Abstract
Chemical investigation of Junceella juncea has resulted in the isolation of three new briaranes designated juncenolides M–O (1–3). The structures of these compounds were determined by spectroscopic analysis including 2D-NMR (COSY, HMBC and NOESY) and HRMS. Compound 1 is a new chlorinated briarane while compound 3 contains a rare methyl ester at C-16. The anti-inflammatory activities tested on superoxide anion generation and elastase release by human neutrophils in response to FMLP/CB were evaluated.
1. Introduction
Gorgonian corals of the genus Junceella (Ellisellidae) are common in subtropical and tropical waters in a number of places around the world, such as the South China Sea and Indo-Pacific Ocean, and are well known as a source of highly oxidized diterpenes of the briarane class (3,8-cyclized cembranoids) [1]. Many in vitro and in vivo studies on diterpenes isolated from gorgonians showed a variety of biological activities including anti-tumor, anti-inflammatory, antiplasmodial, antibacterial, antiviral, antimalarial and antioxidant, as well as ecologically relevant activities such as fish-feeding deterrence. Diterpenes isolated from gorgonian corals have a large structural diversity with 40 different diterpene classes being represented [2]. Recently, three new 8-hydroxybriarane diterpenoids (junceols A–C) and four new briarane diterpenoids (juncenolides H–K) were reported from a chemical investigation of Junceella juncea Pallas collected off the southern Taiwan coast, and some of these metabolites were found to exhibit inhibitory effects on superoxide anion generation and elastase release by human neutrophils [3,4]. Fourteen new briarane diterpenes, juncins O–ZII, were isolated from the EtOH/CH2Cl2 extract of a South China Sea sample of J. juncea and some of them have been shown to exhibit potent antifouling and antifeedant activities [5,6,7]. A bioassay-guided fractionation of the acetone extract of a Taiwanese collection of J. juncea led to the identification of seven new diterpenoids, juncenolides A–G [8,9,10,11]. Moreover, a chemical investigation of the Indian Ocean gorgonian J. juncea resulted in the isolation of eight new briarane-type diterpenoids, juncins G–N [12,13,14]. A new briarane diterpenoid with antifungal activity was also isolated [15]. In continuation of our research programs oriented towards discovering new metabolites from the gorgonians collected off Taiwanese waters, we reinvestigated J. juncea. Examination of different chromatographic fractions of an AcOEt-soluble extract of the Taiwanese J. juncea Pallas resulted in the isolation of three new briaranes, designated juncenolides M–O (1–3) (Figure 1). Their structures were elucidated through detailed spectroscopic analyses, mainly 2D NMR experiments (1H, 1H COSY, HQMC, HMBC). The relative stereochemistry of the chiral centers and the geometry of the double bonds were deduced from NOESY spectra.
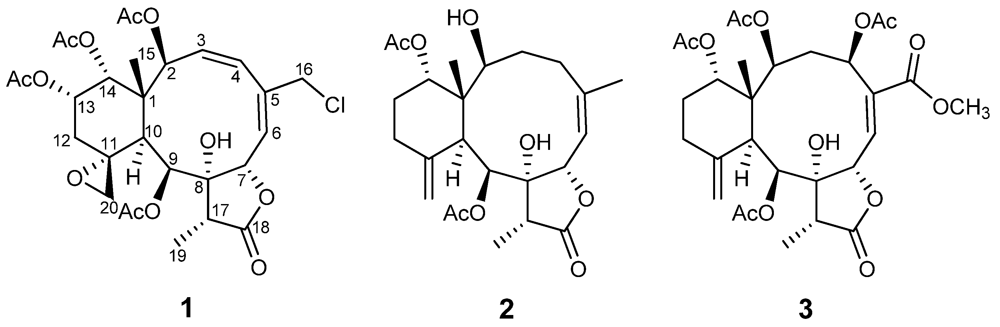
Figure 1.
Structures of compounds 1–3.
Figure 1.
Structures of compounds 1–3.
