Novel One-Pot Green Synthesis of Indolizines Biocatalysed by Candida antarctica Lipases
Abstract
:1. Introduction
2. Results and Discussion
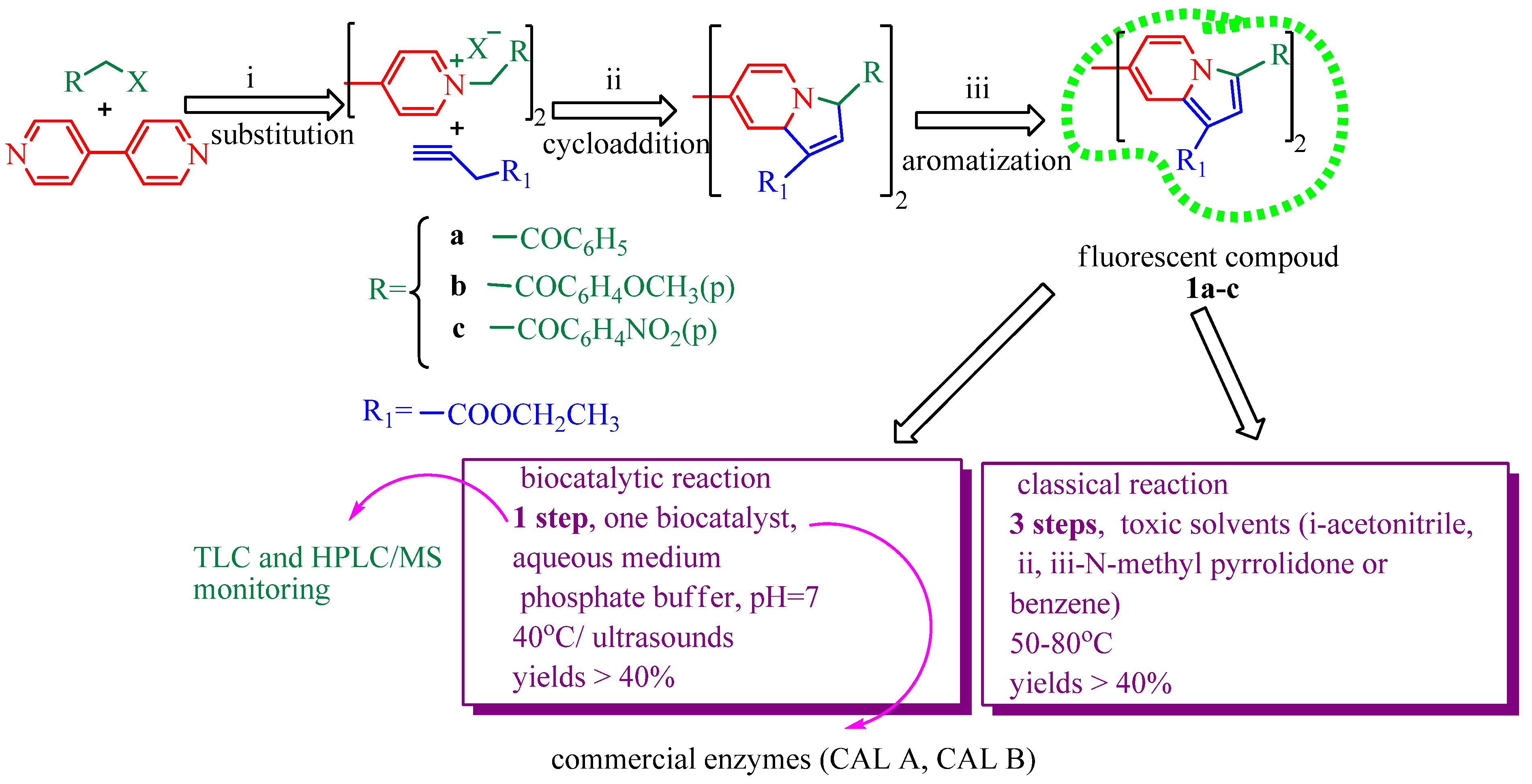
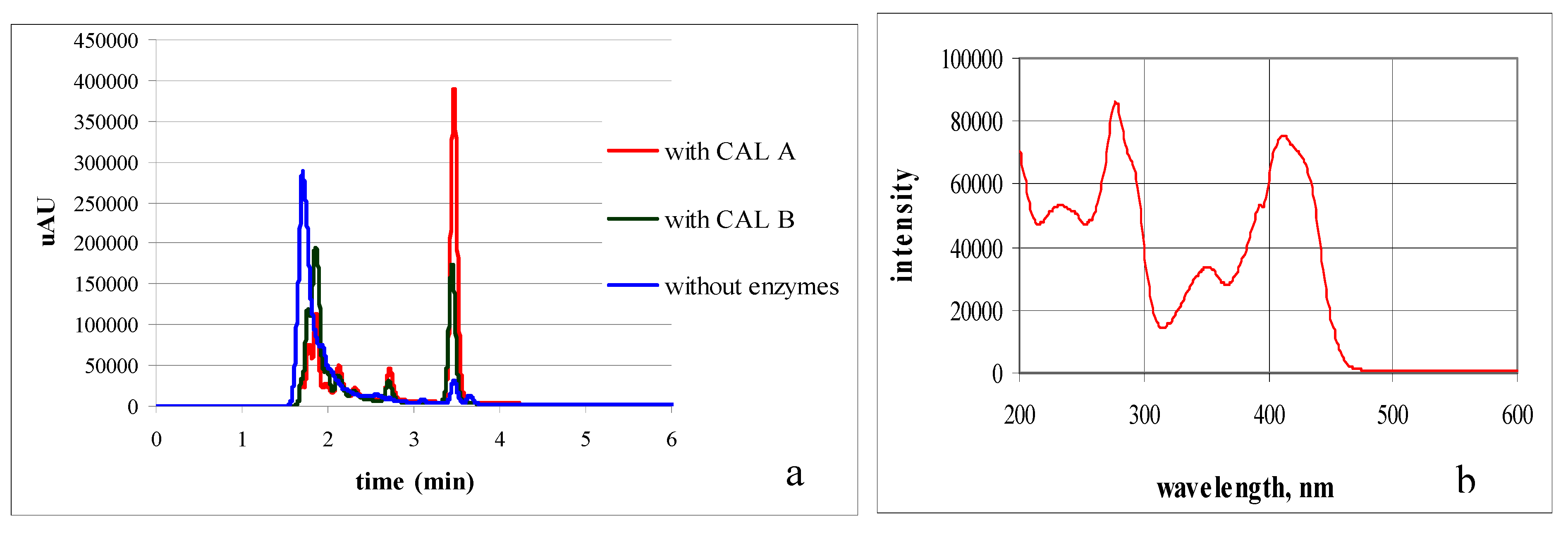
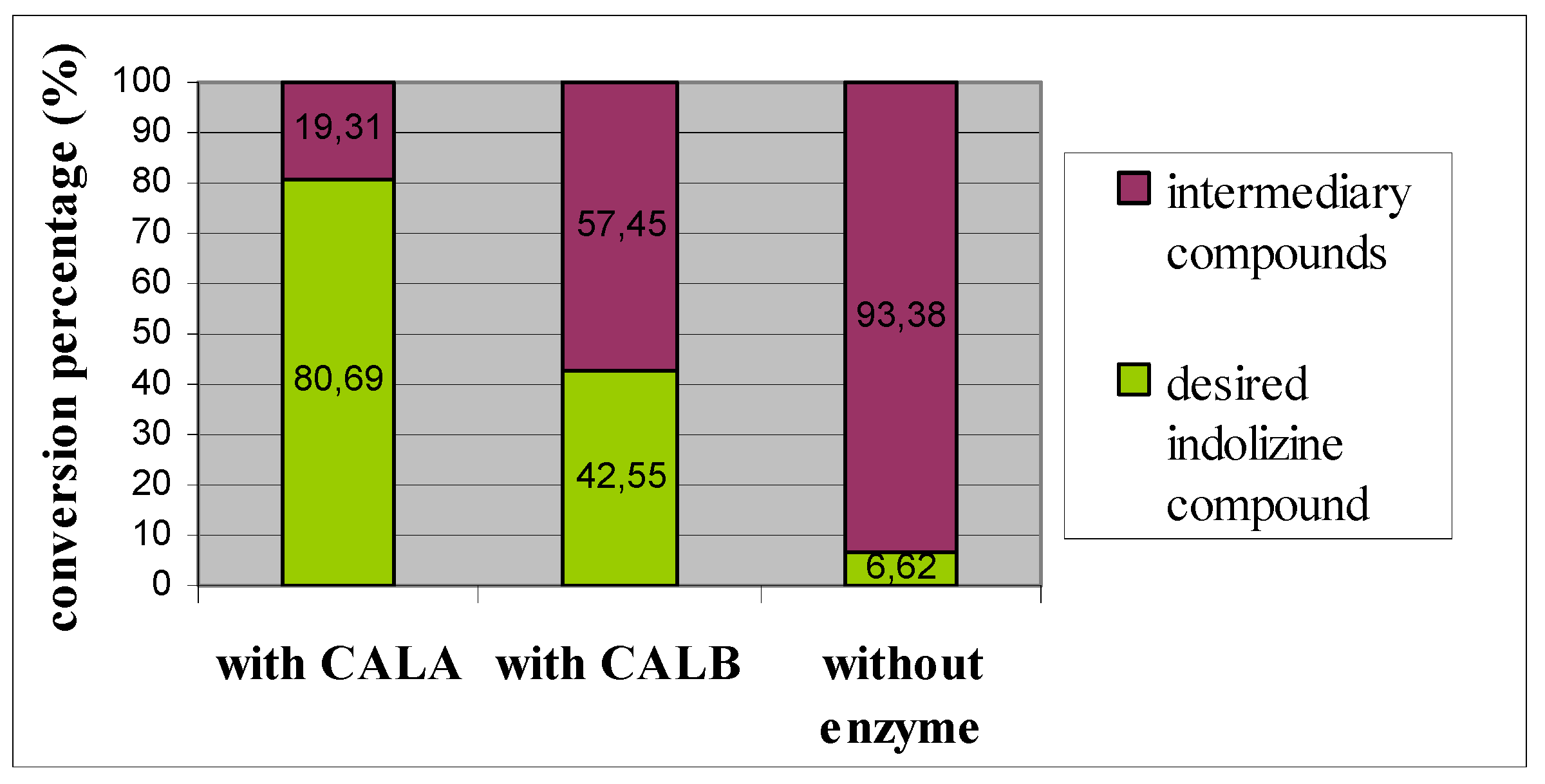
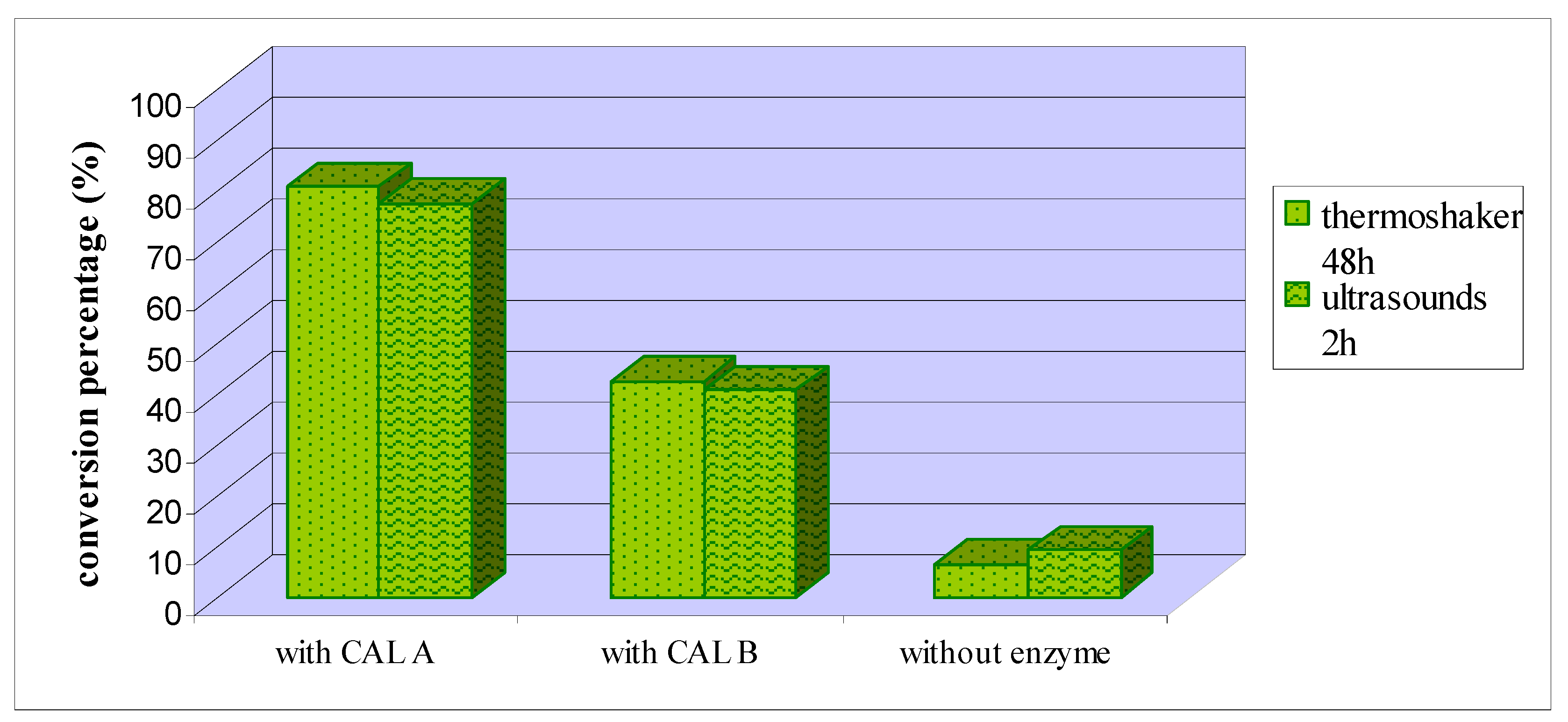
3. Experimental Section
3.1. Enzymes and Chemicals
3.2. Equipment and Chemical Analysis
3.3. Procedure for One-Pot Non-Enzymatic Reactions
3.4. Procedure for One-Pot Enzymatic Reaction
3.5. Procedure for Extraction of Indolizines
3.6. Analysis of the Final Indolizine Products 1a–c
4. Conclusions
Acknowledgements
References
- Flitsch, W. Comprehensive Heterocyclic Chemistry; Katritzky, A.R., Rees, C.W., Eds.; Pergamon Press: Oxford, UK, 1984; Volume 4, p. 443. [Google Scholar]
- Michael, J.P. Indolizidine and quinolizidine alkaloids. Nat. Prod. Rep. 1989, 6, 139–165. [Google Scholar]
- Singh, G.S.; Mmatli, E.E. Recent progress in synthesis and bioactivity studies of indolizines. Eur. J. Med. Chem. 2011, 46, 5237–5257. [Google Scholar] [CrossRef]
- Gundersen, L.-L.; Charnock, C.; Negussie, A.H.; Rise, F.; Teklu, S. Synthesis of indolizine derivatives with selective antibacterial activity against Mycobacterium tuberculosis. Eur. J. Pharm. Sci. 2007, 30, 26–35. [Google Scholar]
