New Steroids from the Soft Coral Nephthea chabrolii
Abstract
:1. Introduction

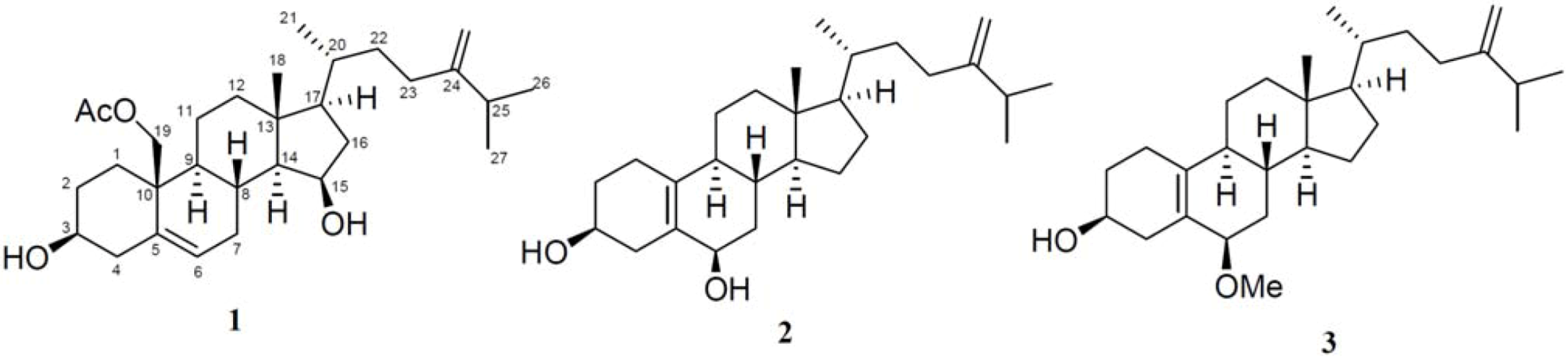
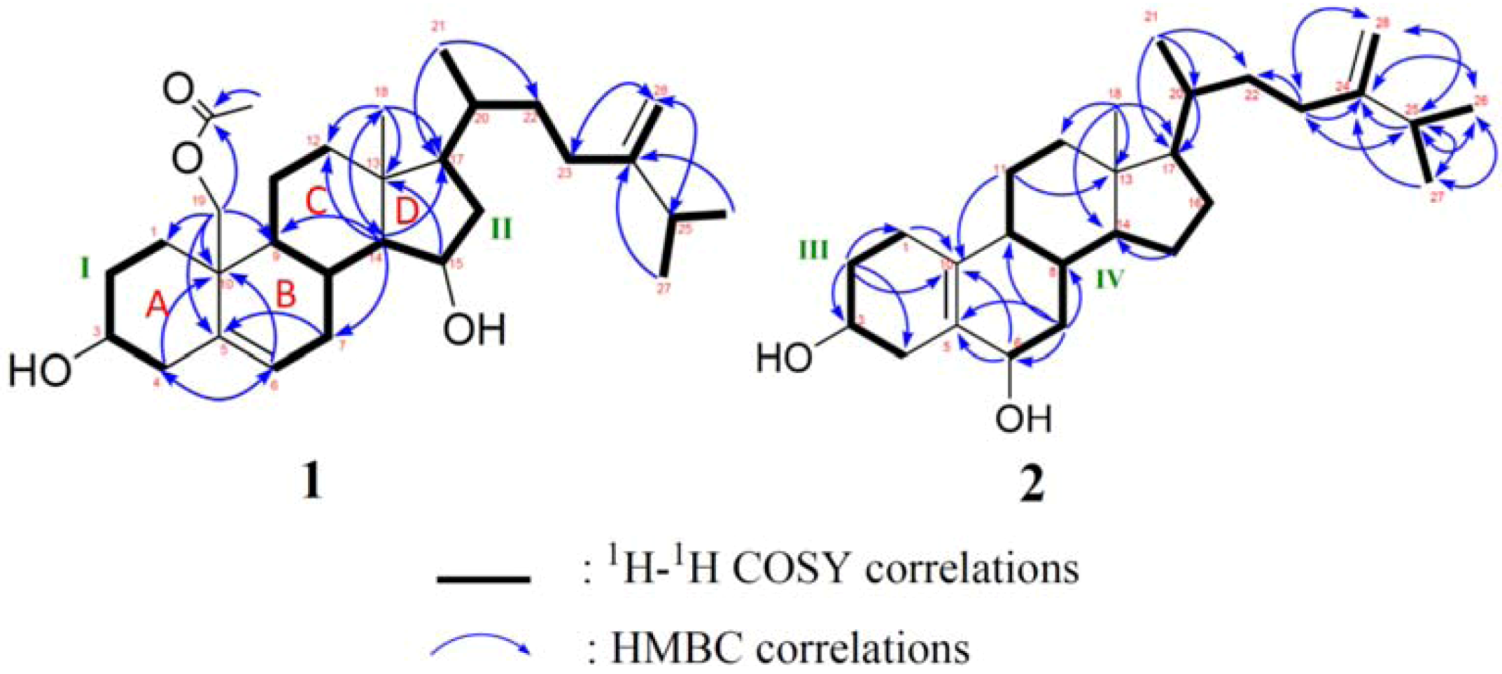
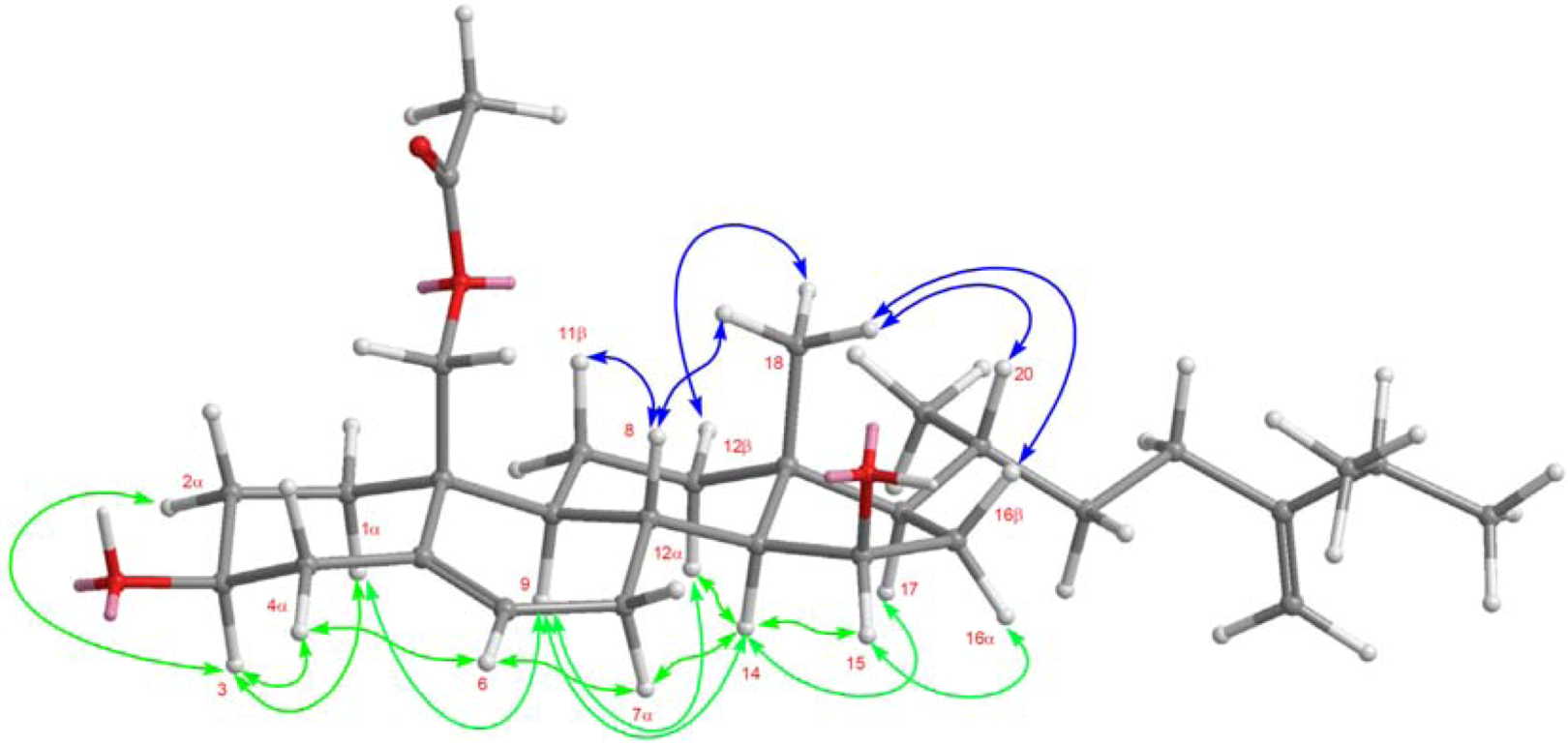
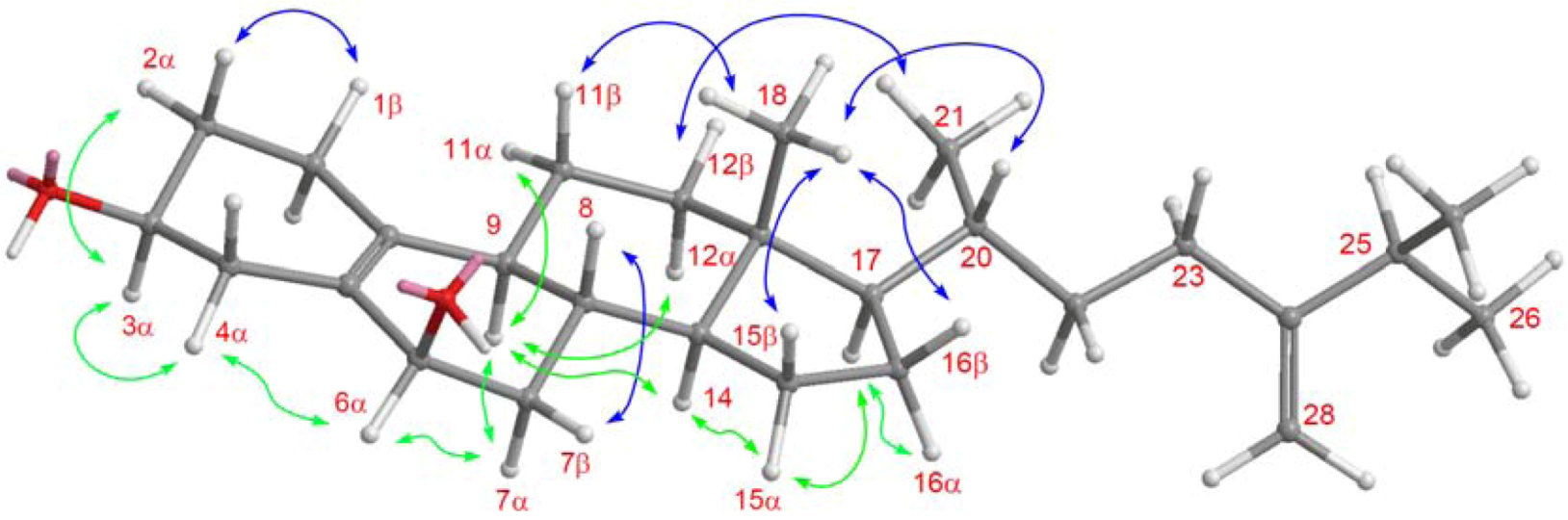
2. Results and Discussion
| position | 1 | 2 | 3 | ||||
|---|---|---|---|---|---|---|---|
| δH a (J in Hz) | δH b (J in Hz) | δC c | δH d (J in Hz) | δC e | δH f (J in Hz) | δC g | |
| 1 | α: 1.10 m | α: 1.09 m | 34.9 | α: 1.90 m | 23.0 | α: 1.88 m | 23.3 |
| β: 2.11 m | β: 2.09 m | β: 2.32 m | β: 2.32 m | ||||
| 2 | α: 1.80 m | α: 1.87 m | 32.5 | α: 1.69 m | 29.7 | α: 1.71 m | 30.0 |
| β: 1.39 m | β: 1.41 m | β: 1.75 m | β: 1.75 m | ||||
| 3 | 3.44 m | 3.57 m | 72.2 | 4.09 m | 65.8 | 4.03 brs | 66.1 |
| 4 | 2.28 m | α: 2.28 m | 43.1 | α: 2.20 m | 36.7 | α: 2.17 m | 37.2 |
| β: 2.26 m | β: 2.40 m | β: 2.36 m | |||||
| 5 | 137.2 | 125.8 | 124.7 | ||||
| 6 | 5.62 d (5.2) | 5.63 d (5.2) | 126.7 | 3.83 brs | 68.6 | 3.31 brs | 78.1 |
| 7 | α: 1.60 m | α: 1.65 m | 31.7 | α: 1.31 m | 36.5 | α: 1.07 m | 30.8 |
| β: 2.32 m | β: 2.23 m | β: 1.82 m | β: 1.98 m | ||||
| 8 | 2.13 m | 2.08 m | 30.0 | 1.50 m | 23.0 | 1.48 m | 33.4 |
| 9 | 1.02 m | 1.02 m | 52.1 | 1.49 m | 46.5 | 1.45 m | 46.4 |
| 10 | 41.1 | 135.4 | 135.6 | ||||
| 11 | 1.58 m | α: 1.50 m | 22.8 | α: 1.84 m | 25.1 | α: 1.82 m | 25.2 |
| β: 1.58 m | β: 1.24 m | β: 1.22 m | |||||
| 12 | α: 1.14 m | α: 1.12 m | 42.7 | α: 1.23 m | 40.2 | α: 1.21 m | 40.2 |