2. Results and Discussion
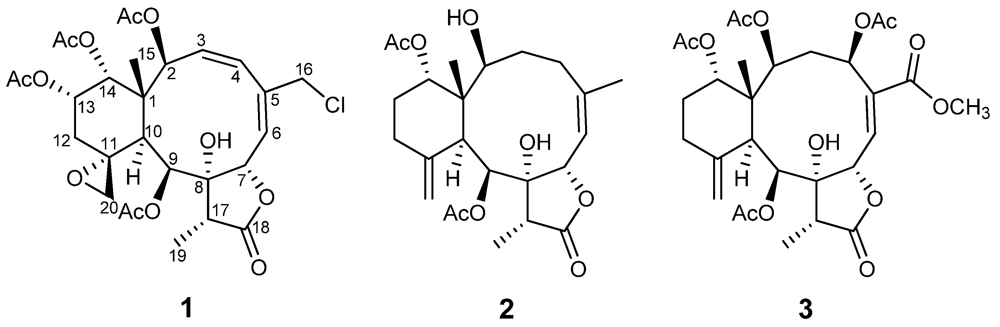
Compound 1 was isolated as a colorless amorphous solid. The molecular formula was determined to be C28H35ClO12 (11 degrees of unsaturation) from the HR-ESI-MS data (m/z 621.1711 ([M + Na]+)), which also showed a M + 2 peak at m/z 623.1685 (3:1), indicating the presence of one chlorine atom. Its IR bands revealed the presence of a hydroxyl group (3402 cm−1), a five-membered lactone (1779 cm−1) and ester groups (1741 cm−1). 1H- and 13C-NMR data (Table 1 and Table 2) indicated the presence of four acetates (δH 2.18, s; 2.07, s; 1.97, s; 1.95, s) and δC 170.2 × 2; 170.0 × 2; 21.6; 21.3; 21.0; 20.8, one carbonyl carbon (δC 175.3), and two double bonds (δC 126.1; 128.1; 131.7; 140.0), suggesting four rings in the structure. The protons of CH2-20 (δH 3.54, br. s; 2.74, br. s), its corresponding carbon (δC 50.2) and the quaternary carbon at δC 58.1, were assigned to an exocyclic epoxide [16]. The presence of a γ-lactone ring was ascertained by the carbonyl carbon at δC 175.3 (C-18), and the O-bearing carbons at δC 78.5 (C-7) and 81.2 (C-8), and confirmed the HMBC correlations (Figure 2) of Me-19/C-8, C-17, C-18 and C-7/C-8, C-18 [16,17]. Four OAc groups were attached to C-2, C-9, C-13 and C-14 by the observation of HMBC correlations (Figure 2). A tertiary methyl signal (δH 1.09, Me-15) correlated with a quaternary carbon (C-1), two oxymethines at δC 74.2 and 73.8, and the CH at δC 41.4, (C-10), implying oxygenation at C-2 and C-14. HMBC correlations of Me-15/C-1, C-2, C-10, C-14, CH2-16/C-4, C-5, C-6, CH-7/C-5, C-8, C-18, CH-9/C-8, C-11, and CH-10/C-1, C-8, C-11, C-20, as well as 1H–1H COSY connectivities between CH-2/CH-3/CH-4, CH-6/CH-7, CH-9/CH-10, CH2-12/CH-13/CH-14 (Figure 1), suggested that compound 1 possesses 8-hydroxybriarane-type diterpenoid skeleton together with an exocyclic epoxy group which was corroborated by HMBC correlations of CH2-12/C-11, C-20.

Table 1.
1H NMR Data of Compounds 1–3. δ in ppm, J in Hz.
| Position | 1 a | 2 b | 3 b |
|---|---|---|---|
| 2 | 5.37 (d, J = 9.6) | 4.94 (overlap) | 4.86 (d, J = 7.6) |
| 3 | 5.62 (t, J = 9.6) | 1.74–1.78 (m) | 2.13–2.16 (m) |
| 2.46–2.50 (m) | 2.72 (t, J = 10.0) | ||
| 4 | 6.35 (d, J = 9.6) | 2.19–2.15 (m) | 5.91–5.94 (m) |
| 2.64 (br. d, J = 13.6) | |||
| 6 | 6.00 (d, J = 9.0) | 5.67 (d, J = 10.4) | 7.06 (d, J = 10.0) |
| 7 | 4.95 (d, J = 9.0) | 5.26 (d, J = 10.4) | 5.62 (d, J = 10.0) |
| 9 | 4.71 (d, J = 4.8) | 5.31 (d, J = 6.0) | 5.56 (d, J = 2.8) |
| 10 | 3.04 (d,J = 4.8) | 3.45 (d, J = 6.0) | 3.25 (d, J = 2.8) |
| 12 | 1.34–1.38 (m) | 2.16–2.19 (m) | 2.18–2.23 (2H, m) |
| 2.48 (d, J = 14.0) | 2.34–2.36 (m) | ||
| 13 | 4.96 (overlap) | 1.82–1.86 (m) | 1.80–1.87 (2H, m) |
| 1.97–1.99 (m) | |||
| 14 | 5.20 (br. s) | 4.59 (br. s) | 4.69 (br. s) |
| 15 | 1.09 (s) | 1.12 (s) | 1.03 (s) |
| 16 | 4.58 (2H, s) | 2.05 (s) | - |
| 17 | 2.26 (q, J = 6.9) | 2.46 (q, J = 6.8) | 2.63 (q, J = 6.8) |
| 19 | 1.13 (d, J = 6.9) | 1.11 (d, J = 6.8) | 1.19 (d, J = 6.8) |
| 20 | 2.74 (br. s) | 4.92 (s) | 4.95 (s) |
| 3.54 (br. s) | 5.05 (s) | 5.05 (s) | |
| AcO-2 | 1.95 (s) | - | 1.97 (s) |
| AcO-4 | - | - | 2.06 (s) |
| AcO-9 | 2.18 (s) | 2.20 (s) | 2.21 (s) |
| AcO-13 | 2.07 (s) | - | - |
| AcO-14 | 1.97 (s) | 1.92 (s) | 1.90 (s) |
| OMe-16 | - | - | 3.83 (s) |
a Recorded in CDCl3 at 300 MHz; b Recorded in CDCl3 at 400 MHz.

Table 2.
