Cytotoxic, Cytostatic and HIV-1 PR Inhibitory Activities of the Soft Coral Litophyton arboreum
Abstract
:1. Introduction
2. Results and Discussion
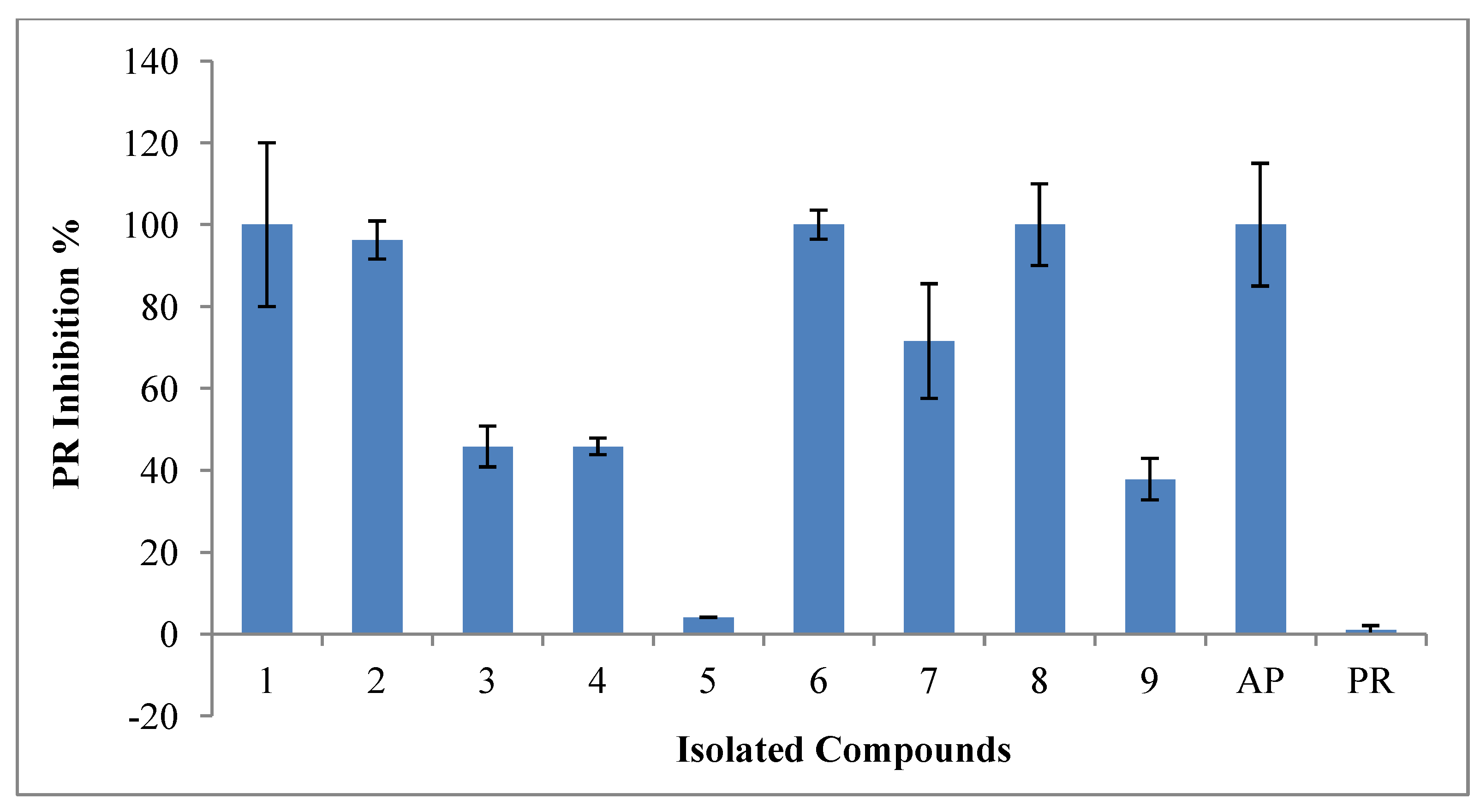
2.1. Bioactivity of Isolated Compounds
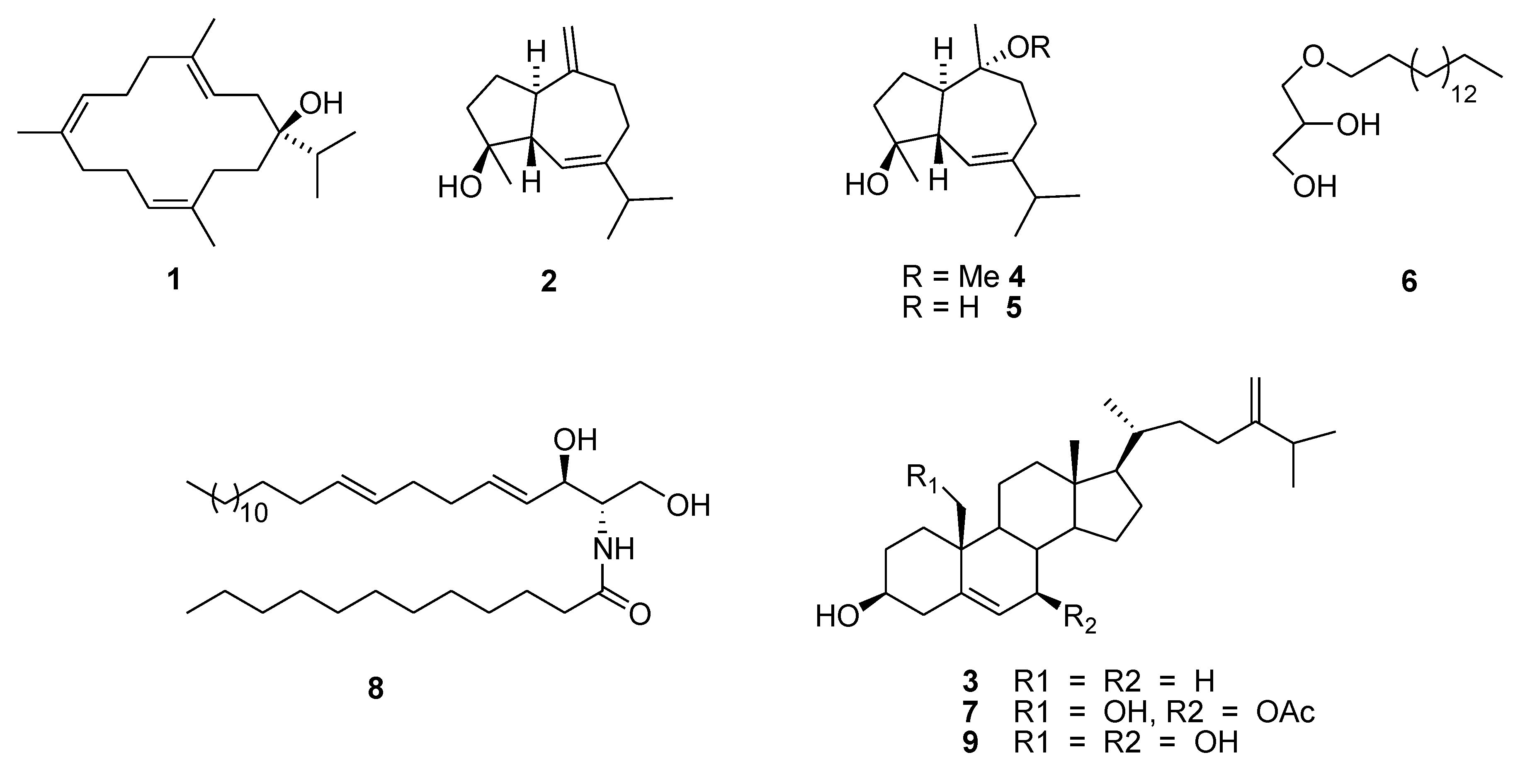
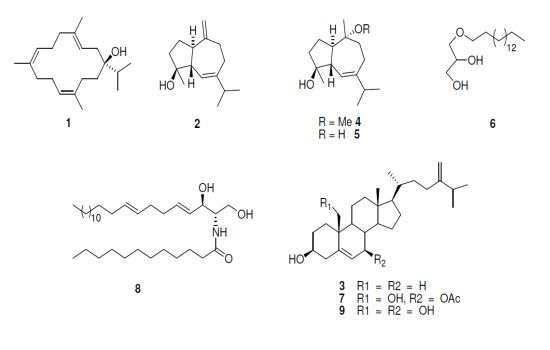
2.2. Cytotoxicity
| Compound | Cytotoxicity IC50 (µM) | SI Values | IC50 (µM) | |||
|---|---|---|---|---|---|---|
| HeLa | Vero | U937 | HeLa | U937 | HIV-1 PR | |
| 1 | 27.5 ± 0.2 | 22 ± 0.2 | 31.7 ± 3.2 | 0.8 | 0.75 | 15.7 ± 0.10 |
| 2 | 30 ± 17.2 | 49 | N/T | 1.6 | N/T | 7.2 ± 0.7 |
| 3 | 48 ± 8.7 | 100 ± 1.2 | N/T | 2.1 | N/T | N/T |
| 4 | 38 ± 0.7 | 49.8 ± 0.5 | 50 ± 0.23 | 1.3 | 1.00 | N/T |
| 5 | >100 | >100 | >100 ± 0.04 | N/A | N/A | N/T |