| β: 2.00 m | β: 2.00 m | β: 2.03 m | β: 2.02 m | ||||
| 13 | 43.3 | 43.1 | 43.1 | ||||
| 14 | 0.82 m | 0.83 m | 63.3 | 1.16 m | 54.8 | 1.13 m | 54.9 |
| 15 | 4.14 td | 4.18 td | 70.5 | α: 1.61 m | 23.6 | α: 1.62 m | 23.6 |
| (5.6, 2.0) | (5.6, 2.0) | β: 1.16 m | β: 1.13 m | ||||
| 16 | α: 2.39 m | α: 2.43 m | 42.1 | α: 1.88 m | 28.3 | α: 1.88 m | 28.3 |
| β: 1.34 m | β: 1.33 m | β: 1.30 m | β: 1.30 m | ||||
| 17 | 1.10 m | 1.09 m | 57.6 | 1.17 m | 56.1 | 1.16 m | 56.2 |
| 18 | 0.97 s | 0.97 s | 15.1 | 0.71 s | 12.2 | 0.70 s | 12.2 |
| 19 | 4.02 d (12.0) | 4.02 d (12.0) | 65.6 | ||||
| 4.53 d (12.0) | 4.47 d (12.0) | ||||||
| 20 | 1.55 m | 1.54 m | 36.7 | 1.43 m | 35.7 | 1.44 m | 34.7 |
| 21 | 0.97 d (6.4) | 0.96 d (6.4) | 19.3 | 0.95 d (6.8) | 18.6 | 0.95 d (6.5) | 18.6 |
| 22 | 1.16 m | 1.15 m | 35.9 | 1.16 m | 34.6 | 1.16 m | 34.7 |
| 1.58 m | 1.54 m | 1.55 m | 1.56 m | ||||
| 23 | 1.92 m | 1.89 m | 32.0 | 1.88 m | 30.9 | 1.88 m | 30.9 |
| 2.11 m | 2.08 m | 2.10 m | 2.11 m | ||||
| 24 | 157.7 | 156.8 | 156.9 | ||||
| 25 | 2.22 m | 2.22 m | 34.9 | 2.22 m | 33.8 | 2.24 m | 33.8 |
| 26 | 1.02 d (6.4) | 1.03 d (6.8) | 22.3 | 1.03 d (6.8) | 22.0 | 1.03 d (7.0) | 22.0 |
| 27 | 1.03 d (6.4) | 1.03 d (6.8) | 22.4 | 1.02 d (6.8) | 21.9 | 1.03 d (7.0) | 21.9 |
| 28 | 4.72 s | 4.72 s | 106.9 | 4.72 s | 105.6 | 4.72 s | 105.6 |
| 4.66 s | 4.66 s | 4.66 s | 4.66 s | ||||
| OAc | 2.04 s | 2.06 s | 21.1 | ||||
| 172.7 | |||||||
| OMe | 3.34 s | 57.0 | |||||
| Compounds | ED50 (μg/mL) | Anti-HCMV (IC50; μg/mL) | |||
