Antinociceptive and Anti-Inflammatory Activities of Crude Methanolic Extract of Red Alga Bryothamnion triquetrum
Abstract
:1. Introduction
2. Results and Discussion
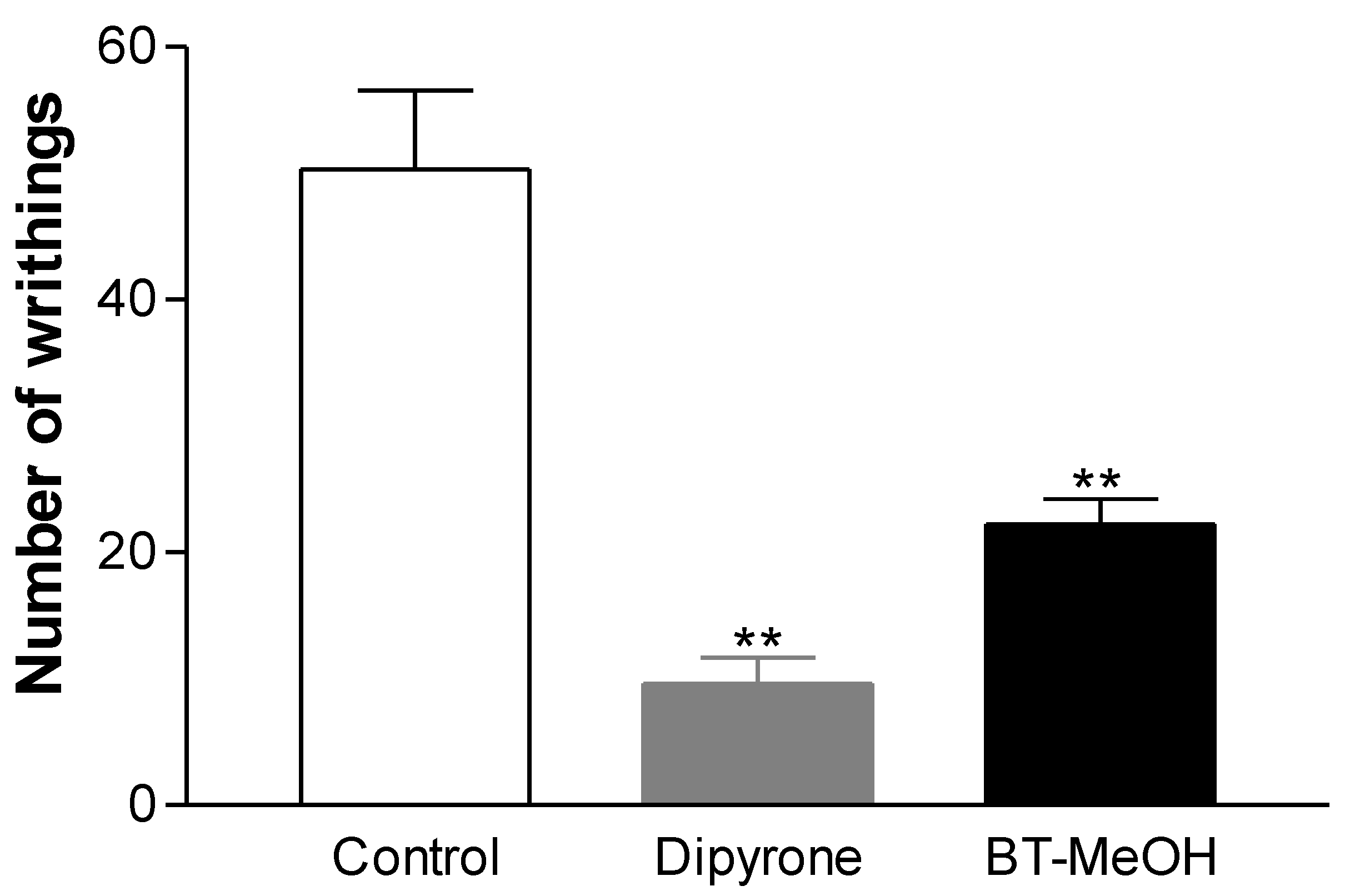
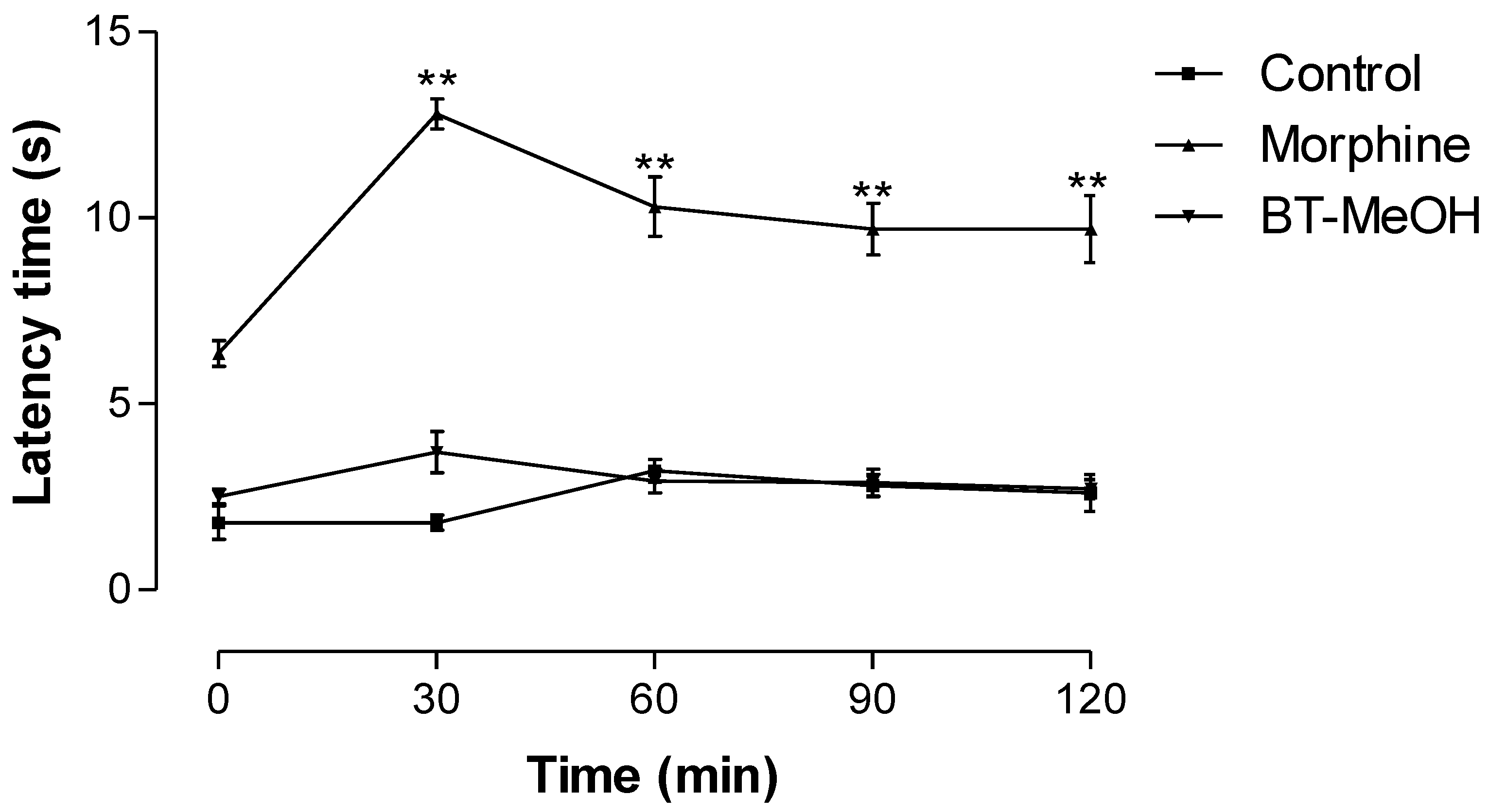

