Pseudoalteromone B: A Novel 15C Compound from a Marine Bacterium Pseudoalteromonas sp. CGH2XX
Abstract
:1. Introduction
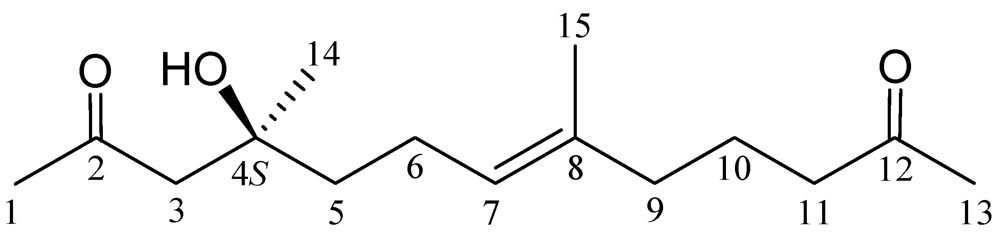
2. Results and Discussion
| Position | δH (J in Hz) | δC, Mult. |
|---|---|---|
| 1 | 2.18 s | 31.9, CH3 |
| 2 | 211.0, qC | |
| 3a/b | 2.58 d (17.2); 2.65 d (17.2) | 52.3, CH2 |
| 4 | 71.5, qC | |
| 5 | 1.51 m | 41.9, CH2 |
| 6 | 2.04 m | 22.5, CH2 |
| 7 | 5.09 tq (7.2, 1.2) | 124.8, CH |
| 8 | 134.6, qC | |
| 9 | 1.96 t (7.2) | 38.8, CH2 |
| 10 | 1.66 quintet (7.2) | 21.8, CH2 |
| 11 | 2.37 t (7.2) | 43.0, CH2 |
| 12 | 209.1, qC | |
| 13 | 2.12 s | 29.9, CH3 |
| 14 | 1.22 s | 26.7, CH3 |
| 15 | 1.58 br s | 15.7, CH3 |
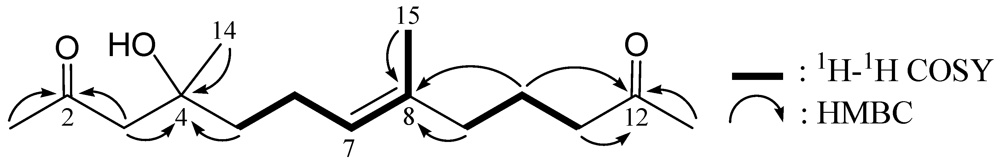
3. Experimental Section
3.1. General Experimental Procedures
3.2. Marine Bacteria Isolation, Culture Conditions and Extract Preparation
3.3. Separation
3.4. Cytotoxicity Testing
3.5. Elastase Release by Human Neutrophils
4. Conclusions
Acknowledgments
References and Notes
- Bowman, J.P. Bioactive compound synthetic capacity and ecological significance of marine bacterial genus Pseudoalteromonas. Mar. Drugs 2007, 5, 220–241. [Google Scholar] [CrossRef]
- Fehér, D.; Barlow, R.S.; Lorenzo, P.S.; Hemscheidt, T.K. A 2-substituted prodiginine, 2-(p-hydroxybenzyl)prodigiosin, from Pseudoalteromonas rubra. J. Nat. Prod. 2008, 71, 1970–1972. [Google Scholar] [CrossRef]
- Fehér, D.; Barlow, R.; McAtee, J.; Hemscheidt, T.K. Highly brominated antimicrobial metabolites from a marine Pseudoalteromonas sp. J. Nat. Prod. 2010, 73, 1963–1966. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Lu, M.-C.; Chang, Y.-C.; Hwang, T.-L.; Wang, W.-H.; Weng, C.-F.; Kuo, J.; Sung, P.-J. Pseudoalteromone A: a novel bioactive ubiquinone from a marine bacterium Pseudoalteromonas sp. CGH2XX (Pseudoalteromonadaceae). Tetrahedron Lett. 2012, 53, 1675–1677. [Google Scholar]
- Chen, I.-H.; Kanai, M.; Shibasaki, M. Copper(I)—Secondary diamine complex-catalyzed enantioselective conjugate boration of linear β,β-disubstituted enones. Org. Lett. 2010, 12, 4098–4101. [Google Scholar] [CrossRef]
- Doxorubicin was used as a reference compound in cytototxicity testing. Doxorubicin showed cytotoxicity toward HCT116, K-562, HL-60, CCRF-CEM, T-47D, and MDA-MB-231 cells (IC50 = 1.8, 0.8, 0.2, 0.1, 1.5 and 1.7 µg/mL).