|---|---|---|---|---|---|
| A549 | HT-29 | P-388 | HEL | ||
| 1 | 6.1 | 8.0 | 1.1 | >100 | >100 |
| 2 | 11.4 | 20.9 | 1.2 | >100 | >100 |
| 3 | 8.7 | 15.3 | 1.0 | >100 | >100 |
| Mithramycin | 0.18 | 0.21 | 0.15 | NT | NT |
| Ganciclovir | NT | NT | NT | NT | 3.3 |
3. Experimental Section
3.1. General Experimental Procedures
3.2. Biological Material
3.3. Extraction and Isolation
3.4. Cytotoxicity Assay
3.5. Anti-HCMV Assay
4. Conclusion
Acknowledgments
References
- Zhang, W.H.; Williams, I.D.; Che, C.T. Chabrolols A, B and C, three new norditerpenes from the soft coral Nephthea chabrolii. Tetrahedron Lett. 2001, 42, 4681–4685. [Google Scholar]
- Hu, J.; Yang, B.; Lin, X.P.; Zhou, X.F.; Yang, X.W.; Long, L.J.; Liu, Y.H. Chemical and biological studies of soft corals of the Nephtheidae family. Chem. Biodivers. 2011, 8, 1011–1032. [Google Scholar] [CrossRef]
- Januar, H.I.; Chasanah, E.; Motti, C.A.; Tapiolas, D.M.; Liptrot, C.H.; Wright, A.D. Cytotoxic cembranes from Indonesian specimens of the soft coral Nephthea sp. Mar. Drugs 2010, 8, 2142–2152. [Google Scholar] [CrossRef]
- Liang, C.H.; Chou, T.H.; Yang, C.C.; Hung, W.J.; Chang, L.C.; Cheng, D.L.; Wang, G.H. Cytotoxic effect of Discosoma sp., Isis hippuris and Nephthea chabrolii on human oral SCC25 cells. J. Taiwan Inst. Chem. Eng. 2010, 41, 333–337. [Google Scholar]
- Duh, C.Y.; Wang, S.K.; Chu, M.J.; Sheu, J.H. Cytotoxic sterols from the soft coral Nephthea erecta. J. Nat. Prod. 1998, 61, 1022–1024. [Google Scholar]