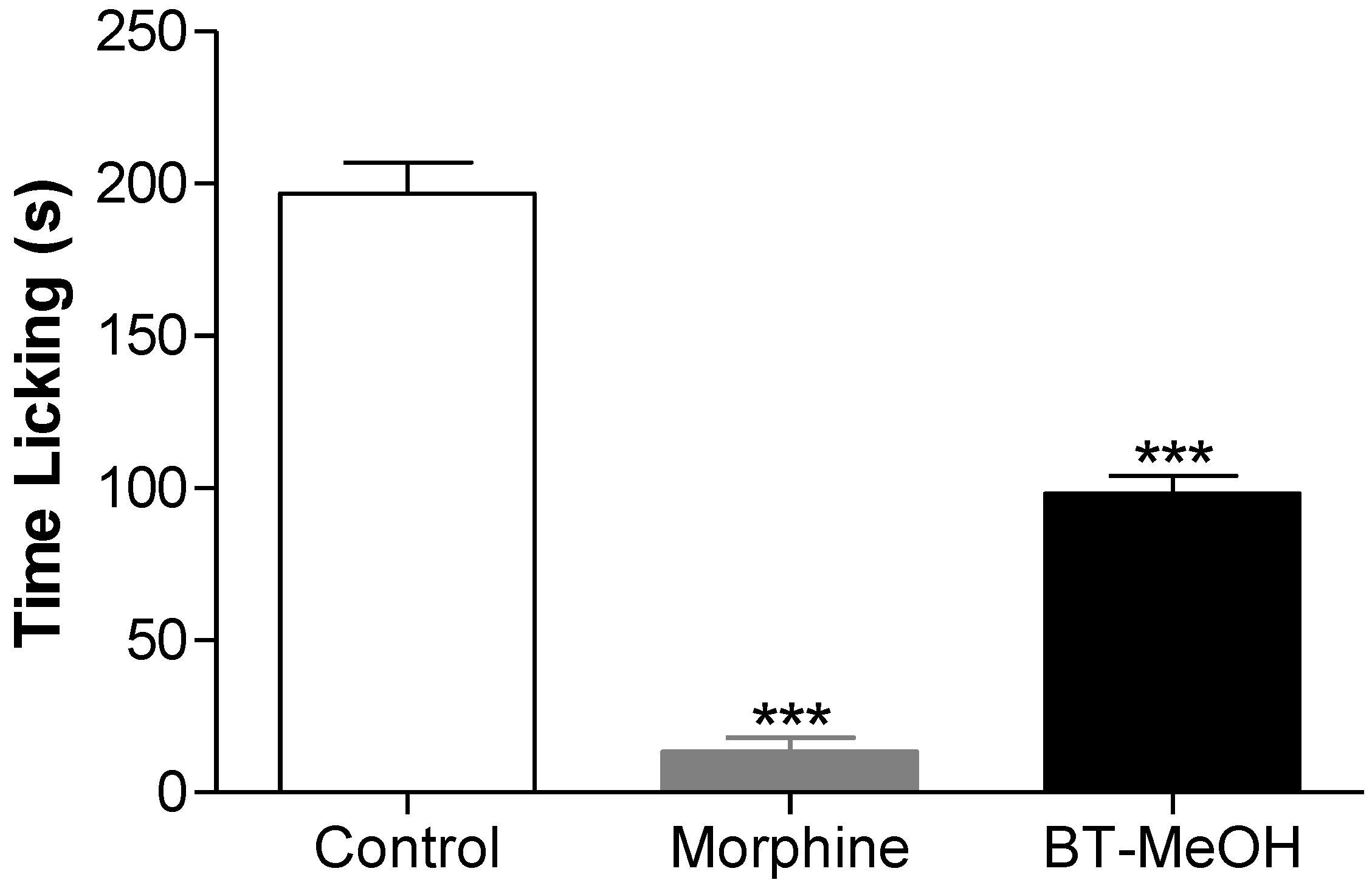
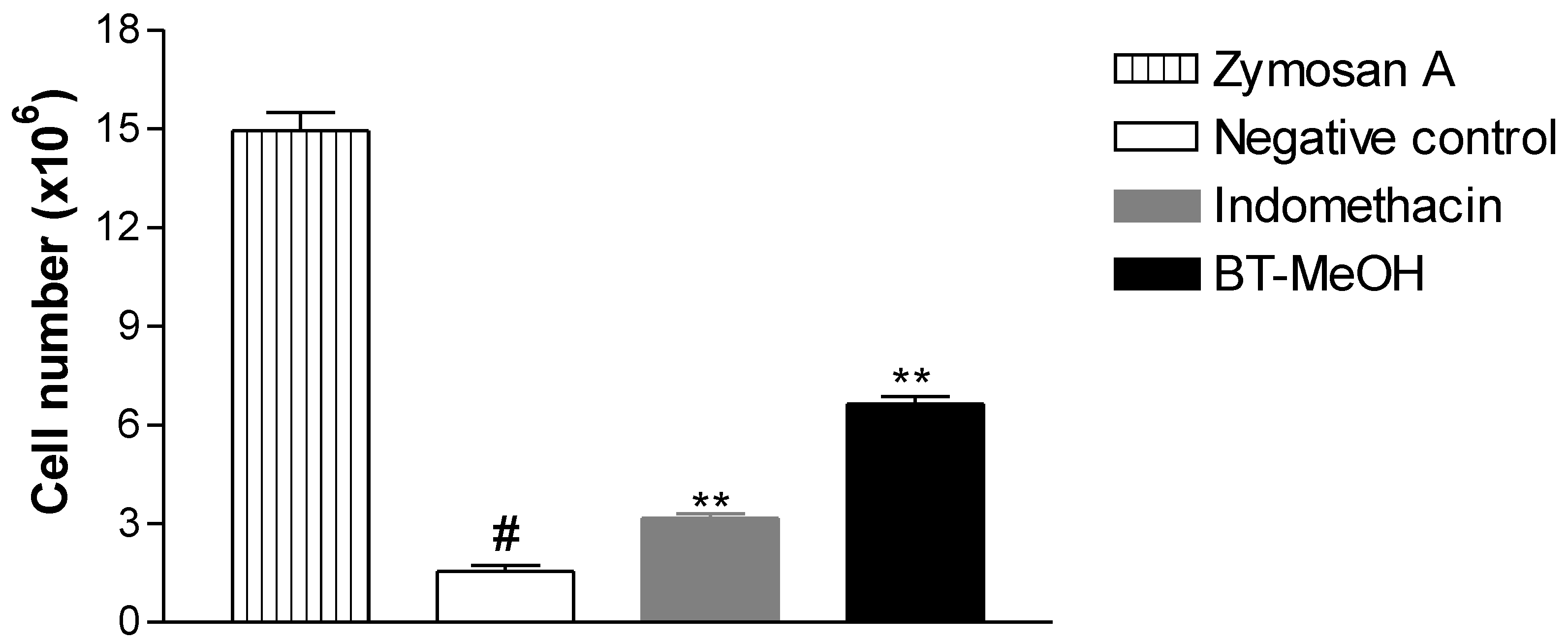
3. Experimental Section
3.1. Plant Material
3.2. Biological Activity Tests
3.2.1. Drugs and Reagents
3.2.2. Animals
3.2.3. Acute Toxicity Study
3.2.4. Acetic Acid-Induced Writhing
3.2.5. Hot-Plate Test
3.2.6. Formalin-Induced Nociception
3.2.7. Glutamate-Induced Nociception
3.2.8. Zymosan A-Induced Peritonitis
3.2.9. Statistical Analysis
4. Conclusions
Acknowledgments
- Sample Availability: Available from the authors.