- Alley, M.C.; Scudiero, D.A.; Monks, A.; Hursey, M.L.; Czerwinski, M.J.; Fine, D.L.; Abbott, B.J.; Mayo, J.G.; Shoemaker, R.H.; Boyd, M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988, 48, 589–601. [Google Scholar]
- Scudiero, D.A.; Shoemaker, R.H.; Paull, K.D.; Monks, A.; Tierney, S.; Nofziger, T.H.; Currens, M.J.; Seniff, D.; Boyd, M.R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988, 48, 4827–4833. [Google Scholar]
- In the in vitro anti-inflammatory bioassay, the inhibitory effect on the release of elastase by activated neutrophils was used as an indicator. For significant activity of pure compounds, an inhibition rate ≥ 40% is required (inhibition rate ≤ 10%, not active; 20% ≥ inhibition rate ≥ 10%, weakly anti-inflammatory; 40% ≥ inhibition rate ≥ 20%, modestly anti-inflammatory). Elastatinal was used as a reference compound in anti-inflammatory activity test (IC50 = 31.9 µg/mL).
- Hwang, T.-L.; Wang, C.-C.; Kuo, Y.-H.; Huang, H.-C.; Wu, Y.-C.; Kuo, L.-M.; Wu, Y.-H. The hederagenin saponin SMG-1 is a natural FMLP receptor inhibitor that suppresses human neutrophil activation. Biochem. Pharmacol. 2010, 80, 1190–1200. [Google Scholar]
- Yu, H.-P.; Hsieh, P.-W.; Chang, Y.-J.; Chung, P.-J.; Kuo, L.-M.; Hwang, T.-L. 2-(2-Fluorobenz-amido)benzoate ethyl ester (EFB-1) inhibits superoxide production by human neutrophils and attenuates hemorrhagic shock-induced organ dysfunction in rats. Free Radic. Biol. Med. 2011, 50, 1737–1748. [Google Scholar] [CrossRef]
- The authors regret that there is an error in pages 1 and 2 of [4] (pages 1675 and 1676 of the issue). In [4], the ubiquinone, pseudoalteromone A, was reported to display an inhibitory effect on the release of elastase (inhibition rate 45.1%) by human nuetrophils at a concentration of 10 µg/mL. However, after detailed collating, we found this data was cited incorrectly. The data (inhibition rate 45.1%) expressed an inhibitory effect of an organic extract from the marine bacterium Pseudoalteromonas sp. CGH2XX on the release of elastase by human nuetrophils as presented in this study. The in vitro anti-inflammatory effects of pseudoalteromone A were tested again. Pseudoalteromone A displayed moderately inhibitory effects on the generation of superoxide anion and the release of elastase (inhibition rates 38.0% and 20.2%) by human neutrophils at a concentration of 10 µg/mL. Diphenyl indonium (DPI) and elastatinal were used as reference compounds in anti-inflammatory activity testing. DPI displayed an inhibitory effect on superoxide anion generation (IC50 = 0.9 µg/mL), and elastatinal exhibited an inhibitory effect on elastase release (IC50 = 31.9 µg/mL) by human neutrophils, respectively. The authors apologize for any inconvenience caused by this error.
- Samples Availability: Not available.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, Y.-H.; Kuo, J.; Su, J.-H.; Hwang, T.-L.; Chen, Y.-H.; Lee, C.-H.; Weng, C.-F.; Sung, P.-J. Pseudoalteromone B: A Novel 15C Compound from a Marine Bacterium Pseudoalteromonas sp. CGH2XX. Mar. Drugs 2012, 10, 1566-1571. https://doi.org/10.3390/md10071566
Chen Y-H, Kuo J, Su J-H, Hwang T-L, Chen Y-H, Lee C-H, Weng C-F, Sung P-J. Pseudoalteromone B: A Novel 15C Compound from a Marine Bacterium Pseudoalteromonas sp. CGH2XX. Marine Drugs. 2012; 10(7):1566-1571. https://doi.org/10.3390/md10071566
Chicago/Turabian StyleChen, Yu-Hsin, Jimmy Kuo, Jui-Hsin Su, Tsong-Long Hwang, Yung-Husan Chen, Chia-Hung Lee, Ching-Feng Weng, and Ping-Jyun Sung. 2012. "Pseudoalteromone B: A Novel 15C Compound from a Marine Bacterium Pseudoalteromonas sp. CGH2XX" Marine Drugs 10, no. 7: 1566-1571. https://doi.org/10.3390/md10071566
APA StyleChen, Y.-H., Kuo, J., Su, J.-H., Hwang, T.-L., Chen, Y.-H., Lee, C.-H., Weng, C.-F., & Sung, P.-J. (2012). Pseudoalteromone B: A Novel 15C Compound from a Marine Bacterium Pseudoalteromonas sp. CGH2XX. Marine Drugs, 10(7), 1566-1571. https://doi.org/10.3390/md10071566







