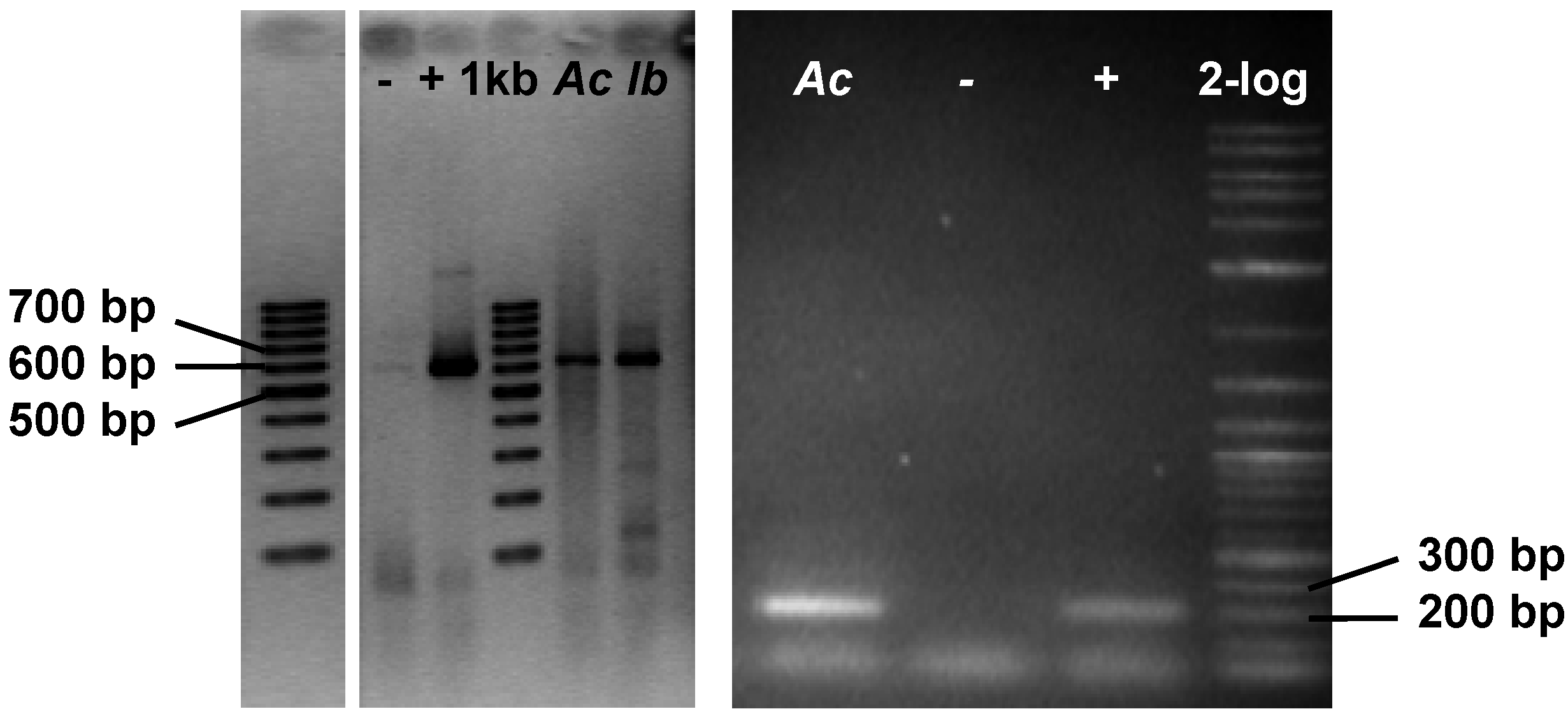
2.3. Genetic Analyses

3. Experimental Section
3.1. Isolation of the Skeletons
3.1.1. Sponge Samples
3.1.2. H2O Extraction
3.1.3. TE100 Extraction
3.1.4. NaOH Extraction
3.1.5. Methanol Extraction of the Purified Skeletons
3.2. HPLC
3.2.1. General Procedures
3.2.2. Identification of Brominated Metabolites
3.2.3. Quantification of Brominated Metabolites
3.3. Light Microscopy
3.4. FTIR Spectroscopy
3.5. NMR Spectroscopy
3.6. Bromine Determination by Potentiometric Titration
3.7. DNA Analysis
3.7.1. Enrichment of Symbiotic Bacteria
3.7.2. Extraction of Metagenomic DNA
3.7.3. Purification of DNA by Size Exclusion Chromatography
3.7.4. Detection of Halogenase Genes
4. Conclusions
- (i)
- Genetic analyses reveal the presence of flavin-dependent halogenase genes in the sponges, A. cavernicola and I. basta. These genes are likely to originate from symbionts of the sponges. This agrees well with the observations of Bayer et al. [23]. Both gene fragments show high similarity to bacterial halogenase genes. It can, therefore, be assumed that the previously identified bromotyrosine derivatives in A. cavernicola and I. basta—aerothionin and bastadins—respectively, are probably of symbiotic origin.