13C-NMR Data of Compounds 1–3.δ in ppm.
| Position | 1 a | 2 b | 3 b |
|---|---|---|---|
| 1 | 46.5 (s) | 46.9 (s) | 47.8 (s) |
| 2 | 74.2 (d) | 75.7 (d) | 72.1 (d) |
| 3 | 131.7 (d) | 31.3 (t) | 37.3 (t) |
| 4 | 128.1 (d) | 29.2 (t) | 67.4 (d) |
| 5 | 140.0 (s) | 144.8 (s) | 136.8 (s) |
| 6 | 126.1 (d) | 120.5 (d) | 139.1 (d) |
| 7 | 78.5 (d) | 77.8 (d) | 76.7 (d) |
| 8 | 80.8 (s) | 83.0 (s) | 82.9 (s) |
| 9 | 64.3 (d) | 71.3 (d) | 72.7 (d) |
| 10 | 35.7 (d) | 41.8 (d) | 42.6 (d) |
| 11 | 58.1 (s) | 150.8 (s) | 150.5 (s) |
| 12 | 34.3 (t) | 26.3 (t) | 29.2 (t) |
| 13 | 67.7 (d) | 26.9 (t) | 27.5 (t) |
| 14 | 73.8 (d) | 74.5 (d) | 74.0 (d) |
| 15 | 14.4 (q) | 15.4 (q) | 14.4 (q) |
| 16 | 44.7 (t) | 27.2 (q) | 166.6 (s) |
| 17 | 43.9 (d) | 42.5 (d) | 43.3 (d) |
| 18 | 175.3 (s) | 175.9 (s) | 175.3 (s) |
| 19 | 6.3 (q) | 6.5 (q) | 6.4 (q) |
| 20 | 50.2 (t) | 113.3 (t) | 113.3 (t) |
| AcO-2 | 170.2 (s) | - | 169.9 (s) |
| 20.8 (q) | 20.8 (q) | ||
| AcO-4 | - | - | 169.6 (s) |
| 21.2 (q) | |||
| AcO-9 | 170.0 (s) | 169.3 (s) | 169.2 (s) |
| 21.6 (q) | 21.7 (q) | 21.7 (q) | |
| AcO-13 | 170.0 (s) | - | - |
| 21.3 (q) | |||
| AcO-14 | 170.2 (s) | 170.4 (s) | 170.5 (s) |
| 21.0 (q) | 21.2 (q) | 21.1 (q) | |
| OMe-16 | - | - | 52.8 (q) |
a Recorded in CDCl3 at 75 MHz; b Recorded in CDCl3 at 100 MHz.
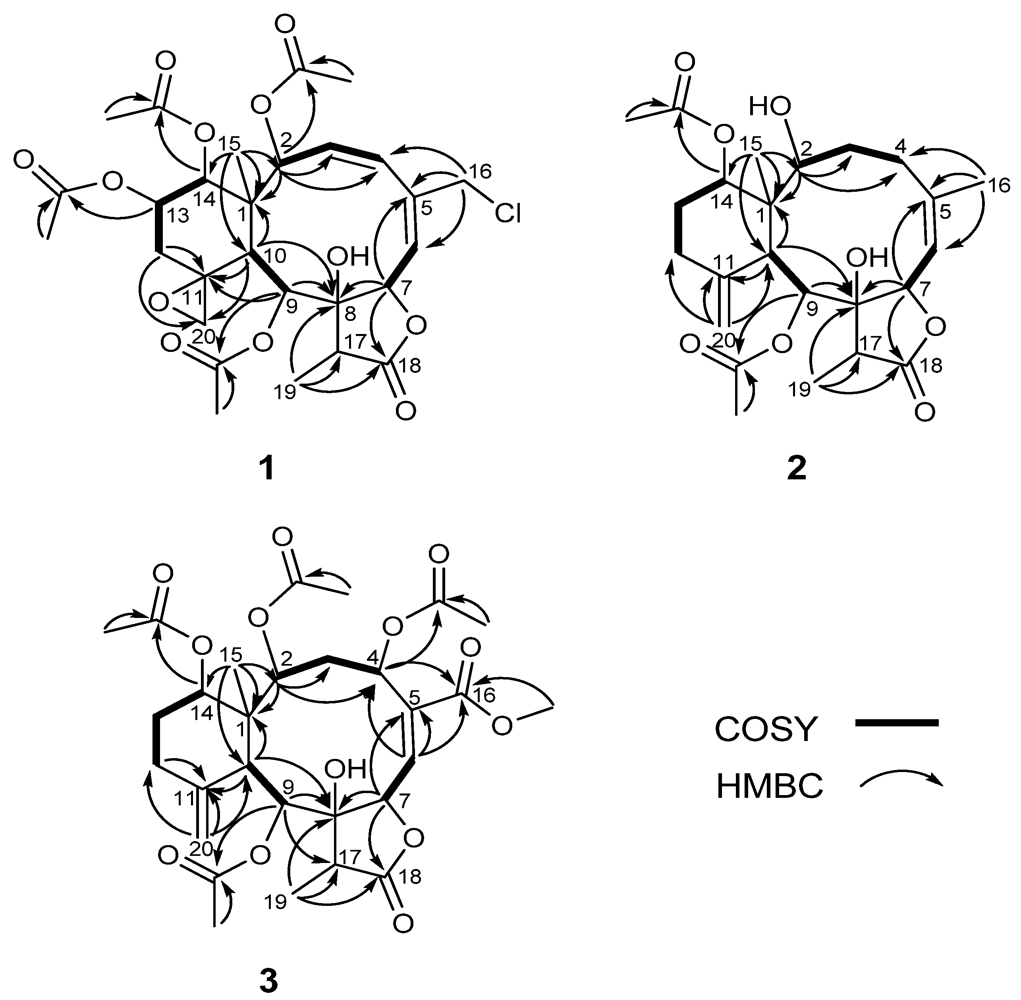
Figure 2.