References
- Kim, S.; Wijesekara, I. Development and biological activities of marine-derived bioactive peptides: A review. J. Funct. Foods 2010, 2, 1–9. [Google Scholar] [CrossRef]
- Almeida, C.L.F.; Falcão, H.S.; Lima, G.R.M.; Montenegro, C.A.; Lira, N.S.; Athayde-Filho, P.F.; Rodrigues, L.C.; Souza, M.F.V.; Barbosa-Filho, J.M.; Batista, L.M. Bioactivities from marine algae of the genus Gracilaria. Int. J. Mol. Sci. 2011, 12, 4550–4573. [Google Scholar] [CrossRef]
- Queiroz, T.M.; Machado, N.T.; Furtado, F.F.; Oliveira-Filho, A.A.; Alustau, M.C.; Figueiredo, C.S.; Miranda, G.E.C.; Barbosa-Filho, J.M.; Braga, V.A.; Medeiros, I.A. Vasorelaxation, induced by Dictyota pulchella (Dictyotaceae), a brown alga, is mediated via inhibition of calcium influx in rats. Mar. Drugs 2011, 9, 2075–2088. [Google Scholar]
- Souza, E.T.; Lira, D.P.; Queiroz, A.C.; Silva, D.J.C.; Aquino, A.B.; Mella, E.A.C.; Lorenzo, V.L.; Miranda, G.E.C.; Araújo-Júnior, J.X.; Chaves, M.C.O.; et al. The antinociceptive and anti-inflammatory activities of caulerpin, a bisindole alkaloid isolated from seaweeds of the genus Caulerpa. Mar. Drugs 2009, 7, 689–704. [Google Scholar] [CrossRef]
- Kijjoa, A.; Sawangwong, P. Drugs and cosmetics from the sea. Mar. Drugs 2004, 2, 73–82. [Google Scholar] [CrossRef]
- La Barre, S.; Potin, P.; Leblanc, C.; Delage, L. The halogenated metabolism of brown algae (Phaeophyta), its biological importance and its environmental significance. Mar. Drugs 2010, 8, 988–1010. [Google Scholar]
- Lira, N.S.; Montes, R.C.; Tavares, J.F.; Silva, M.S.; Cunha, E.V.L.; Athayde-Filho, P.F.; Rodrigues, L.C.; Dias, C.S.; Barbosa-Filho, J.M. Brominated compounds from marine sponges of the genus Aplysina and a compilation of their 13C NMR spectral data. Mar. Drugs 2011, 9, 2316–2368. [Google Scholar] [CrossRef]
- Bergmann, W.; Feeney, R. Contribution to the study of marine sponges. 32. The nucleosides of sponges. J. Org. Chem. 1951, 16, 981–987. [Google Scholar] [CrossRef]
- Kerr, R.G.; Kerr, S.S. Marine natural products as therapeutic agents. Expert. Opin. Ther. Pat. 1999, 9, 1207–1222. [Google Scholar] [CrossRef]
- Jha, R.K.; Zi-rong, X. Biomedical compounds from marine organisms. Mar. Drugs 2004, 2, 123–146. [Google Scholar] [CrossRef]
- Koopmans, M.; Martens, D.; Wijffels, R. Towards commercial production of sponge medicines. Mar. Drugs 2009, 7, 787–802. [Google Scholar] [CrossRef]
- Balan, V.; Nangia-Makker, P.; Raz, A. Galectins as cancer biomarkers. Cancers 2010, 2, 592–610. [Google Scholar] [CrossRef]
- Proksch, P.; Edrada, R.A.; Ebel, R. Drugs from the seas—Current status and microbiological implications. Appl.Microbiol. Biotechnol. 2002, 59, 125–134. [Google Scholar] [CrossRef]
- Mayer, A.M.S.; Glaser, K.B.; Cuevas, C.; Jacobs, R.S.; Kem, W.; Little, R.D.; Mcintosh, J.M.; Newman, D.J.; Potts, B.C.; Shuster, D.E. The odyssey of marine pharmaceuticals: A current pipeline perspective. Trends Pharmacol.Sci. 2010, 31, 255–265. [Google Scholar] [CrossRef]
- Zhang, J.L.; Xia, W.S.; Liu, P.; Cheng, Q.Y.; Tahirou, T.; Gu, W.X.; Li, B. Chitosan modification and pharmaceutical/biomedical applications. Mar. Drugs 2010, 8, 1962–1987. [Google Scholar] [CrossRef]
- O’Sullivan, L.; Murphy, B.; McLoughlin, P.; Duggan, P.; Lawlor, P.G.; Hughes, H.; Gardiner, G.E. Prebiotics from marine macroalgae for human and animal health applications. Mar. Drugs 2010, 8, 2038–2064. [Google Scholar] [CrossRef]
- Matta, C.B.B.; Souza, E.T.; Queiroz, A.C.; Lira, D.P.; Araújo, M.V.; Cavalcante-Silva, L.H.A.; Miranda, G.E.C.; Araújo-Júnior, J.X.; Barbosa-Filho, J.M.; Santos, B.V.O.; et al. Antinociceptive and anti-inflammatory activity from algae of the genus Caulerpa. Mar. Drugs 2011, 9, 307–318. [Google Scholar] [CrossRef]
- Paula, J.C.; Vallim, M.A.; Teixeira, V.L. What are and where are the bioactive terpenoids metabolites from Dictyotaceae (Phaeophyceae). Rev. Bras. Farmacogn. 2011, 21, 216–228. [Google Scholar] [CrossRef]
- Li, Z. Advances in marine microbial symbionts in the China sea and related pharmaceutical metabolites. Mar. Drugs 2009, 7, 113–129. [Google Scholar] [CrossRef]
- Cantillo-Ciau, Z.; Moo-Puc, R.; Quijano, L.; Freile-Pelegrín, Y. The tropical brown alga Lobophora variegata: A source of antiprotozoal compounds. Mar. Drugs 2010, 8, 1292–1304. [Google Scholar]