- Medda, S.; Jaisankar, P.; Manna, R.; Pal, B.; Giri, V.S.; Basu, M.K. Phospholipid microspheres: A novel delivery mode for targeting antileishmanial agent in experimental leishmaniasis. J. Drug Target 2003, 11, 123–128. [Google Scholar] [CrossRef]
- Gupta, S.P.; Mathur, A.N.; Nagappa, A.N.; Kumar, D.; Kumaran, S. A quantitative structure-activityrelationship study on a novel class of calcium-entry blockers: 1-[(4-(Aminoalkoxy)phenyl)sulphonyl]indolizine. Eur. J. Med. Chem. 2003, 38, 867–873. [Google Scholar] [CrossRef]
- Ostby, O.B.; Dalhus, B.; Gundersen, L.-L.; Rise, F.; Bast, A.; Haenen, G.R.M.M. Synthesis of 1-substituted 7-cyano-2,3-diphenylindolizines and evaluation of antioxidant properties. Eur. J. Org. Chem. 2000, 9, 3763–3770. [Google Scholar]
- Teklu, S.; Gundersen, L.-L.; Larsen, T.; Malterud, K.E.; Rise, F. Indolizine 1-sulfonates as potent inhibitors of 15-lipoxygenase from soybeans. Bioorg. Med. Chem. 2005, 13, 3127–3139. [Google Scholar] [CrossRef]
- Prostakov, N.S.; Baktibaev, O.B. Indolizines. Russ. Chem. Rev. 1975, 44, 748–766. [Google Scholar]