Key 1H–1H COSY and HMBC correlations of 1–3.
Figure 2.
Key 1H–1H COSY and HMBC correlations of 1–3.
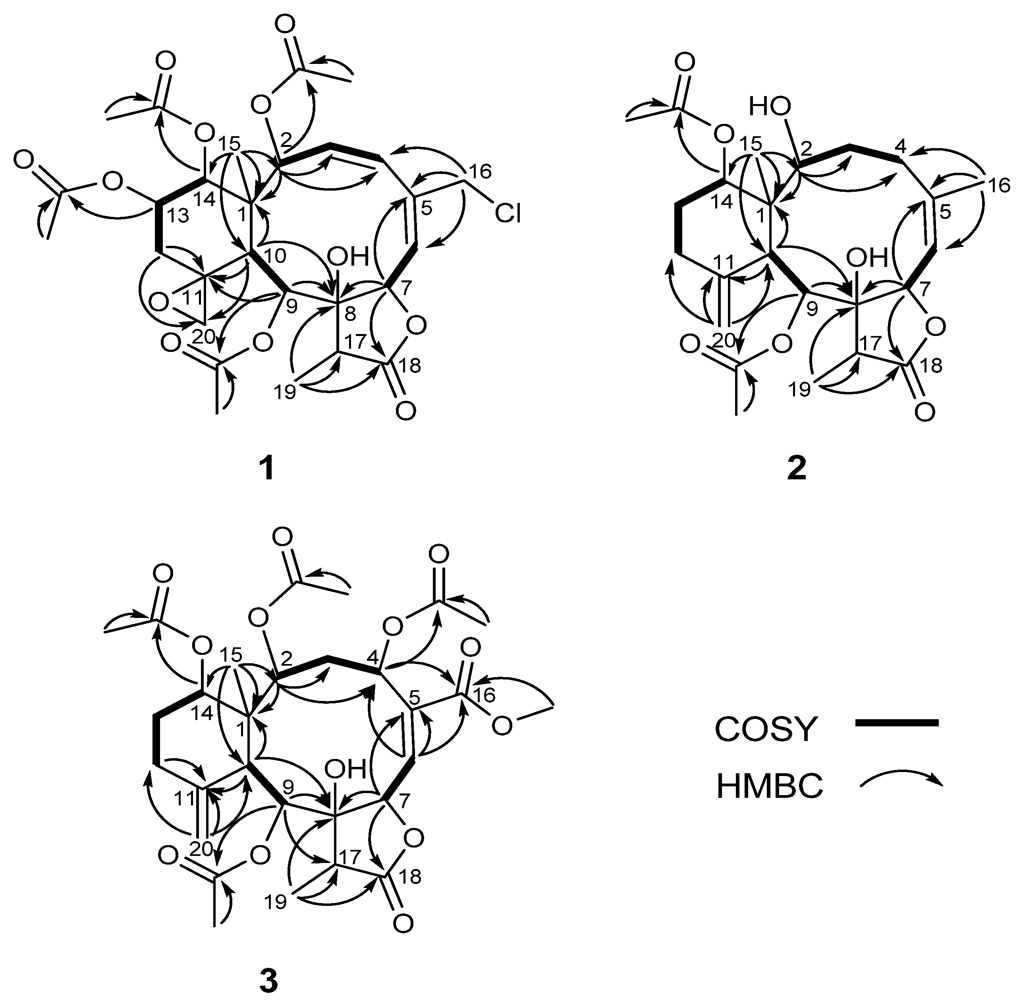
The relative configuration of 1 was determined on the basis of NOESY experiment, MM2 minimized energy calculated molecular modeling (Figure 3), and comparison with other naturally occurring briarane diterpenoids. Briarane-type diterpenoids were previously reported to contain the Me-15 in the β-orientation and H-10 in the α-orientation. As expected, there is no NOE correlation between CH-10 and Me-15. The orientations of CH-10 and the methyl group Me-15 should be opposite. According to MM2 study, we thus concluded that compound 1 had the Me-15 in the β-orientation and CH-10 in the α-orientation as reported [18]. NOESY correlations of CH-10/H-2, CH-9, CHα-12, Me-15/CH2-20, CH-14, CH2-20/CH-13, CHβ-12 and CH-14/CH-13 suggested the β-orientation of CH-13 and CH-14 and α-orientation of CH-2 and CH-9, a β-oriented exocyclic epoxy group attached to cyclohexane moiety as previously assigned from the 1H- and 13C NMR data of C-11 and C-20 [19] and the α-orientation of CH-2. The cis configuration of the C-3/C-4 double bond was suggested by the NOESY correlations (Figure 3) between CH-3/CH-4 and the J value (9.6 Hz). Compound 1 appears to be the chlorinated derivative of juncenolide B isolated from the same species previously [9]. They have the same configuration of chiral centers. Based on the above interpretation, compound 1 is a new chlorinated briarane-type diterpene designated juncenolide M.