- Pangestuti, R.; Kim, S.-K. Neuroprotective effects of marine algae. Mar. Drugs 2011, 9, 803–818. [Google Scholar] [CrossRef]
- Genovese, G.; Tedone, L.; Hamann, M.; Morabito, M. The Mediterranean red alga Asparagopsis: A source of compounds against Leishmania. Mar. Drugs 2009, 7, 361–366. [Google Scholar] [CrossRef]
- Bradbury, J. Docosahexaenoic acid (DHA): An ancient nutrient for the modern human brain. Nutrients 2011, 3, 529–554. [Google Scholar] [CrossRef]
- Dhargalkar, V.K.; Pereira, N. Seaweed: Promising plant of the millennium. Sci. Cult. 2005, 71, 60–66. [Google Scholar]
- Heo, S.J.; Hwang, J.Y.; Choi, J.I.; Han, J.S.; Kim, H.J.; Jeon, Y.J. Diphlorethohydroxycarmalol isolated from Ishige okamurae, a brown algae, a potent [alpha]-glucosidase and [alpha]-amylase inhibitor, alleviates postprandial hyperglycemia in diabetic mice. Eur. J. Pharmacol. 2009, 615, 252–256. [Google Scholar]
- El Gamal, A.A. Biological importance of marine algae. Saudi Pharma. J. 2010, 18, 1–25. [Google Scholar] [CrossRef]
- Raven, P.H.; Evert, R.F.; Curtis, H. Biologia Vegetal, 6th ed; Guanabara Koogan: Rio de Janeiro, Brazil, 2007; p. 906. [Google Scholar]
- Bold, H.C.; Wynne, M.J. Introduction to the Algae Structure and Reproduction, 2nd ed; Prentice-Hall Inc.: Englewood Cliffs, NJ, USA, 1985; pp. 1–33. [Google Scholar]
- Teixeira, V.L.; Kelecom, A.; Gottlieb, O.R. Produtos naturais de algas marinhas. Quím. Nova 1991, 14, 83–90. [Google Scholar]
- Chatter, R.; Cenac, N.; Roussis, V.; Kharrat, R.; Vergnolle, N. Inhibition of sensory afferents activation and visceral pain by a brominated algal diterpene. Neurogastroenterol. Motil. 2012, 24, e336–e343. [Google Scholar] [CrossRef]
- Chatter, R.; Ben Othman, R.; Rabhi, S.; Kladi, M.; Tarhouni, S.; Vagias, C.; Roussis, V.; Guizani-Tabbane, L.; Kharrat, R. In vivo and in vitro anti-inflammatory activity of neorogioltriol, a new diterpene extracted from the red algae Laurencia glandulifera. Mar. Drugs 2011, 9, 1293–1306. [Google Scholar] [CrossRef]
- Wiemer, D.F.; Idler, D.D.; Fenical, W. Vidalols A and B, new anti-inflammatory bromophenols from the Caribbean marine red alga Vidalia obtusaloba. Experientia 1991, 47, 851–853. [Google Scholar]
- Silva, L.M.; Lima, V.; Holanda, M.L.; Pinheiro, P.G.; Rodrigues, J.A.; Lima, M.E.; Benevides, N.M. Antinociceptive and anti-inflammatory activities of lectin from marine red alga Pterocladiella capillacea. Biol. Pharm. Bull. 2010, 33, 830–835. [Google Scholar] [CrossRef]
- Bitencourt, F.S.; Figueiredo, J.G.; Mota, M.R.; Bezerra, C.C.; Silvestre, P.P.; Vale, M.R.; Nascimento, K.S.; Sampaio, A.H.; Nagano, C.S.; Saker-Sampaio, S.; et al. Antinociceptive and anti-inflammatory effects of a mucin-binding agglutinin isolated from the red marine alga Hypnea cervicornis. Naunyn Schmiedebergs Arch. Pharmacol. 2008, 377, 139–148. [Google Scholar]
- Neves, S.A.; Freitas, A.L.; Sousa, B.W.; Rocha, M.L.; Correia, M.V.; Sampaio, D.A.; Viana, G.S. Antinociceptive properties in mice of a lectin isolated from the marine alga Amansia multifida Lamouroux. Braz. J. Med. Biol. Res. 2007, 40, 127–134. [Google Scholar]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef]
- Medzhitov, V.R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef]
- Tracey, I.; Mantyh, P.W. The cerebral signature for pain perception and its modulation. Neuron 2007, 55, 377–391. [Google Scholar] [CrossRef]
- Hua, S.; Cabot, P.J. Mechanisms of peripheral immunecell-mediated analgesia in inflammation: Clinical and therapeutic implications. Cell 2010, 31, 427–433. [Google Scholar]
- Gris, P.; Gauthier, J.; Cheng, P.; Gibson, D.G.; Gris, D.; Laur, O.; Pierson, J.; Wentworth, S.; Nackley, A.G.; Maixner, W.; et al. A novel alternatively spliced isoform of the mu-opioid receptor: functional antagonism. Mol. Pain 2010, 6, 33. [Google Scholar]
- Kummer, C.L.; Coelho, T.C.R.B. Antiinflamatórios não esteróides inibidores da ciclooxigenase-2 (COX-2): Aspectos Atuais. Rev. Bras. Anestesiol. 2002, 52, 498–512. [Google Scholar]
- Vergnolle, N. Postinflammatory visceral sensitivity and pain mechanisms. Eurogastroenterol. Motil. 2008, 20, 73–80. [Google Scholar] [CrossRef]
- Bezerra-Santos, C.R.; Vieira-de-Abreu, A.; Barbosa-Filho, J.M.; Bandeira-Melo, C.; Piuvezam, M.R.; Bozza, P.T. Anti-allergic properties of Cissampelos sympodialis and its isolated alkaloid warifteine. Int. Immunopharmacol. 2006, 6, 1152–1160. [Google Scholar]