| 6 | 23.35 ± 5.8 | 60 ± 1.14 | N/T | 2.6 | N/T | 26.6 ± 2.6 |
| 7 | 5.3 ± 0.6 | 31.3 ± 14.03 | 10.6 ± 0.12 | 7.2 | 2.9 | 4.85 ± 0.18 |
| 8 | 38.17 ± 0.7 | >100 | N/T | 2.6 | 2.95 | 4.8 ± 0.92 |
| 9 | 8 ± 0.5 | 11.4 ± 0.04 | 16.4 ± 1.25 | 1.4 | 1.3 | N/T |
| Positive control | ||||||
| Actinomycin D | 5.1 ± 0.1 | 8.8 ± 2.5 | 1.9 ± 0.865 | 1.7 | 4.72 | |
| Acetyl pepstatin | 8.5 ± 0.72 | |||||
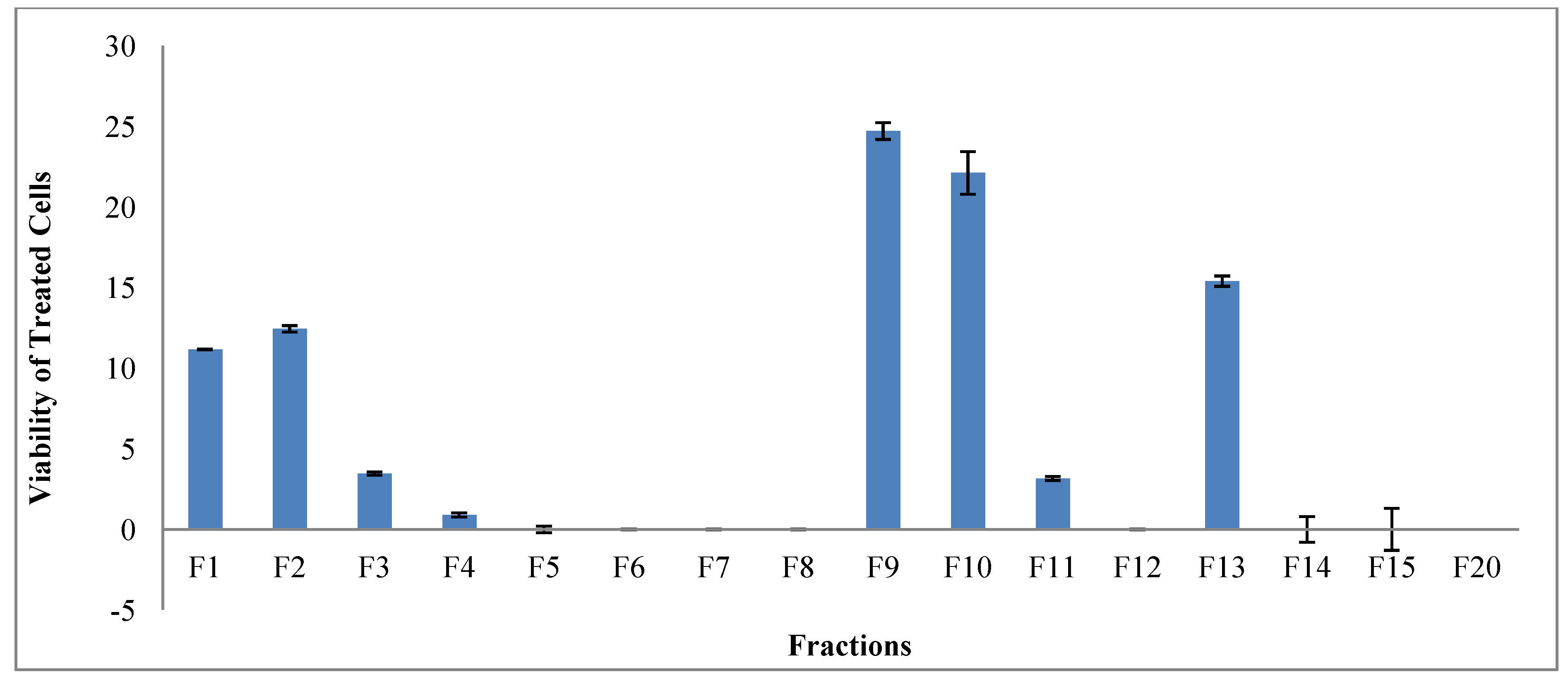
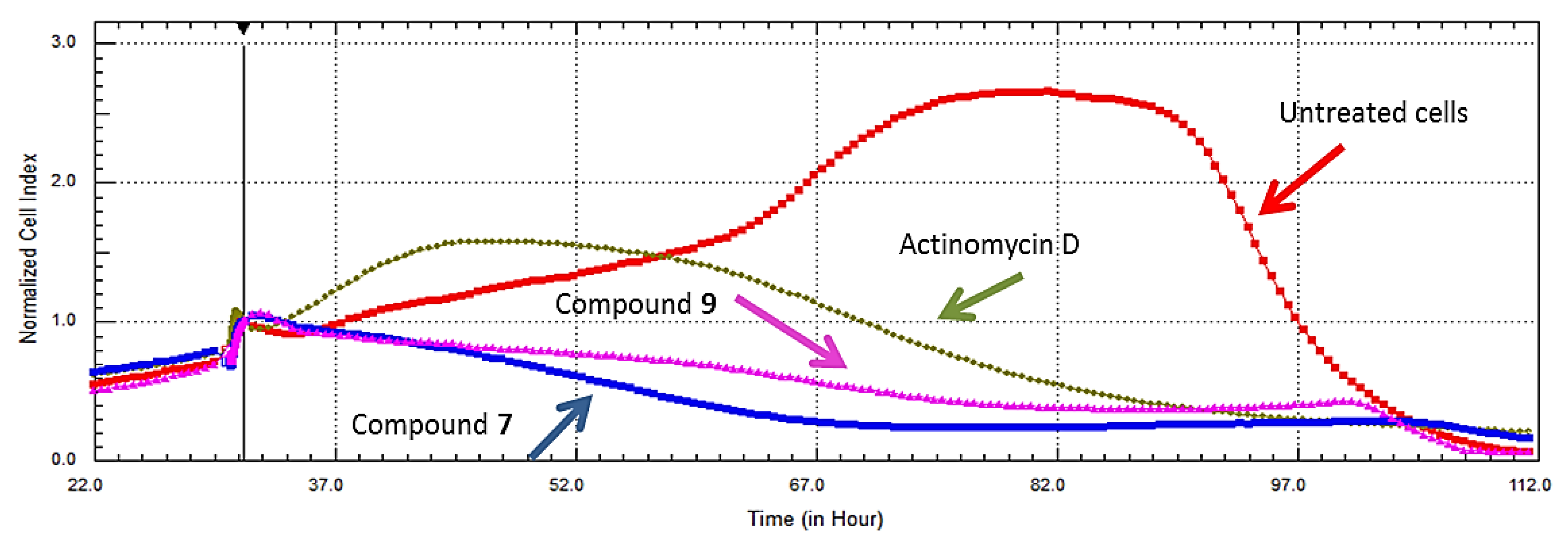
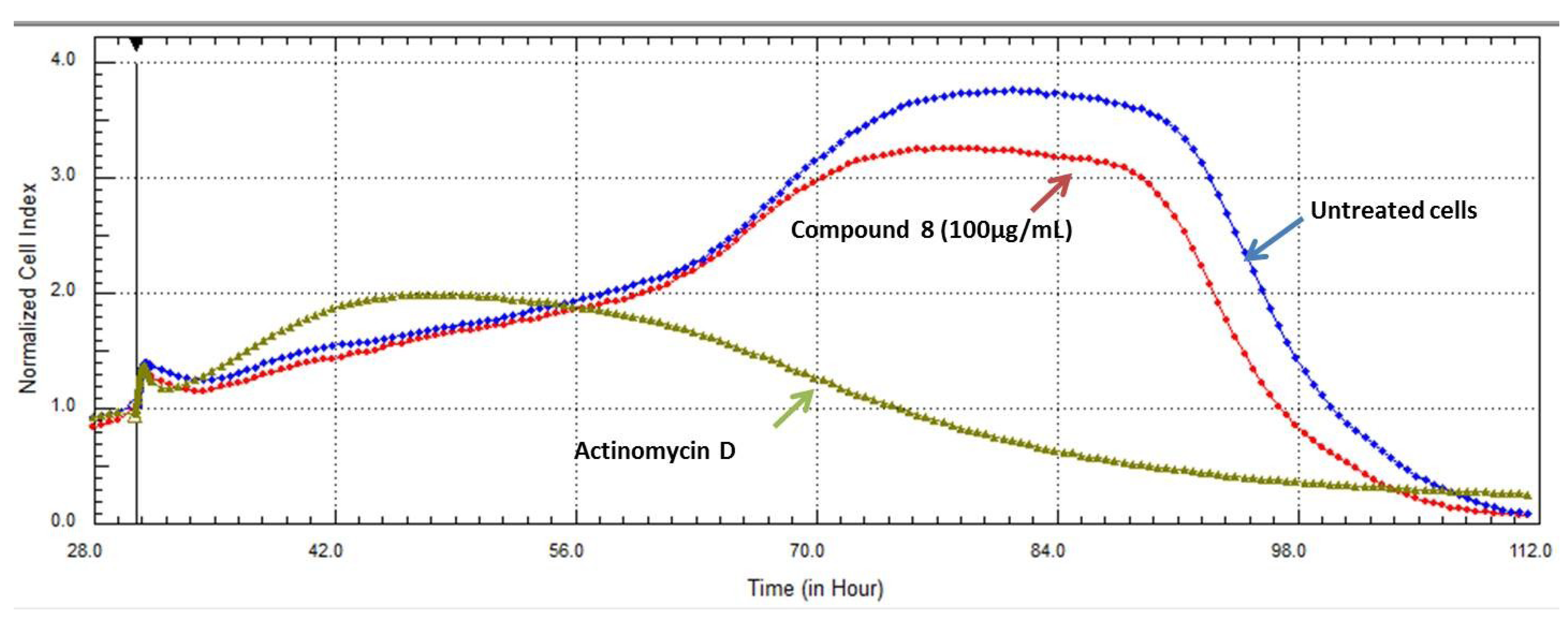
2.3. Real Time Cell Analysis
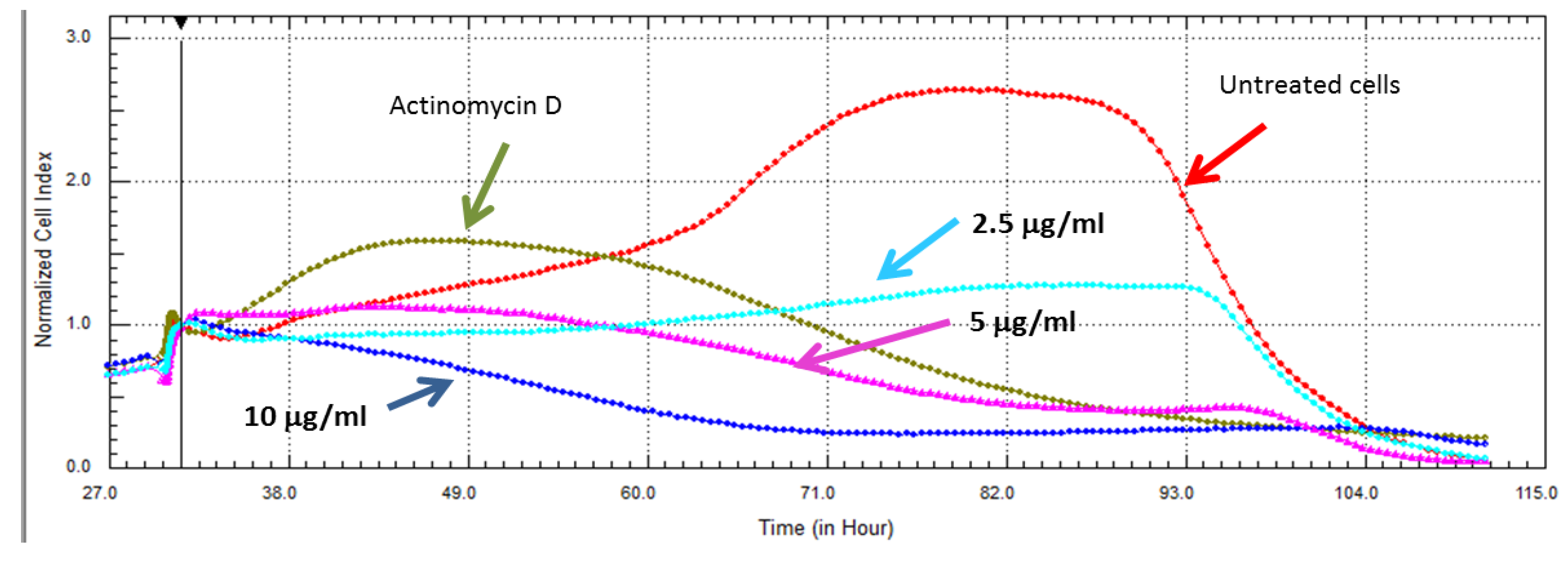
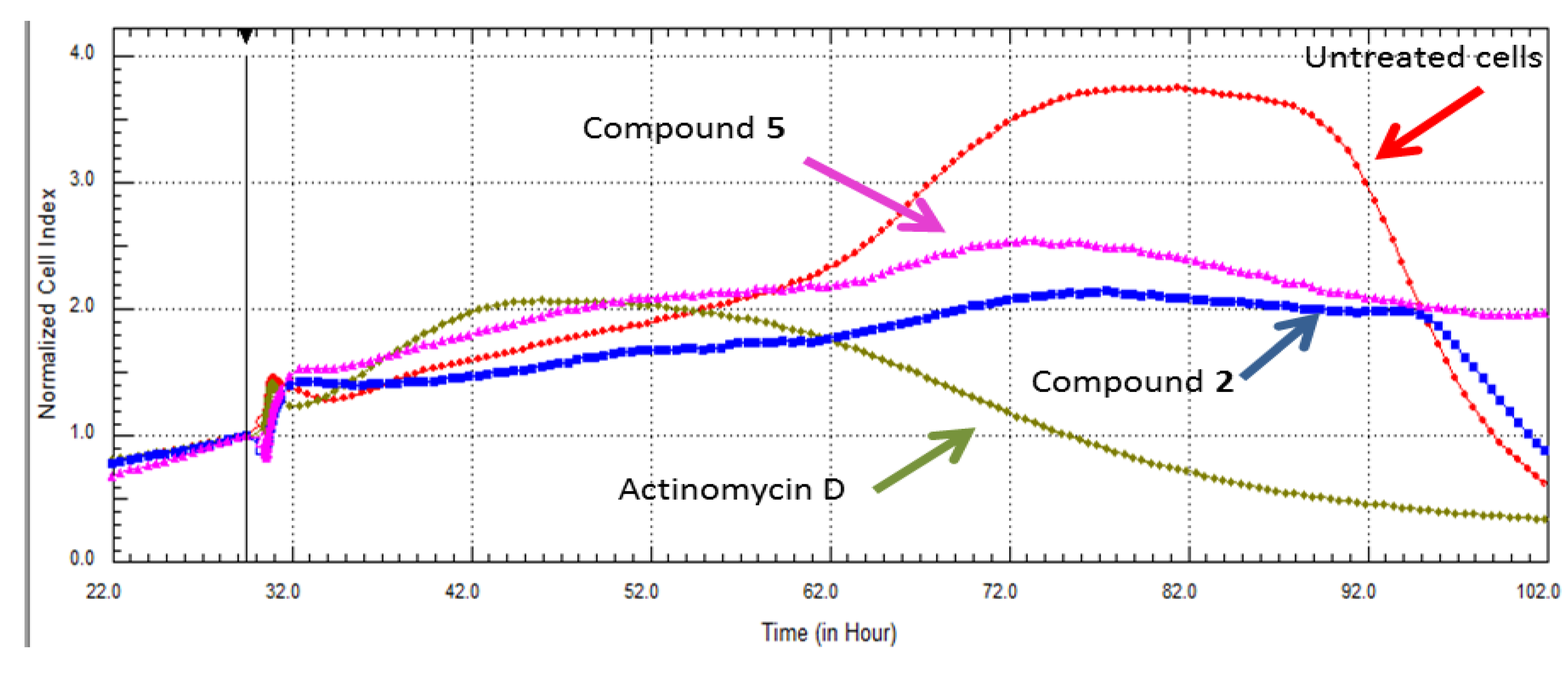
2.4. Inhibitory Activities of HIV-1 Enzymes
2.5. Molecular Docking