- (ii)
- Moreover, our analytical studies show that the majority of the previously identified bromotyrosine derivatives are not associated with the sponge skeletons. They can easily be removed from the skeletons by TE100 or even H2O treatment. This is in line with the aforementioned conclusion that these compounds are produced by symbionts.
- (iii)
- However, a considerable amount of bromine-bearing organic molecules were found to be MeOH-insoluble. These strongly skeleton-associated compounds withstand the established extraction protocol and remain tightly associated with the sponge skeleton. It is tempting to speculate that these compounds are involved in the chemical defense of the skeleton, e.g., by inhibiting chitinases as discussed in the introduction section or by other biological activities. The extraction of these yet unidentified molecules—which may even be covalently bound to the chitin-based scaffolds—without severe chemical damage remains to be the subject of future work, including the elucidation of their ecological function/biological activity and biosynthetic pathway.
Acknowledgments
Conflict of Interest
References
- Blunt, J.W.; Copp, B.R.; Hu, W.P.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2009, 26, 170–244. [Google Scholar] [CrossRef]
- Montaser, R.; Luesch, H. Marine natural products: A new wave of drugs? Future Med. Chem. 2011, 3, 1475–1489. [Google Scholar] [CrossRef]
- Proksch, P.; Putz, A.; Ortlepp, S.; Kjer, J.; Bayer, M. Bioactive natural products from marine sponges and fungal endophytes. Phytochem. Rev. 2010, 9, 475–489. [Google Scholar] [CrossRef]
- Müller, W.E.G. Origin of metazoa: Sponges as living fossils. Naturwissenschaften 1998, 85, 11–25. [Google Scholar] [CrossRef]
- Li, C.-W.; Chen, J.-Y.; Hua, T.-E. Precambrian sponges with cellular structures. Science 1998, 279, 879–882. [Google Scholar] [CrossRef]
- Thoms, C.; Wolff, M.; Padmakumar, K.; Ebel, R.; Proksch, P. Chemical defense of Mediterranean sponges Aplysina cavernicola and Aplysina aerophoba. Z. Naturforsch. C 2004, 59, 113–122. [Google Scholar]
- Thoms, C.; Ebel, R.; Proksch, P. Activated chemical defense in Aplysina sponges revisited. J. Chem. Ecol. 2006, 32, 97–123. [Google Scholar] [CrossRef]
- Paul, V.J.; Ritson-Williams, R.; Sharp, K. Marine chemical ecology in benthic environments. Nat. Prod. Rep. 2011, 28, 345–387. [Google Scholar] [CrossRef]
- Wehner, R.; Gehring, W.J. Zoologie; Georg Thieme Verlag: Stuttgart, Germany, 2007; pp. 698–699. [Google Scholar]
- Bergquist, P.R.; Cook, S.D.C. Order Verongida Bergquist, 1978. In Systema Porifera: A Guide to the Classification of Sponges; Hooper, J.N.A., van Soest, R.W.M., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; Volume I, pp. 1081–1096. [Google Scholar]
- Ehrlich, H.; Maldonado, M.; Spindler, K.-D.; Eckert, C.; Hanke, T.; Born, R.; Goebel, C.; Simon, P.; Heinemann, S.; Worch, H. First evidence of chitin as a component of the skeletal fibers of marine sponges. Part I. Verongidae (Demospongia: Porifera). J. Exp. Zool. B Mol. Dev. Evol. 2007, 308, 347–356. [Google Scholar]
- Brunner, E.; Ehrlich, H.; Schupp, P.; Hedrich, R.; Hunoldt, S.; Kammer, M.; Machill, S.; Paasch, S.; Bazhenov, V.V.; Kurek, D.V.; et al. Chitin-Based scaffolds are an integral part of the skeleton of the marine demosponge Ianthella basta. J. Struct. Biol. 2009, 168, 539–547. [Google Scholar] [CrossRef]