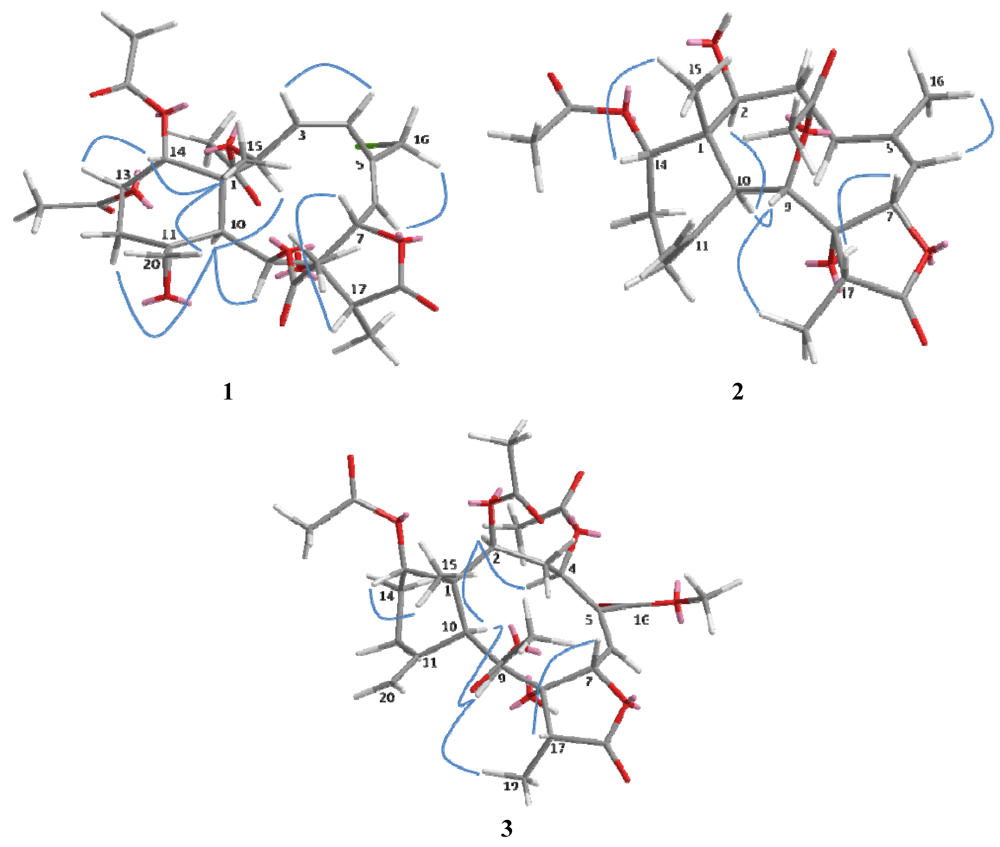
Figure 3.
Key NOESY correlations of compounds 1–3 in molecular modeling.
Figure 3.
Key NOESY correlations of compounds 1–3 in molecular modeling.
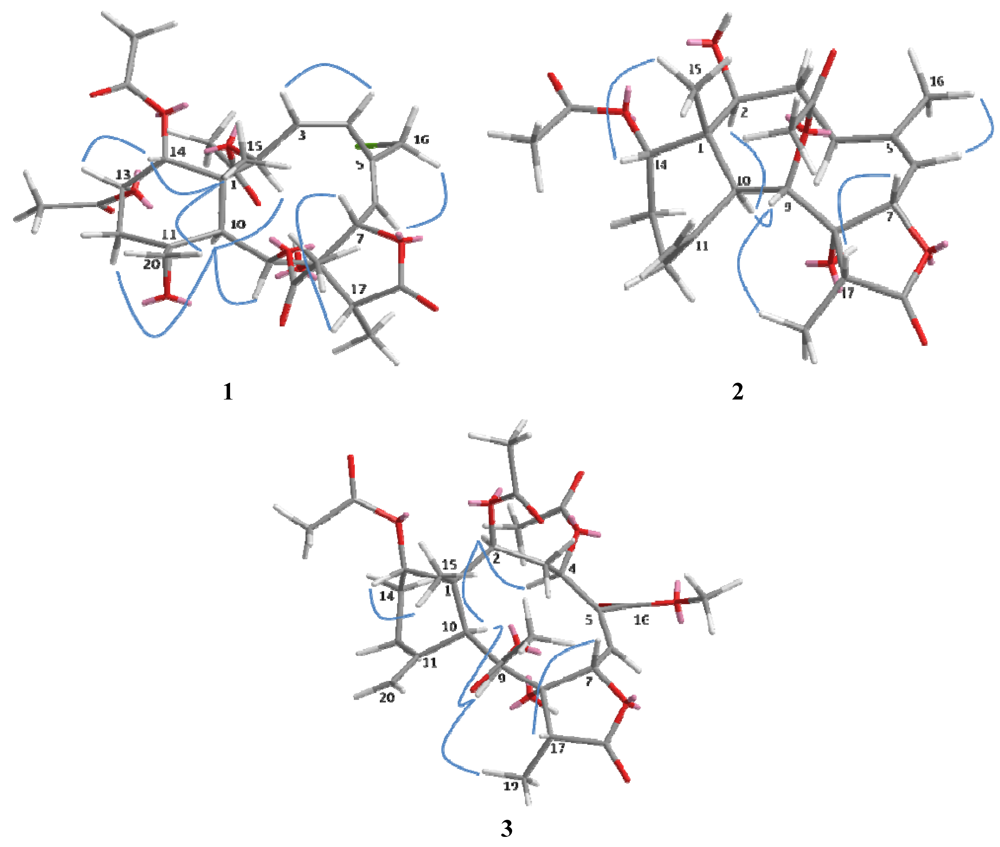
Compound 2, isolated as a colorless solid, and had a molecular formula of C24H34O8, deduced from HR-ESI-MS at m/z 473.2155 ([M + Na]+), showing eight degrees of unsaturation. The presence of hydroxyl, a γ-lactone ring, and ester groups were consistent with IR absorptions at 3481, 1772 and 1732 cm−1. The 1H and 13C NMR (Table 1 and Table 2), revealed the presence of an exomethylene (δC 150.8, 113.3), one trisubstituted double bond (δC 144.8, 120.5), two OAc carbonyl (C=O) (δC 170.4, 169.3) and one γ-lactone carbonyl (C=O) (δC 175.9), which accounted for five degrees of unsaturation and were suggestive of a tricyclic briarane bearing a γ-lactone ring. The carbonyl signal at δC 175.9 (C-18) was ascribed to a γ-lactone ring with the oxymethine at δC 78.3 (C-7) and the O-bearing quaternary carbon at δC 82.2 (C-7). The proton singlets at δH 5.05 and 4.92 (δC 113.3) were assigned to the exocyclic methylene group and correlated to C-10, C-12, and C-11 in the HMBC spectrum (Figure 2), suggesting the presence of a C-11/C-20 double bond. HMBC correlations of Me-15/C-1, C-2, C-14, C-10, Me-16/C-4, C-5, C-6, CH-7/C-5, C-8, C-18, CH-9/C-8, CH-10/C-11, C-1, C8 and the 1H, 1H COSY correlations of CH-2/CH2-3/CH2-4, CH-6/CH-7, CH-9/CH-10, CH2-12/CH2-13/CH-14 revealed the tricyclic skeleton of 2. Furthermore, two OAc groups positioned at C-9 and C-14 were established by the key correlations observed in the HMBC spectrum of 2. NOESY experiment revealed that the absence of correlation between and suggested orientation of and disposition of compound 2. The NOESY correlations (Figure 3) between Me-15/CH-14; CH-10/CH-2, CH-9, CH-9/Me-19, and CH-7/CH-17 were in agreement with the β-orientation of CH-7, CH-14 and CH-17, and α-orientation of CH-2, CH-9 and Me-19. Therefore, compound 2 is a new tricyclic briarane bearing a γ-lactone ring, and was given the name of juncenolide N.