- Costa, H.F.; Bezerra-Santos, C.R.; Barbosa-Filho, J.M.; Martins, M.A.; Piuvezam, M.R. Anti-allergic properties of Cissampelos sympodialis and its isolated alkaloid warifteine. Int. Immunopharmacol. 2008, 8, 519–525. [Google Scholar]
- Vasconcelos, J.F.; Teixeira, M.M.; Barbosa-Filho, J.M.; Agra, M.F.; Nunes, X.P.; Giulietti, A.M.; Ribeiro-dos-Santos, R.; Soares, M.B.P. Effects of umbelliferone in a murine model of allergic airway inflammation. Eur. J. Pharmacol. 2009, 609, 126–131. [Google Scholar]
- Barros, T.A.A.; Freitas, L.A.R.; Filho, J.M.B.; Nunes, X.P.; Giulietti, A.M.; Souza, G.E.; Santos, R.R.; Soares, M.B.P.; Villarreal, C.F. Antinociceptive and anti-inflammatory properties of 7-hydroxycoumarin in experimental animal models: Potential therapeutic for the control of inflammatory chronic pain. J. Pharm. Pharmacol. 2010, 62, 205–213. [Google Scholar]
- Lima, F.O.; Nonato, F.R.; Couto, R.D.; Barbosa Filho, J.M.; Nunes, X.P.; Santos, R.R.; Soares, M.B.P.; Villarreal, C.F. Mechanisms involved in the antinociceptive effects of 7-hydroxycoumarin. J. Nat. Prod. 2011, 74, 596–602. [Google Scholar]
- Leite, C.P.; Araújo, F.L.O.; Melo, C.T.V.; Gutierrez, S.J.C.; Barbosa-Filho, J.M.; Sousa, F.C.F. Anti-inflammatory activity of riparin I (O-methyl-N-benzoyl tyramine) on paw edema models in mice. Inflammat. Res. 2011, 60, 202. [Google Scholar]
- Sousa, F.C.F.; Carvalho, A.M.R.; Leite, C.P.; Rocha, N.F.M.; Rios, E.R.V.; Vasconcelos, L.F.; Melo, C.T.V.; Lima, S.T.; Barbosa-Filho, J.M.; Vasconcelos, S.M.M. Anti-inflammatory activity of riparin II (N-2-hydroxybenzoyl tyramine) in rats. Inflammat. Res. 2011, 60, 206. [Google Scholar]
- Julius, D.; Basbaum, A.I. Molecular mechanisms of nociception. Nature 2001, 413, 203–210. [Google Scholar] [CrossRef]
- Ribeiro, R.A.; Vale, M.L.; Thomazzi, S.M.; Paschoalato, A.B.; Poole, S.; Ferreira, S.H.; Cunha, F.Q. Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by zymosan and acetic acid in mice. Eur. J. Pharmacol. 2000, 387, 111–118. [Google Scholar] [CrossRef]
- Duarte, I.D.G.; Nakamura, M.; Ferreira, S.H. Participation of the sympathetic system in acetic acid-induced writhing in mice. Braz. J. Med. Biol. Res. 1988, 21, 341–343. [Google Scholar]
- Ikeda, Y.; Ueno, A.; Naraba, H.; Oh-Ishi, S. Involvement of vanilloid receptor VR1 and prostanoids in the acid-induced writhing responses of mice. Life Sci. 2001, 69, 2911–2919. [Google Scholar] [CrossRef]
- Hendershot, L.C.; Forsaith, J. Antagonism of the frequency of phenylquinone-induced writhing in the mouse by weak analgesics and nonanalgesics. J. Pharmacol. Exp. Ther. 1959, 125, 237–240. [Google Scholar]
- Chernov, H.I.; Wilson, D.E.; Fowler, W.F.; Plummer, A.J. Non-specificity of the mouse writhing test. Arch. Int. Pharmacodyn. Ther. 1967, 167, 171–178. [Google Scholar]
- Pearl, J.; Aceto, M.D.; Harris, L.S. Prevention of writhing and other effects of narcotics and narcotic antagonists in mice. J. Pharmacol. Exp. Ther. 1968, 160, 217–230. [Google Scholar]
- Loux, J.J.; Smith, S.; Salem, H. Comparative analgesic testing of various compounds in mice using writhing techniques. Arzneim. Forsch. 1978, 28, 1644–1647. [Google Scholar]
- Kuraishi, Y.; Harada, Y.; Aratani, S.; Satoh, M.; Takagi, H. Involvement of the spinal noradrenergic and serotonergic systems in morphine analgesia: the differences in mechanical and thermal algesic tests. Brain Res. 1983, 273, 245–252. [Google Scholar] [CrossRef]
- Le Bars, D.; Gozariu, M.; Cadden, S.W. Animal models of nociception. Pharmacol. Rev. 2001, 53, 597–652. [Google Scholar]
- Miranda, F.G.G.; Vilar, J.C.; Alves, I.A.; Cavalcanti, S.C.; Antoniolli, A.R. Antinociceptive and antiedematogenic properties and acute toxicity of Tabebuia avellanedae Lor. ex Griseb. inner bark aqueous extract. BMC Pharmacol. 2001, 1, 1–16. [Google Scholar]
- El Habazi, K.; Aboufatima, R.; Benharref, A.Z.; Chait, A.; Dalal, A. Study on the antinociceptive effects of Thymus broussonetii Boiss extracts in mice and rats. J. Ethnopharmacol. 2006, 107, 406–411. [Google Scholar]
- Hunskaar, S.; Hole, K. The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain 1987, 30, 103–114. [Google Scholar] [CrossRef]