- Duh, C.Y.; Wang, S.K.; Weng, Y.L.; Chiang, M.Y.; Bai, C.F. Cytotoxic terpenoids from the Formosan soft coral Nephthea brassica. J. Nat. Prod. 1999, 62, 1518–1521. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Wang, S.K.; Wen, Z.H.; Dai, C.F.; Duh, C.Y. Three new eudesmanoids from the Formosan soft coral Nephthea erecta. J. Asian Nat. Prod. Res. 2009, 11, 967–973. [Google Scholar]
- Su, J.H.; Ahmed, A.F.; Sung, P.J.; Wu, Y.C.; Sheu, J.H. Meroditerpenoids from a Formosan soft coral Nephthea chabrolii. J. Nat. Prod. 2005, 68, 1651–1655. [Google Scholar]
- Shao, Z.Y.; Zhu, D.Y.; Guo, Y.W. Nanjiols A–C, new steroids from the Chinese soft coral Nephthea bayeri. J. Nat. Prod. 2002, 65, 1675–1677. [Google Scholar] [CrossRef]
- Wang, S.K.; Duh, C.Y. Nardosinane sesquiterpenoids from the Formosan soft coral Nephthea elongata. Chem. Pharm. Bull. 2007, 55, 762–765. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Dai, C.F.; Duh, C.Y. New 4-methylated and 19-oxygenated steroids from the Formosan soft coral Nephthea erecta. Steroids 2007, 72, 653–659. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Huang, Y.C.; Wen, Z.H.; Hsu, C.H.; Wang, S.K.; Dai, C.F.; Duh, C.Y. New 19-oxygenated and 4-methylated steroids from the Formosan soft coral Nephthea chabrolii. Steroids 2009, 74, 543–547. [Google Scholar]
- Huang, Y.C.; Wen, Z.H.; Wang, S.K.; Hsu, C.H.; Duh, C.Y. New anti-inflammatory 4-methylated steroids from the Formosan soft coral Nephthea chabrolii. Steroids 2008, 73, 1181–1186. [Google Scholar]