- Kakehi, A. Reactions of pyridinium N-ylides and their related pyridinium salts. Heterocycles 2012, 85, 1529–1577. [Google Scholar] [CrossRef]
- Druta, I.; Dinica, R.; Bacu, E.; Humelnicu, I. Synthesis of 7,7′-bis-Indolizines by the reaction of 4,4′-bipyridinium-ylides with activated alkynes. Tetrahedron 1998, 54, 10811–10818. [Google Scholar] [CrossRef]
- Dinica, R.M.; Pettinari, C. Synthesis of substituted, 7,7′-bis-Indolizines via 1,3-dipolar cycloaddition under microwave irradiation. Synlett 2000, 7, 1013–1015. [Google Scholar]
- Furdui, B.; Dinica, R.M.; Druta, I.; Demeunynck, M. Improved synthesis of cationic pyridinium-substituted indolizines. Synthesis 2006, 16, 2640–2642. [Google Scholar]
- Zhang, C.; Kim, S.-K. Research and application of marine microbial enzymes: Status and prospects. Mar. Drugs 2010, 8, 1920–1934. [Google Scholar] [CrossRef]
- Sanchez-Amat, A.; Solano, F.; Lucas-Elío, P. Finding new enzymes from bacterial physiology: A successful approach illustrated by the detection of novel oxidases in Marinomonas mediterranea. Mar. Drugs 2010, 8, 519–541. [Google Scholar] [CrossRef]
- Ferreira, H.V.; Rocha, L.C.; Severino, R.P.; Porto, A.L.M. Syntheses of enantiopure aliphatic secondary alcohols and acetates by bioresolution with lipase B from Candida antarctica. Molecules 2012, 17, 8955–8967. [Google Scholar] [CrossRef]