- Tjølsen, A.; Berge, O.G.; Hunskaar, S.; Rosland, J.H.; Hole, K. The formalin test: An evaluation of the method. Pain 1992, 51, 5–17. [Google Scholar] [CrossRef]
- Granados-Soto, V.; Alonso-Lopez, R.; Asomoza-Espinosa, R.; Rufino, M.O.; Gomes-Lopes, L.D.; Ferreira, S.H. Participation of COX, IL-1β and TNF-α in formalin-induced inflammatory pain. Proceed. Western Pharmacol. Soc. 2001, 44, 15–17. [Google Scholar]
- Beirith, A.; Santos, A.R.; Calixto, J.B. Mechanisms underlying the nociception and paw oedema caused by injection of glutamate into the mouse paw. Brain Res. 2002, 924, 219–228. [Google Scholar] [CrossRef]
- Aanonsen, L.M.; Wilcox, G.L. Excitatory amino acid receptors and nociceptive neurotransmission in rat spinal cord. Pain 1990, 41, 309–321. [Google Scholar] [CrossRef]
- Ferreira, J.; Santos, A.R.S.; Calixto, J.B. The role of systemic, spinal and supraspinal L-arginine-nitric oxide-cGMP pathway in thermal hyperalgesia caused by intrathecal injection of glutamate in mice. Neuropharmacology 1999, 38, 835–842. [Google Scholar] [CrossRef]
- Mao, J.; Price, D.D.; Hayes, R.L.; Lu, J.; Mayer, D.J. Differential roles of NMDA and non-NMDA receptor activation in induction and maintenance of thermal hyperalgesia in rats with painful peripheral mononeuropathy. Brain Res. 1992, 598, 271–278. [Google Scholar] [CrossRef]
- Sato, M.; Sano, H.; Iwaki, D.; Kudo, K.; Konishi, M.; Takahashi, H.; Takahashi, T.; Imaizumi, H.; Asai, Y.; Kuroki, Y. Direct binding of toll-like receptor 2 to zymosan, and zymosan-induced NF-κB activation and TNF-α secretion are down-regulated by lung collectin surfactant protein A. J. Immunol. 2003, 171, 417–425. [Google Scholar]
- Daum, T.; Rohrbach, M.S. Zymosan induces selective release of arachidonic acid from rabbit alveolar macrophages via stimulation of a β-glucan receptor. FEBS Lett. 1992, 309, 119–122. [Google Scholar] [CrossRef]
- Noble, P.W.; Henson, P.M.; Lucas, C.; Mora-Worms, M.; Carre, P.C.; Riches, D.W.H. Transforming growth factor-β primes macrophages to express inflammatory gene products in response to particulate stimuli by an autocrine/paracrine mechanism. J. Immunol. 1993, 151, 979–989. [Google Scholar]
- Okazaki, M.; Chiba, N.; Adachi, Y.; Ohno, N.; Yadomae, T. Signal transduction pathway on-glucans-triggered hydrogen peroxide production by murine peritoneal macrophages in vitro. Biol. Pharm. Bull. 1996, 19, 18–23. [Google Scholar] [CrossRef]
- Hsieh, G.C.; Chandran, P.; Salyers, A.K.; Pai, M.; Zhu, C.Z.; Wensink, E.J.; Witte, D.G.; Miller, T.R.; Mikusa, J.P.; Baker, S.J.; et al. H4 receptor antagonism exhibits anti-nociceptive effects in inflammatory and neuropathic pain models in rats. Pharmacol. Biochem. Behav. 2010, 95, 41–50. [Google Scholar]
- Strakhova, M.I.; Cuff, C.A.; Manelli, A.M.; Carr, T.L.; Witte, D.G.; Baranowski, J.L.; Vortherms, T.A.; Miller, T.R.; Rundell, L.; McPherson, M.J.; et al. In vitro and in vivo characterization of A-940894: A potent histamine H4 receptor antagonist with anti-inflammatory properties. Br. J. Pharmacol. 2009, 157, 44–54. [Google Scholar]
- Bitencourt, M.A.O.; Dantas, G.R.; Lira, D.P.; Barbosa-Filho, J.M.; Miranda, G.E.C.; Santos, B.V.O.; Souto, J.T. Aqueous and methanolic extracts of Caulerpa mexicana suppress the cell migration and ear edema induced by inflammatory agents. Mar. Drugs 2011, 9, 1332–1345. [Google Scholar] [CrossRef]
- Vidal, A.; Fallarero, A.; Andrade-Wartha, E.R.S.; Silva, A.M.O.; Lima, A.; Torres, R.P.; Vuorela, P.; Mancini-Filho, J. Composición química y actividad antioxidante del alga marina roja Bryothamnion triquetrum (S.G.Gmelin) Howe. Rev. Bras. Cienc. Farm. 2006, 42, 509–600. [Google Scholar]
- Zubia, M.; Robledo, D.; Freile-Pelegrin, Y. Antioxidant activities in tropical marine macroalgae from the Yucatan Peninsula, Mexico. J. Appl. Phycol. 2007, 19, 449–458. [Google Scholar] [CrossRef]
- Fallarero, A.; Loikkanen, J.J.; Mansito, P.T.; Castañeda, O.; Vidal, A. Effects of aqueous extracts of Halimeda incrassata (Ellis) Lamouroux and Bryothamnion triquetrum (S.G. Gmelin) Howe on hydrogen peroxide and methyl mercury-induced oxidative stress in GT1-7 mouse hypothalamic immortalized cells. Phytomedicine 2003, 10, 39–47. [Google Scholar] [CrossRef]
- Maia, R.M.; Moura, C.W.N.; Bispo, V.S.; Santos, J.L.A.; Santana, R.S.; Matos, H.R. Avaliação do sequestro do óxido nítrico (NO) pelo extrato metanólico da alga Bryothamnion triquetrum (Gmelin) Howe. Rev. Bras. Farmacogn. 2010, 20, 489–493. [Google Scholar]