The molecular formula of 3 was established as C29H38O13 from the molecular peak at m/z 617.2212 [M + Na]+ in the HR-ESI-MS. The NMR data (Table 1 and Table 2) revealed the basic features of a 8-hyoxybriarane type diterpenoid with a γ-lactone, one exomethylene double bond, one trisubstituted double bond (Table 1 and Table 2), and four acetate esters. The C-20/C-11 exomethylene double bond was assigned with the aid of HMBC correlations of CH2-20/C-10, C-11, C-12. The signal of CH-6 showed correlations to C-4, C-5 and C-16 in HMBC, revealing the C-5/C-6 trisubstituted double bond, and a COOMe group attached to C-5 (Figure 2). The four acetates were deduced to be located at C-2, C-4, C-9, and C-14 by HMBC correlations of the oxymethines at δH 4.86 (CH-2), 5.93 (CH-4), 5.56 (CH-13), and 4.69 (CH-14) to their respective acetate carbonyls. The 1H,1H COSY connectivities (Figure 2) of CH-2/CH2-3/CH-4, CH-6/CH-7, CH-9/CH-10, CH2-12/CH2-13/CH-14, as well as HMBC correlations of Me-15/C-1, C-2, C-10, C-14, CH-4/C-16, CH-7/C-8, C-18, CH-9/C-8, C-17, CH-10/C-1, C-8, C-11, CH2-12/C-11 and Me-19/C-8, C-17, C-18, confirmed the tricyclic skeleton of 3. The NOESY experiments (Figure 3) showed the relative configuration of compound 3. Due to the α-orientation of CH-10, the methyl group Me-15 at the ring junction should be β-oriented as no NOE correlation was observed between CH-10 and Me-15. NOESY spectrum clearly displayed the interactions between Me-15/CH-14, CH-10/CH-9, CH-2, CH-2/CH-4, and CH-9/CH-19, indicating that the OAc at C-2, C-4 and C-9 are β-oriented, whereas the OAc at C-14 is in the α-position. Thus, compound 3 is a new briarane ester with a γ-lactone skeleton, and designated juncenolide O.
The isolated briaranes 1–3 were tested on inhibitory effects of superoxide anion generation and elastase release by human neutrophils in response to FMLP/CB at a concentration of 10 μg/mL. As illustrated in Table 3, compounds 2 and 3 showed moderate inhibitory activities against elastase release with 29.0 ± 5.6%, and 35.9 ± 7.4%, respectively. Furthermore, compound 3 also exhibited moderate inhibitory activity against superoxide anion with 27.6 ± 7.0%.

Table 3.
Effects of compounds 1–3 on superoxide anion generation and elastase release by human neutrophils in response to FMLP/CB a.
| Compounds | Superoxide anion | Elastase release |
| Inhibition (%) b | Inhibition (%) | |
| 1 | 7.6 ± 2.8 | 15.9 ± 5.5 |
| 2 | 6.7 ± 2.9 | 29.0 ± 5.6 |
| 3 | 27.6 ± 7.0 | 35.9 ± 7.4 |
| Genistein | 65.0 ± 5.7 | 51.6 ± 5.9 |
a Results are presented as mean ± S.E.M. (n = 3); b Percent of inhibition at 10 μg/mL.