- Raminelli, C.; Kagohara, E.; Pellizari, V.H.; Comasseto, J.V.; Andrade, L.H.; Porto, A.L.M. Biotransformations of Mannich bases and propiophenones by Brazilian microorganisms and enzymatic resolution of phenylpropanols by lipase from Candida antarctica (Novozym 435). Enzyme Microb. Technol. 2007, 40, 362–369. [Google Scholar] [CrossRef]
- Cardillo, G.; Gennari, A.; Gentilucci, L.; Mosconi, E.; Tolomelli, A.; Troisi, S. Synthesis of chiral non-racemic intermediates and Arg-Gly-Asp mimetics by CaLB-catalyzed resolution. Tetrahedron Asymmetry 2010, 2, 96–102. [Google Scholar]
- Georgescu, E.; Caira, M.R.; Georgescu, F.; Draghici, B.; Popa, M.M.; Dumitrascu, F. One-pot, three-component synthesis of a library of new pyrrolo[1,2-a]quinoline derivatives. Synlett 2009, 11, 1795–1799. [Google Scholar]
- Bora, U.; Saikia, A.; Boruah, R.C. A novel microwave-mediated one-pot synthesis of indolizines via a three-component reaction. Org. Lett. 2003, 5, 435–438. [Google Scholar] [CrossRef]
- Siddiqui, S.K.; Cavicchioli, R. Improved thermal stability and activity in the cold-adapted lipase B from Candida antarctica following chemical modification with oxidized, polysaccharides. Extremophiles 2005, 9, 471–476. [Google Scholar] [CrossRef]