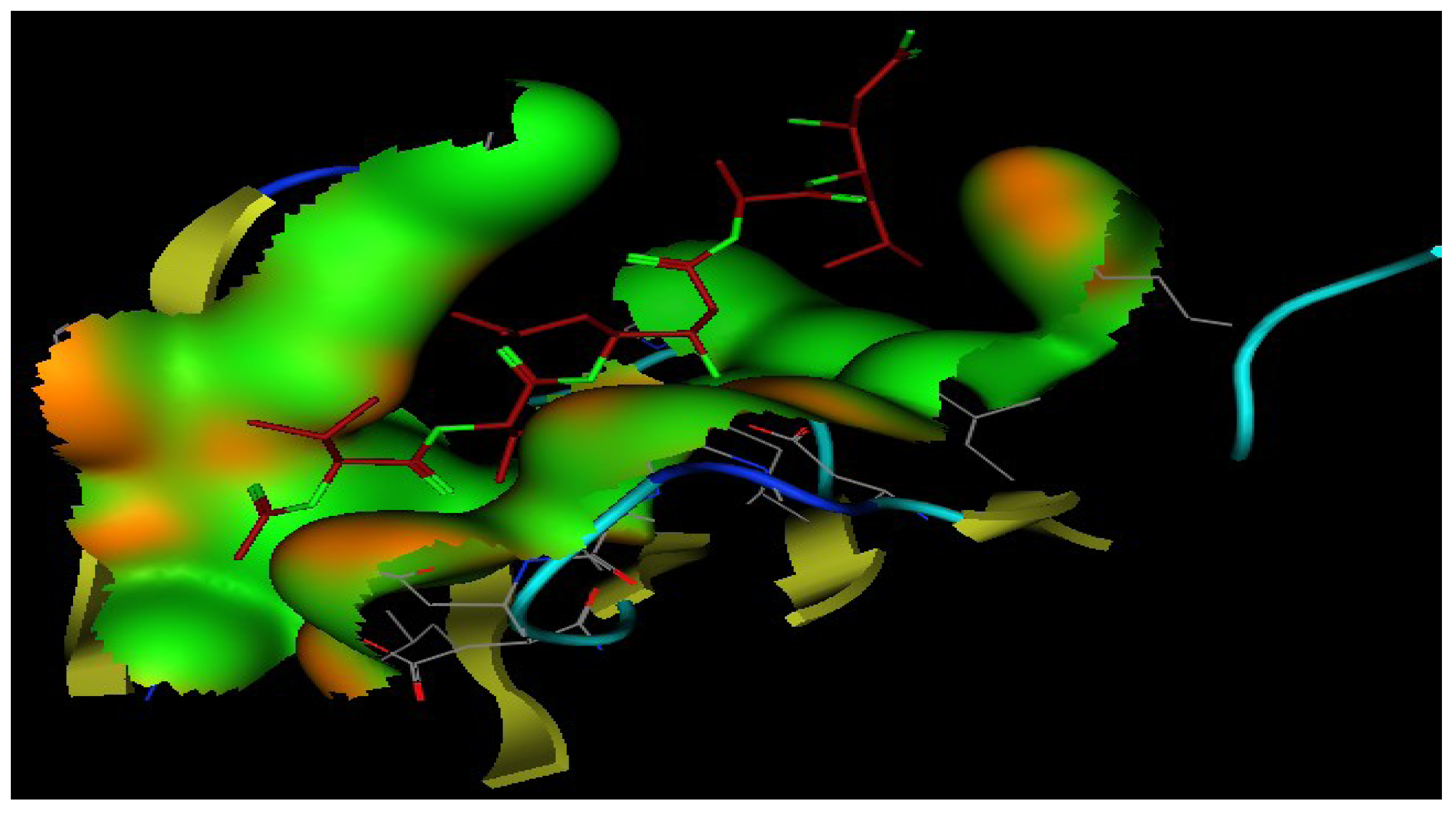
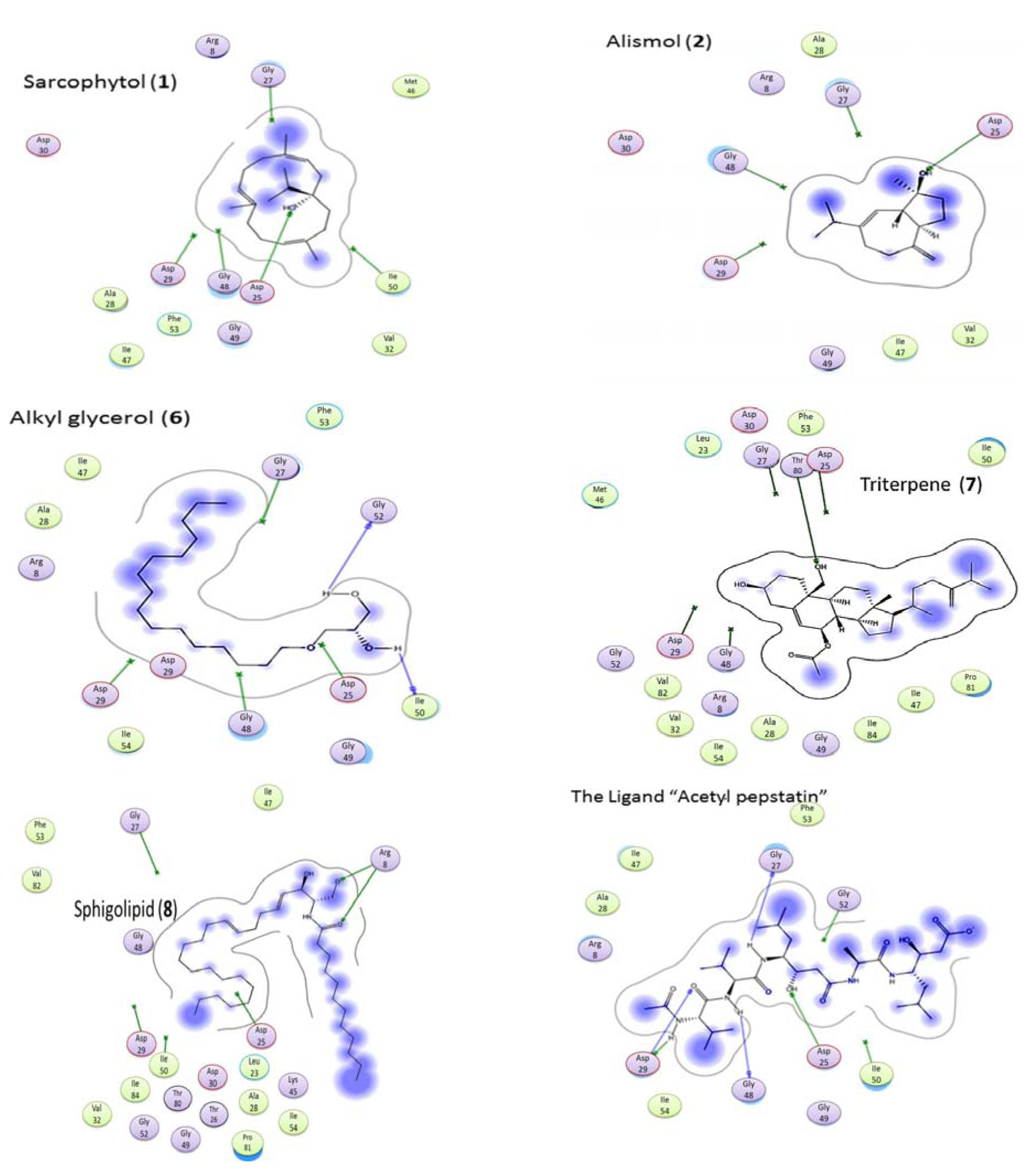
| Ligand/Compound | Residue | Type | Distance | Score |
|---|---|---|---|---|
| Ligand | ASP 25 | H-don | 1.99 | −24.79 |
| ASP 25 | H-don | 2.72 | ||
| GLY 27 | H-don | 2.16 | ||
| ASP 29 | H-don | 1.95 | ||
| GLY 48 | H-don | 1.94 | ||
| ASP 25 | H-acc | 1.99 | ||
| ASP 25 | H-acc | 2.72 | ||
| ASP 29 | H-acc | 2.81 | ||
| 1 | ASP 25 | H-don | 2.45 | −14.44 |
| ASP 25 | H-acc | 2.45 | ||
| 2 | ASP 25 | H-don | 2.37 | −11.14 |
| ASP 25 | H-acc | 2.37 | ||
| 6 | ILE 50 | H-don | 1.50 | −17.16 |
| GLY 52 | H-don | 1.65 | ||
| 7 | THR 80 | H-don | 2.75 | −18.36 |
| THR 80 | H-acc | 2.75 | ||
| 8 | ARG 8 | H-Acc | 2.61 | −15.92 |
| ARG 8 | H-acc | 3.01 |
3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Purification of the Active Constituents
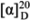 +34 (c.0.76 CHCl3,); 1H NMR, (200 MHz, CDCl3) δH 5.25 (1H, t, J = 6.8 Hz, H-3); 4.91 (1H, t, J = 7.8 Hz, H-11); 4.89 (1H, t, J = 6.8 Hz, H-7); 1.61 (3H, s, Me-20); 1.59 (3H, m, H-19), 1.56 (3H, s, Me-18); 0.96, 0.94 (3H each, d, J = 6.8; Me-16, 17). 13C-NMR (50 MHz, CDCl3) δC 136.7 (C-4), 135.5 (C-12), 133.3 (C-8), 125.9 (CH, C-7), 123.2 (CH, C-11), 120.9 (CH, C-3), 76.9 (C-1), 39.9 (CH2, C-9), 39.5 (CH2, C-5), 35.0 (CH2, C-14), 34.8 (CH2, C-2), 34.6 (CH, C-15), 33.5 (CH2, C-13), 24.8 (CH2, C-6), 23.7 (CH2, C-10), 16.9 (CH3, C-17), 16.6 (CH3, C-16), 16.2 (CH3, C-20), 15.4 (CH3, C-19), 15.2 (CH3, C-18) [12].