3. Experimental Section
3.1. General
Column chromatography (CC); silica gel 60 (Merck, Darmstadt, Germany) and Sephadex LH-20 (Amersham Pharmacia Biotech AB, Uppsala, Sweden). Prep. TLC: pre-coated silica gel plates (Merck; silica gel 60 F-254, 1 mm). LiChrospher Si 60 (5 μm, 250-10, Merck) and LiChrospher 100 RP-18e (5 μm, 250-10, Merck) were used for NP-HPLC and RP-HPLC (Merck, Darmstadt, Germany), respectively. Spray reagent: p-anisaldehyde reagent with 5% H2SO4. Optical rotations: Jasco DIP-1000 polarimeter. UV Spectra: Hitachi U-3210 spectrometer; λmax (log ε) in nm. IR Spectra: Hitachi T-2001 spectrometer; in cm−1. 1H-, 13C-NMR, COSY, HMQC, HMBC, and NOESY experiments: Bruker Avance 300 NMR spectrometer or Varian MR 400 NMR spectrometer, SiMe4 as internal standard; δ in ppm, coupling constants J in Hz. LRESIMS and HRESIMS: JEOL JMS-HX 110 mass spectrometer; in m/z.
3.2. Animal Material
The gorgonian Junceella juncea Pallas (Ellisellidae) was collected in Tai-Tong County, Taiwan, by scuba diving at a depth of 15 m, in November 2006. The fresh gorgonian was immediately frozen after collection and kept at −20 °C until processed. This species was identified by one of the authors (C.-C.L). A voucher specimen (WSG-5) was deposited in the School of Pharmacy, College of Medicine, National Taiwan University, Taiwan.
3.3. Extraction and Isolation
The outer grey layer of the gorgonian (1.4 kg, wet weight) was extracted with acetone (3 × 500 mL) at r.t., and the acetone extract was concentrated under vacuum. The crude extract (8 g) was partitioned between AcOEt and H2O (1:1). The AcOEt-soluble portion (4.9 g) was subjected to column chromatography (SiO2, n-Hexane/AcOEt 10:1–0:1; TLC (GF254) monitoring) giving fractions 1-16. Fr. 12 (195 mg) was subjected to a NP-HPLC (CH2Cl2/MeOH, 150:1), affording Fr. 12a (12 mg) which was further purified by RP-HPLC (MeOH/H2O/CH3CN, 70:25:5) yielding compound 1 (6 mg). Fr. 16 (105 mg) was separated by NP-HPLC (CH2Cl2/MeOH, 80:1), giving Fr. 16a (38 mg) which was subjected to RP-HPLC (MeOH/H2O/CH3CN, 55:40:5), yielding Fr. 16b (10 mg) that was further purified by RP-HPLC (MeOH/H2O/CH3CN, 55:40:5) furnishing compounds 2 (4 mg) and 3 (2 mg).
Juncenolide M (1): colorless amorphous solid; [α]D25 = −42 (c 0.05, CH2Cl2); UV (MeOH): 221 (3.20); IR (neat): 3402 (OH), 2930, 2853, 1779 (C=O γ-lactone), 1741 (C=O ester) cm−1; 1H-NMR (300 MHz, CDCl3) data, see Table 1; 13C-NMR (75 MHz, CDCl3) data, see Table 2; HR-ESI-MS [M + Na]+ m/z 621.1711 (calcd. 621.1715, C28H35ClO12Na).
Juncenolide N (2): colorless amorphous solid; [α]D25 = −60 (c 0.05, CH2Cl2); UV (MeOH): 204 (3.90); IR (neat): 3481 (OH), 1772 (C=O γ-lactone), 1732 (C=O ester) cm−1; 1H-NMR (400 MHz, CDCl3) data, see Table 1; 13C-NMR (100 MHz, CDCl3) data, see Table 2; HR-ESI-MS [M + Na]+ m/z 473.2155 (calcd. 473.2151, C24H34O8Na).
Juncenolide O (3): colorless amorphous solid; [α]D25 = +4 (c 0.05, CH2Cl2); UV (MeOH): 220 (3.80), 205 (3.90); IR (neat): 3423 (OH), 3020, 2921, 2850, 1780 (C=O γ-lactone), 1738 (C=O ester) cm−1; 1H-NMR (400 MHz, CDCl3) data, see Table 1; 13C-NMR (100 MHz, CDCl3) data, see Table 2; HR-ESI-MS [M + Na]+ m/z 617.2212 (calcd. 617.2210, C29H38O13Na).
3.4. Anti-Inflammatory Assays
Neutrophils were obtained by means of dextran sedimentation and Ficoll centrifugation. Superoxide generation and elastase release were carried out according to a procedure described previously [20]. Superoxide anion production was assayed by monitoring the superoxide dismutase-inhibitable reduction of ferricytochrome c. Elastase release experiments were performed using MeO-Suc-Ala-Ala-Pro-Valp-nitroanilide as the elastase substrate. Genistein was used as a positive control.
4. Conclusions
Three new diterpenoids, named juncenolides M–O (1–3), were isolated from the Taiwanese gorgonian Junceella juncea Pallas. Compound 1 is a new chlorinated briarane, compound 2 is a new brierane with a free hydroxy at C-2, while compound 3 contains a rare methyl ester at C-5. The anti-inflammatory activities tested on superoxide anion generation and elastase release by human neutrophils in response to FMLP/CB were evaluated. As a result, compounds 2 and 3 showed moderate inhibitory activities against elastase release at 10 μg/mL.