- Ishii, T.; Zhan, Z.Q.; Vairappan, C.S. A new cembrane diterpene from the Bornean soft coral Nephthea sp. Molecules 2010, 15, 3857–3862. [Google Scholar] [CrossRef]
- El-Gamal, A.A.H.; Wang, S.K.; Dai, C.F.; Duh, C.Y. New nardosinanes and 19-oxygenated ergosterols from the soft coral Nephthea armata collected in Taiwan. J. Nat. Prod. 2004, 67, 1455–1458. [Google Scholar] [CrossRef]
- Handayani, D.; Edrada, R.A.; Proksch, P.; Wray, V.; Witte, L.; van Ofwegen, L.; Kunzmann, A. New oxygenated sesquiterpenes from the Indonesian soft coral Nephthea chabrolii. J. Nat. Prod. 1997, 60, 716–718. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Huang, K.J.; Wang, S.K.; Wen, Z.H.; Hsu, C.H.; Dai, C.F.; Duh, C.Y. New terpenoids from the soft corals Sinularia capillosa and Nephthea chabrolii. Org. Lett. 2009, 11, 4830–4833. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Huang, Y.C.; Wen, Z.H.; Chiou, S.F.; Wang, S.K.; Hsu, C.H.; Dai, C.F.; Duh, C.Y. Novel sesquiterpenes and norergosterol from the soft corals Nephthea erecta and Nephthea chabrolii. Tetrahedron Lett. 2009, 50, 802–806. [Google Scholar]
- Su, J.H.; Lin, F.Y.; Huang, H.C.; Dai, C.F.; Wu, Y.C.; Hu, W.P.; Hsu, C.H.; Sheu, J.H. Novel steroids from the soft coral Nephthea chabrolii. Tetrahedron 2007, 63, 703–707. [Google Scholar]
- El-Gamal, A.A.H.; Wang, S.K.; Duh, C.Y. Prenylbicyclogermacrane diterpenoids from the Formosan soft coral Nephthea elongata. Chem. Pharm. Bull. 2007, 55, 890–893. [Google Scholar] [CrossRef]