+34 (c.0.76 CHCl3,); 1H NMR, (200 MHz, CDCl3) δH 5.25 (1H, t, J = 6.8 Hz, H-3); 4.91 (1H, t, J = 7.8 Hz, H-11); 4.89 (1H, t, J = 6.8 Hz, H-7); 1.61 (3H, s, Me-20); 1.59 (3H, m, H-19), 1.56 (3H, s, Me-18); 0.96, 0.94 (3H each, d, J = 6.8; Me-16, 17). 13C-NMR (50 MHz, CDCl3) δC 136.7 (C-4), 135.5 (C-12), 133.3 (C-8), 125.9 (CH, C-7), 123.2 (CH, C-11), 120.9 (CH, C-3), 76.9 (C-1), 39.9 (CH2, C-9), 39.5 (CH2, C-5), 35.0 (CH2, C-14), 34.8 (CH2, C-2), 34.6 (CH, C-15), 33.5 (CH2, C-13), 24.8 (CH2, C-6), 23.7 (CH2, C-10), 16.9 (CH3, C-17), 16.6 (CH3, C-16), 16.2 (CH3, C-20), 15.4 (CH3, C-19), 15.2 (CH3, C-18) [12].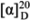 +4.9 (c.0.2 CHCl3,); 1H NMR (200 MHz, CDCl3): δH 5.56 (1H, s, H-6), 4.69,4.64 (1H each, s, H2-14); 1.17 (3H, s, Me-15), 0.93, 0.92 (3H each, d, J = 6.6 Hz, Me-13, 14); 13C NMR (50 MHz, CDCl3): δC 153.9 (C-10), 149.3 (C-7), 121.4 (CH, C-6), 106.4(CH2, C-15), 80.4 (C-4), 54.6 (CH, C-1), 47.0 (CH, C-5), 40.0 (CH2, C-3), 37.3 (CH, C-11), 36.9 (CH2, C-9), 29.8 (CH2, C-8), 24.6 (CH2, C-2), 23.8 (CH3, C-14), 21.3 (CH3, C-13), 21.1(CH3, C-12) [13,14].
+4.9 (c.0.2 CHCl3,); 1H NMR (200 MHz, CDCl3): δH 5.56 (1H, s, H-6), 4.69,4.64 (1H each, s, H2-14); 1.17 (3H, s, Me-15), 0.93, 0.92 (3H each, d, J = 6.6 Hz, Me-13, 14); 13C NMR (50 MHz, CDCl3): δC 153.9 (C-10), 149.3 (C-7), 121.4 (CH, C-6), 106.4(CH2, C-15), 80.4 (C-4), 54.6 (CH, C-1), 47.0 (CH, C-5), 40.0 (CH2, C-3), 37.3 (CH, C-11), 36.9 (CH2, C-9), 29.8 (CH2, C-8), 24.6 (CH2, C-2), 23.8 (CH3, C-14), 21.3 (CH3, C-13), 21.1(CH3, C-12) [13,14].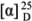 +7.2° (c 0.3 CHCl3); 1H NMR (400 MHz, CDCl3) δH 0.65 (3H, br s, H-18), 0.92 (3H, d, J= 6.18, H-21), 0.97 (3H, br s, H-19), 1.00 (3H, d, J = Hz, H-27), 0.98, 1.00 (3H, d, J= Hz, H-26), 3.51 (1H, br s, H-3), 4.63 (1H, s, H-28a), 4.69 (1H, s, H-28b), 5.32 (1H, s, H-6). 13C NMR (100 MHz, CDCl3) data: δC 157.3 (C-24), 141.2 (C-5), 122.1 (CH, C-6), 106.4 (CH2, C-28), 72.1 (CH, C-3), 57.2 (CH, C-14), 56.4 (CH, C-17), 50.5 (CH, C-9), 42.6 (C-13), 42.8 (CH2, C-4), 40.1 (CH2, C-12), 37.7 (CH2, C-1), 36.2 (C-10), 35.7 (CH, C-20), 35.1 (CH2, C-23), 34.2 (CH, C-25), 32.3 (CH, C-8), 32.0 (CH2, C-7), 32.0 (CH2, C-2), 31.4 (CH2, C-22), 28.6 (CH2, C-16), 24.7 (CH2, C-15), 22.4 (CH3, C-27), 22.3 (CH3, C-26), 21.5 (CH2, C-11), 19.8 (CH3, C-19), 19.1 (CH3, C-21), 12.3 (CH3, C-18) [15].
+7.2° (c 0.3 CHCl3); 1H NMR (400 MHz, CDCl3) δH 0.65 (3H, br s, H-18), 0.92 (3H, d, J= 6.18, H-21), 0.97 (3H, br s, H-19), 1.00 (3H, d, J = Hz, H-27), 0.98, 1.00 (3H, d, J= Hz, H-26), 3.51 (1H, br s, H-3), 4.63 (1H, s, H-28a), 4.69 (1H, s, H-28b), 5.32 (1H, s, H-6). 13C NMR (100 MHz, CDCl3) data: δC 157.3 (C-24), 141.2 (C-5), 122.1 (CH, C-6), 106.4 (CH2, C-28), 72.1 (CH, C-3), 57.2 (CH, C-14), 56.4 (CH, C-17), 50.5 (CH, C-9), 42.6 (C-13), 42.8 (CH2, C-4), 40.1 (CH2, C-12), 37.7 (CH2, C-1), 36.2 (C-10), 35.7 (CH, C-20), 35.1 (CH2, C-23), 34.2 (CH, C-25), 32.3 (CH, C-8), 32.0 (CH2, C-7), 32.0 (CH2, C-2), 31.4 (CH2, C-22), 28.6 (CH2, C-16), 24.7 (CH2, C-15), 22.4 (CH3, C-27), 22.3 (CH3, C-26), 21.5 (CH2, C-11), 19.8 (CH3, C-19), 19.1 (CH3, C-21), 12.3 (CH3, C-18) [15].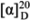 +5.9° (c 0.3 CHCl3). 1H NMR (200 MHz, CDCl3) δH 5.41 (1H, br s, H-6), 3.12 (OMe); 1.13, 1.14 (3H each, s, Me-14, 15); 0.93, 0.90 (3H each, d, J = 6.6 Hz, Me-12, 13); 13C NMR (50 MHz, CDCl3): δC 149.5(C-7), 121.2 (CH, C-6), 80.1 (C-4), 79.2(C-10), 50.0 (CH, C-5), 48.6 (OCH3), 47.8 (CH, C-1), 40.4 (CH2, C-3), 37.1(CH, C-11), 35.4(CH2, C-9), 24.5(CH2, C-8), 22.3(CH3, C-14), 21.6 (CH2, C-2), 21.5 (CH3, C-13), 21.2 (CH3, C-12), 17.9 (CH3, C-15) [13,16].