- Vidal, A.N.; Motidome, M.; Mancini Filho, J.; Fallarero Linares, A.; Tanae, M.M.; Torres, L.M.B.; Lapa, A.J. Actividad antioxidante y ácidos fenólicos del alga marina Bryothamnion triquetrum (S.G.Gmelim) Howe. Rev. Bras. Cienc. Farm. 2001, 37, 373–382. [Google Scholar]
- Wang, Z.Q.; Porreca, F.; Cuzzocrea, S.; Galen, K.; Lightfoot, R.; Masini, E.; Muscoli, C.; Mollace, V.; Ndengele, M.; Ischiropoulos, H.; et al. A newly identified role for superoxide in inflammatory pain. J. Pharmacol. Exp. Ther. 2004, 309, 869–878. [Google Scholar] [CrossRef]
- Hacimuftuoglu, A.; Handy, C.R.; Goettl, V.M.; Lin, C.G.; Dane, S.; Stephens, R.L., Jr. Antioxidants attenuate multiple phases of formalin-induced nociceptive response in mice. Behav. Brain Res. 2006, 173, 211–216. [Google Scholar]
- Viana, G.S.B.; Freitas, A.L.P.; Lima, M.M.L.; Vieira, L.A.P.; Andrade, M.C.H.; Benevides, N.M.B. Antinociceptive activity of sulfated carbohydrates from the red algae Bryothamnion seaforthii (Turner) Kütz. and B. triquetrum (S.G. Gmel.) M. Howe. Braz. J. Med. Biol. Res. 2002, 35, 713–722. [Google Scholar]
- Vieira, L.A.P.; Freitas, A.L.P.; Feitosa, J.P.A.; Silva, D.C.; Viana, G.S.B. The alga Bryothamnion seaforthii contains carbohydrates with antinociceptive activity. Braz. J. Med. Biol. Res. 2004, 37, 1071–1079. [Google Scholar] [CrossRef]
- Romay, Ch.; González, R.; Ledón, N.; Remirez, D.; Rimbau, V. C-phycocyanin: A biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Curr. Protein Pept. Sci. 2003, 4, 207–216. [Google Scholar] [CrossRef]
- Shih, C.M.; Cheng, S.N.; Wong, C.S.; Kuo, Y.L.; Chou, T.C. Antiinflammatory and antihyperalgesic activity of C-phycocyanin. Anesth. Analg. 2009, 108, 1303–1310. [Google Scholar] [CrossRef]
- Species Link. Available online: http://www.specieslink.com (accessed on 20 June 2012).
- Almeida, R.N.; Falcão, A.C.G.M.; Diniz, R.S.T.; Quintanas-Júnior, L.J.; Polari, R.M.; Barbosa-Filho, J.M.; Agra, M.F.; Duarte, J.C.; Ferreira, C.D.; Antoniolli, A.R.; Araújo, C.C. Metodologia para avaliação de plantas com atividade no Sistema Nervoso Central e alguns dados experimentais. Rev. Bras. Farm. 1999, 80, 72–76. [Google Scholar]
- Collier, H.O.J.; Dinneen, L.C.; Johnson, C.A.; Schneider, C. The abdominal constriction response and its suppression by analgesic drugs in mice. Br. J. Pharmacol. 1968, 32, 285–310. [Google Scholar]
- Doherty, N.X.; Poubelle, P.; Borgeat, P.; Beave, T.H.; Westrich, G.L.; Schrader, N.L. Intraperitoneal injection of zymosan in mice induces pain, inflammation and the synthesis of peptidoleukotrienes and prostaglandin E2. Prostaglandins 1985, 30, 769–789. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cavalcante-Silva, L.H.A.; Barbosa Brito da Matta, C.; De Araújo, M.V.; Barbosa-Filho, J.M.; Pereira de Lira, D.; De Oliveira Santos, B.V.; De Miranda, G.E.C.; Alexandre-Moreira, M.S. Antinociceptive and Anti-Inflammatory Activities of Crude Methanolic Extract of Red Alga Bryothamnion triquetrum. Mar. Drugs 2012, 10, 1977-1992. https://doi.org/10.3390/md10091977
Cavalcante-Silva LHA, Barbosa Brito da Matta C, De Araújo MV, Barbosa-Filho JM, Pereira de Lira D, De Oliveira Santos BV, De Miranda GEC, Alexandre-Moreira MS. Antinociceptive and Anti-Inflammatory Activities of Crude Methanolic Extract of Red Alga Bryothamnion triquetrum. Marine Drugs. 2012; 10(9):1977-1992. https://doi.org/10.3390/md10091977
Chicago/Turabian StyleCavalcante-Silva, Luiz Henrique Agra, Carolina Barbosa Brito da Matta, Morgana Vital De Araújo, José Maria Barbosa-Filho, Daysianne Pereira de Lira, Bárbara Viviana De Oliveira Santos, George Emmanuel C. De Miranda, and Magna Suzana Alexandre-Moreira. 2012. "Antinociceptive and Anti-Inflammatory Activities of Crude Methanolic Extract of Red Alga Bryothamnion triquetrum" Marine Drugs 10, no. 9: 1977-1992. https://doi.org/10.3390/md10091977
APA StyleCavalcante-Silva, L. H. A., Barbosa Brito da Matta, C., De Araújo, M. V., Barbosa-Filho, J. M., Pereira de Lira, D., De Oliveira Santos, B. V., De Miranda, G. E. C., & Alexandre-Moreira, M. S. (2012). Antinociceptive and Anti-Inflammatory Activities of Crude Methanolic Extract of Red Alga Bryothamnion triquetrum. Marine Drugs, 10(9), 1977-1992. https://doi.org/10.3390/md10091977