- Uriz, M.-J.; Turon, X.; Becerro, M.A.; Agell, G. Siliceous spicules and skeleton frameworks in sponges: Origin, diversity, ultrastructural patterns, and biological functions. Microsc. Res. Tech. 2003, 62, 279–299. [Google Scholar] [CrossRef]
- Ehrlich, H.; Ilan, M.; Maldonado, M.; Muricy, G.; Bavestrello, G.; Kljajic, Z.; Carballo, J.L.; Shiaparelli, S.; Ereskovsky, A.V.; Schupp, P.; et al. Three-Dimensional chitin-based scaffolds from Verongida sponges (Demospongiae: Porifera). Part I. Isolation and identification of chitin. Int. J. Biol. Macromol. 2010, 47, 132–140. [Google Scholar] [CrossRef]
- Teeyapant, R.; Woerdenbag, H.; Kreis, P.; Hacker, J.; Wray, V.; Witte, L.; Proksch, P. Antibiotic and cytotoxic activity of brominated compounds from the marine sponge Verongia aerophoba. Z. Naturforsch. C 1993, 48, 939–945. [Google Scholar]
- Faulkner, D.J. Marine pharmacology. Antonie Van Leeuwenhoek 2000, 77, 135–145. [Google Scholar] [CrossRef]
- Weiss, B.; Ebel, R.; Elbrächter, M.; Kirchner, M.; Proksch, P. Defence metabolites from the marine sponge Verongia aerophoba. Biochem. Syst. Ecol. 1996, 24, 1–12. [Google Scholar] [CrossRef]
- Tabudravu, J.N.; Eijsink, V.G.H.; Gooday, G.W.; Jaspars, M.; Komander, D.; Legg, M.; Synstad, B.; van Aalten, D.M.F. Psammaplin A, a Chitinase Inhibitor Isolated from the Fijian Marine Sponge Aplysinella rhax. Bioorg. Med. Chem. 2002, 10, 1123–1128. [Google Scholar] [CrossRef]
- Thomson, J.E.; Barrow, K.D.; Faulkner, D.J. Localization of two brominated metabolites, aerothionin and homoaerothionin, in spherulous cells of the marine sponge Aplysina fistularis (=Verongia thiona). Acta Zool. 1981, 64, 199–210. [Google Scholar]
- Turon, X.; Becerro, M.A.; Uriz, M.J. Distribution of brominated compounds within the sponge Aplysina aerophoba: Coupling X-ray microanalysis with cryofixation techniques. Cell Tissue Res. 2000, 301, 311–322. [Google Scholar] [CrossRef]
- Tymiak, A.A.; Rinehart, L.R., Jr. Biosynthesis of dibromotyrosine-derived antimicrobial compounds by the marine sponge Aplysina fistularis (Verongia aurea). J. Am. Chem. Soc. 1981, 103, 6763–6765. [Google Scholar] [CrossRef]
- Leone-Stumpf, D. Synthesis and Chromatography of [RuCp]+-labelled Diaryl Ether Peptoids as Precursors of the Bastadins from the Marine Sponge Ianthella basta. PhD Thesis, Combined Faculties for the Natural Sciences and for Mathematics, Ruperto-Carola University of Heidelberg, Heidelberg, Germany, November 2011. [Google Scholar]
- Bayer, K.; Scheuermayer, M.; Fieseler, L.; Hentschel, U. Genomic mining for novel FADH2-dependent halogenases in marine sponge-associated microbial consortia. Mar. Biotechnol. 2013, 15, 63–72. [Google Scholar]
- Van Pée, K.-H. Enzymatic chlorination and bromination. Methods Enzymol. 2012, 516, 237–257. [Google Scholar] [CrossRef]
- Chow, T.Y.K. Purification of yeast—E. coli shuttle plasmid suitable for high transformation frequency in E. coli. Nucleic Acids Res. 1989, 17, 8391. [Google Scholar] [CrossRef]