+5.9° (c 0.3 CHCl3). 1H NMR (200 MHz, CDCl3) δH 5.41 (1H, br s, H-6), 3.12 (OMe); 1.13, 1.14 (3H each, s, Me-14, 15); 0.93, 0.90 (3H each, d, J = 6.6 Hz, Me-12, 13); 13C NMR (50 MHz, CDCl3): δC 149.5(C-7), 121.2 (CH, C-6), 80.1 (C-4), 79.2(C-10), 50.0 (CH, C-5), 48.6 (OCH3), 47.8 (CH, C-1), 40.4 (CH2, C-3), 37.1(CH, C-11), 35.4(CH2, C-9), 24.5(CH2, C-8), 22.3(CH3, C-14), 21.6 (CH2, C-2), 21.5 (CH3, C-13), 21.2 (CH3, C-12), 17.9 (CH3, C-15) [13,16].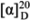 +9.3 (c 0.9 CHCl3,) 1H NMR (400 MHz, CDCl3) δH 5.44 (1H, br d, J = 3.0 Hz, H-6), 0.98, 1.0 (3H each, d, J = 6.9 Hz, H-12, 13), 1.25, 128 (3H each, s, H-14,15);13C NMR (100 MHz, CDCl3): δC 149.4(C-7), 121.3 (CH, C-6), 80.0 (C-4), 75.2 (C-10), 50.5 (CH, C-1), 50.1 (CH, C-5), 42.5 (CH2, C-9), 40.3 (CH2, C-3), 37.2 (CH, C-11), 25.0 (CH2, C-8), 22.4 (CH3, C-14), 21.4 (CH2, C-2), 21.3 (CH3, C-15), 21.2 (CH3, C-13), 21.1 (CH3, C-12).
+9.3 (c 0.9 CHCl3,) 1H NMR (400 MHz, CDCl3) δH 5.44 (1H, br d, J = 3.0 Hz, H-6), 0.98, 1.0 (3H each, d, J = 6.9 Hz, H-12, 13), 1.25, 128 (3H each, s, H-14,15);13C NMR (100 MHz, CDCl3): δC 149.4(C-7), 121.3 (CH, C-6), 80.0 (C-4), 75.2 (C-10), 50.5 (CH, C-1), 50.1 (CH, C-5), 42.5 (CH2, C-9), 40.3 (CH2, C-3), 37.2 (CH, C-11), 25.0 (CH2, C-8), 22.4 (CH3, C-14), 21.4 (CH2, C-2), 21.3 (CH3, C-15), 21.2 (CH3, C-13), 21.1 (CH3, C-12).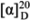 +7.2° (c 0.3 CHCl3); 1H NMR (CDCl3, 200 MHz) δH, 3.83 (1 H, m, H-2), 3.72, 3.64 (2H, m, H-1), 3.45 (4H, m, H-1′, 3), 1.52 (2H, m, 2′-H2), 1.26 (26H, br s, CH2), 0.84 (3H, t, J = 6.6 Hz, CH3); 13C NMR (50 MHz, CDCl3): δC 72.4 (CH2-1′), 71.8 (CH2-1), 70.5 (CH-2), 64.2 (CH2-3), 31.9 (CH2-13′), 29.7-29.3 (CH2′s), 26.0 (CH2-3′), 22.7 (CH2-14′), 14.1 (CH3). HRESIMS: 317.3045 [M + H]+ corresponding to C19H41O3, calculated for 317.3057 [13,14].
+7.2° (c 0.3 CHCl3); 1H NMR (CDCl3, 200 MHz) δH, 3.83 (1 H, m, H-2), 3.72, 3.64 (2H, m, H-1), 3.45 (4H, m, H-1′, 3), 1.52 (2H, m, 2′-H2), 1.26 (26H, br s, CH2), 0.84 (3H, t, J = 6.6 Hz, CH3); 13C NMR (50 MHz, CDCl3): δC 72.4 (CH2-1′), 71.8 (CH2-1), 70.5 (CH-2), 64.2 (CH2-3), 31.9 (CH2-13′), 29.7-29.3 (CH2′s), 26.0 (CH2-3′), 22.7 (CH2-14′), 14.1 (CH3). HRESIMS: 317.3045 [M + H]+ corresponding to C19H41O3, calculated for 317.3057 [13,14].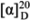 +6.7° (c 0.5 CHCl3); 1H NMR (400 MHz, CDCl3): δH 5.56 (1H, t, J = 2 Hz, H-6), 4.95 (1H, dt, J = 2.2, 2.2, 8.5 Hz, H-7α), 4.70 (1H, t, J = 1.3 Hz, HA-28), 4.64 (1H, dd, J = 1.3 and 1.7 Hz, HB-28), 3.87 (1H, d, J = 11.5 Hz, HA-19), 3.64 (1H, d, J = 11.5 Hz, HB-19), 3.59 (1H, tt, J = 11.2 4.7 Hz, H-3α), 2.38 (1H, ddd, J = 13.5, 4.7, 2.4 Hz, H-4α), 2.01 (3H, s, OAc), 1.01 (3H, d, J = 6.8 Hz, Me-26), 1.00 (3H, d, J = 6.8 Hz, Me-27), 0.93 (3H, d, J = 6.8 Hz, Me-21), 0.73 (3H, s, Me-18). 13C NMR (100 MHz, CDCl3): δC 171.4 (MeCOO-), 156.7 (C-24), 140.1 (C-5), 126.6 (CH, C-6), 106.0 (CH2, C-28), 75.2 (CH, C-7), 70.8 (CH, C-3), 62.8 (CH2, C-19), 56.5 (CH, C-14), 55.2 (CH, C-17), 48.5 (CH, C-9), 43.1 (C-13), 41.6 (CH2, C-4), 41.4 (qC, C-10), 39.7 (CH2, C-12), 37.8 (CH, C-8), 35.6 (CH, C-20), 34.6 (CH2, C-22), 33.7 (CH, C-25), 33.1 (CH2, C-1), 31.7 (CH2, C-2), 30.9 (CH2, C-23), 28.3 (CH2, C-16), 24.9 (CH2, C-15), 22.0 (CH3, C-26), 21.8 (CH3, C-27), 21.7 (CH3, CH3COO), 21.6 (CH2, C-11), 18.7 (CH3, C-21), 12.1 (CH3, C-18) [18].
+6.7° (c 0.5 CHCl3); 1H NMR (400 MHz, CDCl3): δH 5.56 (1H, t, J = 2 Hz, H-6), 4.95 (1H, dt, J = 2.2, 2.2, 8.5 Hz, H-7α), 4.70 (1H, t, J = 1.3 Hz, HA-28), 4.64 (1H, dd, J = 1.3 and 1.7 Hz, HB-28), 3.87 (1H, d, J = 11.5 Hz, HA-19), 3.64 (1H, d, J = 11.5 Hz, HB-19), 3.59 (1H, tt, J = 11.2 4.7 Hz, H-3α), 2.38 (1H, ddd, J = 13.5, 4.7, 2.4 Hz, H-4α), 2.01 (3H, s, OAc), 1.01 (3H, d, J = 6.8 Hz, Me-26), 1.00 (3H, d, J = 6.8 Hz, Me-27), 0.93 (3H, d, J = 6.8 Hz, Me-21), 0.73 (3H, s, Me-18). 13C NMR (100 MHz, CDCl3): δC 171.4 (MeCOO-), 156.7 (C-24), 140.1 (C-5), 126.6 (CH, C-6), 106.0 (CH2, C-28), 75.2 (CH, C-7), 70.8 (CH, C-3), 62.8 (CH2, C-19), 56.5 (CH, C-14), 55.2 (CH, C-17), 48.5 (CH, C-9), 43.1 (C-13), 41.6 (CH2, C-4), 41.4 (qC, C-10), 39.7 (CH2, C-12), 37.8 (CH, C-8), 35.6 (CH, C-20), 34.6 (CH2, C-22), 33.7 (CH, C-25), 33.1 (CH2, C-1), 31.7 (CH2, C-2), 30.9 (CH2, C-23), 28.3 (CH2, C-16), 24.9 (CH2, C-15), 22.0 (CH3, C-26), 21.8 (CH3, C-27), 21.7 (CH3, CH3COO), 21.6 (CH2, C-11), 18.7 (CH3, C-21), 12.1 (CH3, C-18) [18].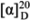 +5.2° (c 0.2 MeOH). 1H-NMR [200 MHz, CDCl3/CD3OD (1:3)] δH 5.44 (1H, s, H-6), 4.67, 4.61 (1H each, br s, H2-28) 3.54, 3.80 (1H each, d, J = 11.8 Hz, H-19), 3.60 (1H, br s, H-7), 3.42 (1H, m, H-3), 0.94 (6H, d, J = 7.0 Hz, Me-26, 27), 0.92 (3H, d, J= 6.6, Me-21), 0.72 (3H, br s, Me-18),. 13C-NMR [50 MHz, CDCl3/CD3OD (1:3)] δC 157.7 (C-24), 140.0 (C-5), 131.0 (CH, C-6), 106.9 (CH2, C-28), 73.3 (CH, C-7), 72.0 (CH, C-3), 63.4 (CH2, C-19), 58.6 (C-14), 56.8 (CH, C-17), 49.8 (CH, C-9), 44.3 (C-10), 42.7 (CH2, C-4), 42.4 (CH, C-8), 42.4 (C-13), 41.5 (CH2, C-12), 37.0 (CH, C-20), 36.0 (CH2, C-22), 34.9 (CH, C-25), 34.0 (CH2, C-1), 32.6 (CH, C-2), 32.1 (CH2, C-23), 29.7 (CH2, C-16) 27.2 (CH2, C-15), 22.9 (CH2, C-11), 22.5 (CH3, C-26), 22.3 (CH3, C-27), 19.3 (CH3, C-21), 12.6 (CH3, C-18) [20].