- El-Gamal, A.A.H.; Wang, S.K.; Dai, C.F.; Chen, I.G.; Duh, C.Y. Prenylbicyclogermacrane diterpenoids from the Formosan soft coral Nephthea pacifica. J. Nat. Prod. 2005, 68, 74–77. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Wen, Z.H.; Wang, S.K.; Chiang, M.Y.; El-Gamal, A.A.H.; Dai, C.F.; Duh, C.Y. Revision of the absolute configuration at C(23) of lanostanoids and isolation of secondary metabolites from Formosan soft coral Nephthea erecta. Chem. Biodivers. 2009, 6, 86–95. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Dai, C.F.; Duh, C.Y. Sesquiterpenoids and artificial 19-oxygenated steroids from the Formosan soft coral Nephthea erecta. J. Nat. Prod. 2007, 70, 1449–1453. [Google Scholar] [CrossRef]
- Blackman, A.J.; Bowden, B.F.; Coll, J.C.; Frick, B.; Mahendran, M.; Mitchell, S.J. Studies of Australian soft corals XXIX several new cembranoid diterpenes from Nephthea brassica and related diterpenes from a Sarcophyton species. Aust. J. Chem. 1982, 35, 1873–1880. [Google Scholar]
- Su, J.H.; Dai, C.F.; Huang, H.H.; Wu, Y.C.; Sung, P.J.; Hsu, C.H.; Sheu, J.H. Terpenoid-related metabolites from a Formosan soft coral Nephthea chabrolii. Chem. Pharm. Bull. 2007, 55, 594–597. [Google Scholar] [CrossRef]
- Wang, S.-K.; Puu, S.-Y.; Duh, C.-Y. New 19-Oxygenated steroids from the soft coral Nephthea chabrolii. Mar. Drugs 2012, 10, 1288–1296. [Google Scholar] [CrossRef]