- Bollet, C.; Gevaudan, M.J.; de Lamballerie, X.; Zandotti, C.; de Micco, P. A simple method for the isolation of chromosomal DNA from Gram positive or acid-fast bacteria. Nucleic Acids Res. 1991, 19, 1955. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Zhang, F.; An, H.; Chen, S.; Li, H.; Wang, P.; Wang, X.; Wang, Y.; Yang, H. Investigation on the morphology of precipitated chemicals from TE buffer on solid substrates. Surf. Rev. Lett. 2007, 14, 1121–1128. [Google Scholar] [CrossRef]
- Ouyang, Y.; Dai, S.; Xie, L.; Ravi Kumar, M.S.; Sun, W.; Sun, H.; Tang, D.; Li, X. Isolation of high molecular weight DNA from marine sponge bacteria for BAC library construction. Mar. Biotechnol. 2010, 12, 318–325. [Google Scholar] [CrossRef]
- Schöniger, W. Eine mikroanalytische Schnellbestimmung von Halogenen in organischen Substanzen. Mikrochem. Acta 1995, 1, 123–129. [Google Scholar]
- Ebada, S.S.; Edrada, R.A.; Lin, W.; Proksch, P. Methods of isolation, purification and structural elucidation of bioactive secondary metabolites from marine invertebrates. Nat. Protoc. 2008, 3, 1820–1831. [Google Scholar] [CrossRef]
- Pelzer, S.; Süßmuth, R.; Heckmann, D.; Recktenwald, J.; Huber, P.; Jung, G.; Wohlleben, W. Identification and analysis of the balhimycin biosynthetic gene cluster and its use for manipulating glycopeptide biosynthesis in Amycalotopsis mediterranei DSM5908. Antimicrob. Agents Chemother. 1999, 43, 1565–1573. [Google Scholar]
- Institut für angewandte Hydrobiologie; HYDRA AG; HYDRA Institut für Meereswissenschaften AG; HYDRA Büro für Gewässerökologie Mürle & Ortlepp GbR; HYDRA Wiesloch—Dipl-Biol. Andreas Becker. Available online: http://www.hydra-institute.com (accessed on 10 April 2013).
- Fung, B.M.; Khitrin, A.K.; Ermolaev, J. An improved broadband decoupling sequence for liquid crystals and solids. J. Magn. Reson. 2002, 142, 97–101. [Google Scholar]
- Schirmer, A.; Gadkari, R.; Reeves, C.D.; Ibrahim, F.; DeLong, E.F.; Hutchinson, C.R. Metagenomic analysis reveals diverse polyketide synthase gene clusters in microorganisms associated with the marine sponge Discodermia dissoluta. Appl. Environ. Microbiol. 2005, 71, 4840–4849. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kunze, K.; Niemann, H.; Ueberlein, S.; Schulze, R.; Ehrlich, H.; Brunner, E.; Proksch, P.; Pée, K.-H.V. Brominated Skeletal Components of the Marine Demosponges, Aplysina cavernicola and Ianthella basta: Analytical and Biochemical Investigations. Mar. Drugs 2013, 11, 1271-1287. https://doi.org/10.3390/md11041271
Kunze K, Niemann H, Ueberlein S, Schulze R, Ehrlich H, Brunner E, Proksch P, Pée K-HV. Brominated Skeletal Components of the Marine Demosponges, Aplysina cavernicola and Ianthella basta: Analytical and Biochemical Investigations. Marine Drugs. 2013; 11(4):1271-1287. https://doi.org/10.3390/md11041271
Chicago/Turabian StyleKunze, Kurt, Hendrik Niemann, Susanne Ueberlein, Renate Schulze, Hermann Ehrlich, Eike Brunner, Peter Proksch, and Karl-Heinz Van Pée. 2013. "Brominated Skeletal Components of the Marine Demosponges, Aplysina cavernicola and Ianthella basta: Analytical and Biochemical Investigations" Marine Drugs 11, no. 4: 1271-1287. https://doi.org/10.3390/md11041271
APA StyleKunze, K., Niemann, H., Ueberlein, S., Schulze, R., Ehrlich, H., Brunner, E., Proksch, P., & Pée, K.-H. V. (2013). Brominated Skeletal Components of the Marine Demosponges, Aplysina cavernicola and Ianthella basta: Analytical and Biochemical Investigations. Marine Drugs, 11(4), 1271-1287. https://doi.org/10.3390/md11041271