+5.2° (c 0.2 MeOH). 1H-NMR [200 MHz, CDCl3/CD3OD (1:3)] δH 5.44 (1H, s, H-6), 4.67, 4.61 (1H each, br s, H2-28) 3.54, 3.80 (1H each, d, J = 11.8 Hz, H-19), 3.60 (1H, br s, H-7), 3.42 (1H, m, H-3), 0.94 (6H, d, J = 7.0 Hz, Me-26, 27), 0.92 (3H, d, J= 6.6, Me-21), 0.72 (3H, br s, Me-18),. 13C-NMR [50 MHz, CDCl3/CD3OD (1:3)] δC 157.7 (C-24), 140.0 (C-5), 131.0 (CH, C-6), 106.9 (CH2, C-28), 73.3 (CH, C-7), 72.0 (CH, C-3), 63.4 (CH2, C-19), 58.6 (C-14), 56.8 (CH, C-17), 49.8 (CH, C-9), 44.3 (C-10), 42.7 (CH2, C-4), 42.4 (CH, C-8), 42.4 (C-13), 41.5 (CH2, C-12), 37.0 (CH, C-20), 36.0 (CH2, C-22), 34.9 (CH, C-25), 34.0 (CH2, C-1), 32.6 (CH, C-2), 32.1 (CH2, C-23), 29.7 (CH2, C-16) 27.2 (CH2, C-15), 22.9 (CH2, C-11), 22.5 (CH3, C-26), 22.3 (CH3, C-27), 19.3 (CH3, C-21), 12.6 (CH3, C-18) [20].3.4. HIV-1 Direct Enzyme Assays
3.5. Cytotoxicity
3.6. Real-Time Cell Electronic Sensing (RT-CES) xCELLigence
3.7. Molecular Docking
4. Conclusions
Abbreviations
| XTT | (2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide) |
| ART | Antiretroviral therapy |
| HAART | highly active antiretroviral therapy |
| SI | Selectivity index |
| RT-CES | Real time cell electronic sensing |
| CI | cell index |
| AP | acetyl pepstatin |
| EIMS | Electron ionized mass spectrometry |
Acknowledgments
Conflicts of Interest
References
- Global HIV/AIDS response: Epidemic update and health sector progress towards universal access: Progress report 2011. Available online: http://whqlibdoc.who.int/publications/2011/9789241502986_eng.pdf (accessed on 10 June 2012).
- Logsdon, B.C.; Vickrey, J.F.; Martin, P.; Proteasa, G.; Koepke, J.I.; Terlecky, S.R.; Wawrzak, Z.; Winters, M.A.; Merigan, T.C.; Kovari, L.C. Crystal structures of a multidrug-resistant human immunodeficiency virus type 1 protease reveal an expanded active-site cavity. J. Virol. 2004, 78, 3123–3132. [Google Scholar] [CrossRef]
- Leonardo, A.M.; de Souza, A.M.T.; Rodrigues, C.R.; Paixão, I.C.N.P.; Teixeira, V.L.; Castro, H.C. HIV-1 Reverse Transcriptase: A potential target for marine products. Rev. Bras. Farmacogn. 2012, 22, 881–888. [Google Scholar] [CrossRef]
- Chow, W.A.; Jiang, C.; Guan, M. Anti-HIV drugs for cancer therapeutics: Back to the future? Lancet Oncol. 2009, 10, 61–71. [Google Scholar] [CrossRef]
- Gochfeld, D.J.; El Sayed, K.A.; Yousaf, M.; Hu, J.F.; Bartyzel, P.; Dunbar, D.C.; Wilkins, S.P.; Zjawiony, J.K.; Schinazi, R.F.; Schlueter, W.S.; et al. Marine natural products as lead anti-HIV agents. Mini Rev. Med. Chem. 2003, 5, 401–424. [Google Scholar]
- Zhong, H.J.; Mac, V.P.; Cheng, Z.; Chan, D.S. Discovery of a natural product inhibitor targeting protein neddylation by structure-based virtual screening. Biochimie 2012, 49, 2457–2460. [Google Scholar]
- Shaker, K.H.; Mueller, M.; Ghani, M.A.; Dahse, H.-M.; Seifert, K. Terpenes from the Soft Corals Litophyton arboreum and Sarcophyton ehrenbergi. Chem. Biodivers. 2010, 7, 2007–2015. [Google Scholar] [CrossRef]
- Grote, D.; Dahse, H.-M.; Seifert, K. Furanocembranoids from the soft corals Sinularia asterolobata and Litophyton arboretum. Chem. Biodivers. 2008, 5, 2449–2456. [Google Scholar] [CrossRef]
- Rezanka, T.V.; Dembitsky, V.M. γ-Lactones from the soft corals Sarcophyton trocheliophorum and Lithophyton arboretum. Tetrahedron 2001, 57, 8743–8749. [Google Scholar] [CrossRef]
- Li, R.; Wang, S.; Tan, G.; Long, K. Two polyhydroxylated steroids from the Chinese soft coral Litophyton arboretum. Steroids 1994, 59, 503–505. [Google Scholar] [CrossRef]
- Le Roux, K.A.; Hussein, A.A.; Lall, N. In vitro chemo-preventative activity of Crotalaria agatiflora subspecies agatiflora Schweinf. J. Ethnopharmacol. 2011, 138, 748–755. [Google Scholar] [CrossRef]
- Pardhy, R.S.; Bhattacharya, S.C. Structure of serratol, a new diterpene cembranoid alcohol from Boswellia serrata Roxb. Ind. J. Chem. B 1978, 16B, 171–173. [Google Scholar]
- Oshima, Y.; Iwakawa, T.; Hikino, H. Alismol and alismoxide, sesquiterpenoids of Alisma rhizomes. Pytochemistry 1983, 22, 183–185. [Google Scholar] [CrossRef]
- Penga, G.-P.; Tiana, G.; Huanga, X.-F.; Loub, F.-C. Guaiane-type sesquiterpenoids from Alisma orientalis. Phytochemistry 2003, 63, 877–881. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Hsieh, Y.T.; Wen, Z.H.; Wu, Y.C.; Sheu, J.H. Polyoxygenated Sterols from the Formosan Soft Coral Sinularia gibberosa. J. Nat. Prod. 2006, 69, 1275–1279. [Google Scholar] [CrossRef]
- Jin, H.-G.; Jin, Q.; Kim, A.R.; Choi, H.; Lee, J.H.; Kim, Y.S.; Lee, D.G.; Woo, E.-R. A New Triterpenoid from Alisma orientale and their antibacterial effect. Arch. Pharm. Res. 2012, 35, 1919–1926. [Google Scholar] [CrossRef]
- Chao, C.-H.; Huang, H.-C.; Wu, Y.-C.; Lu, C.-K.; Dai, C.-F.; Sheu, J.-H. Glycolipids from the formosan soft coral Lobophytum crissum. Chem. Pharm. Bull. 2007, 55, 1720–1723. [Google Scholar] [CrossRef]
- Faheem, A.; Yen, C.; Yam, W.S. Chemical Constituents and Biological Properties of the Marine Soft Coral Nephthea sp. Trop. J. Pharma. Res. 2012, 11, 485–498. [Google Scholar]
- Chebaane, K.; Guyot, M. Occurrence of erythro-docosasphinga-4,8-dienine, as an ester, in Anemonia sulcata. Tetrahedron Lett. 1986, 27, 1495–1496. [Google Scholar] [CrossRef]
- Bortolotto, M.; Braekman, J.C.; Daloze, D.; Losman, D.; Tursch, B. Chemical studies of marine invertebrates. XXIII. A novel polyhydroxylated sterol from the soft coral Litophyton viridis (Coelenterata, Octocorallia, Alcyonacea). Steroids 1976, 28, 461–466. [Google Scholar] [CrossRef]
- Yamahara, J.; Matsuda, H.; Murakami, H.; Fujimura, H. The active principle of alismatis rhizoma which inhibits contractile responses in aorta. Chem. Pharm. Bull. 1986, 34, 4422–4424. [Google Scholar] [CrossRef]
- Matsuda, H.; Kobayashi, G.; Yamahara, J.; Fujimura, H.; Kurahashi, K.; Fujiwara, M. Effects of alismol isolated from Alismatis rhizoma on calcium-induced contraction in the rabbit thoracic aorta. Life Sci. 1987, 41, 1845–1852. [Google Scholar] [CrossRef]