- Geran, R.I.; Greenberg, N.H.; MacDonald, M.M.; Schumacher, A.M.; Abbott, B.J. Protocols for screening chemical agents and natural products against animal tumors and other biological syatems. Cancer Chemother. Rep. 1972, 3, 1–91. [Google Scholar]
- Hou, R.-S.; Duh, C.-Y.; Chiang, M.Y.; Lin, C.-N. Sinugibberol, a new cytotoxic cembranoid diterpene from the soft coral Sinularia gibberosa. J. Nat. Prod. 1995, 58, 1126–1130. [Google Scholar] [CrossRef]
- Stevens, M.; Balzarini, J.; Tabarrini, O.; Andrei, G.; Snoeck, R.; Cecchetti, V.; Fravolini, A.; de Clercq, E.; Pannecouque, C. Cell-dependent interference of a series of new 6-aminoquinolone derivatives with viral (HIV/CMV) transactivation. J. Antimicrob. Chemother. 2005, 56, 847–855. [Google Scholar] [CrossRef]
- Samples Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, S.-K.; Puu, S.-Y.; Duh, C.-Y. New Steroids from the Soft Coral Nephthea chabrolii. Mar. Drugs 2013, 11, 571-580. https://doi.org/10.3390/md11020571
Wang S-K, Puu S-Y, Duh C-Y. New Steroids from the Soft Coral Nephthea chabrolii. Marine Drugs. 2013; 11(2):571-580. https://doi.org/10.3390/md11020571
Chicago/Turabian StyleWang, Shang-Kwei, Shyh-Yueh Puu, and Chang-Yih Duh. 2013. "New Steroids from the Soft Coral Nephthea chabrolii" Marine Drugs 11, no. 2: 571-580. https://doi.org/10.3390/md11020571
APA StyleWang, S.-K., Puu, S.-Y., & Duh, C.-Y. (2013). New Steroids from the Soft Coral Nephthea chabrolii. Marine Drugs, 11(2), 571-580. https://doi.org/10.3390/md11020571