- Samples Availability: Available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dinica, R.M.; Furdui, B.; Ghinea, I.O.; Bahrim, G.; Bonte, S.; Demeunynck, M. Novel One-Pot Green Synthesis of Indolizines Biocatalysed by Candida antarctica Lipases. Mar. Drugs 2013, 11, 431-439. https://doi.org/10.3390/md11020431
Dinica RM, Furdui B, Ghinea IO, Bahrim G, Bonte S, Demeunynck M. Novel One-Pot Green Synthesis of Indolizines Biocatalysed by Candida antarctica Lipases. Marine Drugs. 2013; 11(2):431-439. https://doi.org/10.3390/md11020431
Chicago/Turabian StyleDinica, Rodica Mihaela, Bianca Furdui, Ioana Otilia Ghinea, Gabriela Bahrim, Simon Bonte, and Martine Demeunynck. 2013. "Novel One-Pot Green Synthesis of Indolizines Biocatalysed by Candida antarctica Lipases" Marine Drugs 11, no. 2: 431-439. https://doi.org/10.3390/md11020431
APA StyleDinica, R. M., Furdui, B., Ghinea, I. O., Bahrim, G., Bonte, S., & Demeunynck, M. (2013). Novel One-Pot Green Synthesis of Indolizines Biocatalysed by Candida antarctica Lipases. Marine Drugs, 11(2), 431-439. https://doi.org/10.3390/md11020431