- Yamahara, J.; Kobayashi, G.; Iwamoto, M.; Matsuda, H.; Fujimura, H. The effect of alismol isolated from Alismatis rhizoma on experimental hypertensive models in rats. Phytother Res. 1989, 3, 57–60. [Google Scholar] [CrossRef]
- Xu, J.; Jin, D.; Shi, D.; Ma, Y.; Yang, B.; Zhao, P.; Guo, Y. Sesquiterpenes from Vladimiria souliei and their inhibitory effects on NO production. Fitoterapia 2011, 82, 508–511. [Google Scholar] [CrossRef]
- Prayong, P.; Barusrux, S.; Weerapreeyakul, N. Cytotoxic activity screening of some indigenous Thai plants. Fitoterapia 2008, 79, 598–601. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; El-Shemy, H.A. Efficacy assessment for several natural products with potential cytotoxic activity against breast and cervix cancers. J. Arid Land Stud. 2012, 22, 107–110. [Google Scholar]
- Zhang, W.; Liu, W.K.; Che, C.-T. Polyhydroxylated steroids and other constituents of the soft coral Nephthea chabroli. Chem. Pharm. Bull. 2003, 51, 1009–1011. [Google Scholar] [CrossRef]
- Duh, C.-Y.; Wang, S.-K.; Chu, M.-J.; Sheu, J.-H. Cytotoxic Sterols from the Soft Coral Nephthea erecta. J. Nat. Prod. 1998, 61, 1022–1024. [Google Scholar] [CrossRef]
- El Sayed, K.A.; Bartyzel, P.; Shen, X.; Perry, T.L.; Zjawiony, J.K.; Hamann, M.T. Marine natural products as antituberculosis agents. Tetrahedron 2000, 56, 949–953. [Google Scholar] [CrossRef]
- Hu, Q.; Zhao, J.; Xu, S. Effects of 3β,7β,19-trihydroxy-24-methylene-Δ5-cholesten on the immune system of mice. Redai Haiyang 1998, 17, 75–79. [Google Scholar]
- Xu, S.; Zhao, J.; Hu, Q.; Wang, Z.; Li, R. Study on antioxidation of 24-methylene-3β,7β,19-trihydroxy-5-cholestene. Chin. Pharmacol. Bull. 1997, 13, 81–84. [Google Scholar]
- Duax, W.L.; Griffin, J.F.; Rohrer, D.C.; Weeks, C.M. Conformational analysis of sterols: Comparison of x-ray crystallographic observations with data from other sources. Lipids 1980, 15, 783–792. [Google Scholar] [CrossRef]
- Emil, P.; Daniela, C.; Michael, E.; Clay, C.; Jean, D. Lipophilicity Parameters and Biological Activity in a Series of Compounds with Potential Cardiovascular Applications. Croat. Chem. Acta 2004, 77, 1–2. [Google Scholar]
- Fonteh, P.N.; Keter, F.K.; Meyer, D. New bis(thiosemicarbazonate) gold(III) complexes inhibit HIV replication at cytostatic concentrations: Potential for incorporation into virostatic cocktails. J. Inorg. Biochem. 2011, 105, 1173–1180. [Google Scholar] [CrossRef]
- Cheenpracha, S.; Karalai, C.; Ponglimanont, C.; Subhadhirasakul, S.; Tewtrakul, S. Anti-HIV-1 protease activity of compounds from Boesenbergia pandurata. Bioorg. Med. Chem. 2006, 15, 1710–1714. [Google Scholar]
- Marastoni, M.; Salvadori, S.; Bortolotti, F.; Tomatis, R. HIV-1 protease inhibitors containing a novel C2 symmetrical hydroxyalkylgem-diamino core structure. J. Pept. Res. 1997, 49, 538–544. [Google Scholar]
- Yang, H.; Nkeze, J.; Zhao, R.Y. Effects of HIV-1 protease on cellular functions and their potential applications in antiretroviral therapy. Cell Biosci. 2012, 4, 2–32. [Google Scholar]
- Verma, S.P. HIV: A raft-targeting approach for prevention and therapy using plant-derived compounds. Curr. Drug Targets 2009, 10, 51–59. [Google Scholar] [CrossRef]
- Lam, T.L.; Lam, M.L.; Au, T.K.; Ip, D.T.; Ng, T.B.; Fong, W.P.; Wan, D.C. A comparison of human immunodeficiency virus type-1 protease inhibition activities by the aqueous and methanol extracts of Chinese medicinal herbs. Life Sci. 2000, 69, 2889–2896. [Google Scholar]
- Zheng, Y.T.; Chan, W.L.; Chan, P.; Huang, H.; Tam, S.C. Enhancement of the antiherpetic effect of trichosanthin by acyclovir and interferon. FEBS Lett. 2001, 496, 139–142. [Google Scholar] [CrossRef]
- Roche Diagnostics GmbH. Introduction of the RTCA SP Instrument. RTCA SP Instrument Operator’s Manual A; Acea Biosciences, Inc.: San Diego, CA, USA, 2008; pp. 14–16. [Google Scholar]
- Wan, Q.; Rumpf, D.; Schricker, S.R.; Mariotti, A.; Culbertson, B.M. Influence of hyperbranched multi-methacrylates for dental neat resins on proliferation of human gingival fibroblasts. Biomacromolecules 2001, 1, 217–222. [Google Scholar]
- Atienza, J.M.; Zhu, J.; Wang, X.; Xu, X.; Abbasi, Y. Dynamic monitoring of cell adhesion and spreading on microelectronic sensor arrays. J. Biomol. Screen 2005, 10, 795–805. [Google Scholar] [CrossRef]
- Giaever, I.; Keese, C.R. A morphological biosensor for mammalian cells. Nature 1993, 366, 591–592. [Google Scholar] [CrossRef]
- Fitzgerald, P.M.; McKeever, B.M.; VanMiddlesworth, J.F.; Springer, J.P.; Heimbach, J.C.; Leu, C.T.; Herber, W.K.; Dixon, R.A.; Darke, P.L. Crystallographic analysis of a complex between human immunodeficiency virus type 1 protease and acetyl-pepstatin at 2.0-A resolution. J. Biol. Chem. 1990, 265, 14209–14219. [Google Scholar]
- Slattery, M.; Starmer, J.; Paul, V. Temporal and spatial variation in defensive metabolites of the tropical Pacific soft corals Sinularia maxima and S. polydactyla. J. Mar. Biol. 2001, 138, 1183–1193. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ellithey, M.S.; Lall, N.; Hussein, A.A.; Meyer, D. Cytotoxic, Cytostatic and HIV-1 PR Inhibitory Activities of the Soft Coral Litophyton arboreum. Mar. Drugs 2013, 11, 4917-4936. https://doi.org/10.3390/md11124917
Ellithey MS, Lall N, Hussein AA, Meyer D. Cytotoxic, Cytostatic and HIV-1 PR Inhibitory Activities of the Soft Coral Litophyton arboreum. Marine Drugs. 2013; 11(12):4917-4936. https://doi.org/10.3390/md11124917
Chicago/Turabian StyleEllithey, Mona S., Namrita Lall, Ahmed A. Hussein, and Debra Meyer. 2013. "Cytotoxic, Cytostatic and HIV-1 PR Inhibitory Activities of the Soft Coral Litophyton arboreum" Marine Drugs 11, no. 12: 4917-4936. https://doi.org/10.3390/md11124917
APA StyleEllithey, M. S., Lall, N., Hussein, A. A., & Meyer, D. (2013). Cytotoxic, Cytostatic and HIV-1 PR Inhibitory Activities of the Soft Coral Litophyton arboreum. Marine Drugs, 11(12), 4917-4936. https://doi.org/10.3390/md11124917