Acknowledgements
The authors thank the National Science Council, Taiwan (grant No. NSC-100-2113-M-002-0013) for providing financial support.
References
- Sung, P.-J.; Gwo, H.-H.; Fan, T.-Y.; Li, J.-J.; Dong, J.; Han, C.-C.; Wu, S.-L.; Fang, L.-S. Natural product chemistry of gorgonian corals of the genus Junceella. Biochem. Syst. Ecol. 2004, 32, 185–196. [Google Scholar] [CrossRef]
- Berrue, F.; Kerr, R.G. Diterpenes from gorgonian corals. Nat. Prod. Rep. 2009, 26, 681–710. [Google Scholar] [CrossRef]
- Sung, P.-J.; Pai, C.-H.; Su, Y.-D.; Hwang, T.-L.; Kuo, F.-W.; Fan, T.-Y.; Li, J.-J. New 8-hydroxybriarane diterpenoids from the gorgonians Junceella juncea and Junceella fragilis. Tetrahedron 2008, 64, 4224–4232. [Google Scholar]
- Wang, S.-S.; Chen, Y.-H.; Chang, J.-Y.; Hwang, T.-L.; Chen, C.-H.; Khalil, A.T.; Shen, Y.-C. Juncenolides H–K, new briarane diterpenoids from Junceella juncea. Helv. Chim. Acta 2009, 92, 2092–2100. [Google Scholar] [CrossRef]
- Qi, S.-H.; Zhang, S.; Huang, H.; Xiao, Z.-H.; Huang, J.-S.; Li, Q.-X. New briaranes from the south China sea gorgonian Junceella juncea. J. Nat. Prod. 2004, 67, 1907–1910. [Google Scholar] [CrossRef]
- Qi, S.-H.; Zhang, S.; Qian, B.-Y.; Xiao, Z.-H.; Li, M.-Y. Ten new antifouling briarane diterpenoids from the south China sea gorgonian Junceella juncea. Tetrahedron 2006, 62, 9123–9130. [Google Scholar]
- Qi, S.-H.; Zhang, S.; Qian, B.-Y. Antifeedant and antifouling briaranes from the South China Sea gorgonian Junceella juncea. Chem. Nat. Compd. 2009, 45, 49–54. [Google Scholar] [CrossRef]
- Shen, Y.-C.; Lin, Y.-C.; Chiang, M.Y. Juncenolide A, a new briarane from the Taiwanese gorgonian Junceella juncea. J. Nat. Prod. 2002, 65, 54–56. [Google Scholar]
- Shen, Y.-C.; Lin, Y.-C.; Ko, C.-L.; Wang, L.-T. New briarane from the Taiwanese gorgonian Junceella juncea. J. Nat. Prod. 2003, 66, 302–305. [Google Scholar] [CrossRef]
- Shen, Y.-C.; Lin, Y.-C.; Huang, Y.-L. Juncenolide E, a new briarane from Taiwanese gorgonian Junceella juncea. J. Chin. Chem. Soc. 2003, 50, 1267–1270. [Google Scholar]
- Lin, Y.-C.; Huang, Y.-L.; Khalil, A.T.; Chen, M.-H.; Shen, Y.-C. Juncenolides F and G, two new briarane diterpenoids from Taiwanese gorgonian Junceella juncea. Chem. Pharm. Bull. 2005, 53, 128–130. [Google Scholar] [CrossRef]
- Anjaneyulu, A.S.R.; Rao, N.S.K. Juncins G and H: New briarane diterpenoids of the Indian Ocean gorgonian Junceella juncea Pallas. J. Chem. Soc. Perkin Trans. I 1997. [Google Scholar]
- Anjaneyulu, A.S.R.; Rao, V.L.; Sastry, V.G.; Venugopal Mukku, J.R.V.; Schmitz, F.J. Juncins I–M, five new briarane diterpenoids from the Indian Ocean gorgonian Junceella juncea Pallas. J. Nat. Prod. 2003, 66, 507–510. [Google Scholar] [CrossRef]
- Sung, P.-J.; Fan, T.-Y.; Fang, L.-S.; She, J.-H.; Wu, S.-L.; Wang, G.-H.; Lin, M.-R. Juncin N, a new briarane-type diterpenoid from the gorgonian coral Junceella juncea. Heterocycles 2003, 61, 587–592. [Google Scholar] [CrossRef]
- Murthy, Y.L.N.; Mallika, D.; Rajack, A.; Reddy, G.D. A new antifungal briarane diterpenoid from the gorgonian Junceella juncea Pallas. Bioorg. Med. Chem. Lett. 2011, 21, 7522–7525. [Google Scholar]
- He, H.-Y.; Faulkner, D.J. New chlorinated diterpenes from the gorgonian Junceella gemmacea. Tetrahedron 1991, 47, 3271–3280. [Google Scholar] [CrossRef]
- Hamann, M.T.; Harrison, K.N.; Carroll, A.R.; Scheuer, P.J. Briarane diterpenes from Micronesian gorgonians. Heterocycles 1996, 42, 325–331. [Google Scholar] [CrossRef]
- Subrahmanyam, C.; Kulatheeswaran, R.; Ward, R.S. Briarane diterpenes from the Indian Ocean gorgonian Gorgonella umbraculum. J. Nat. Prod. 1998, 61, 1120–1122. [Google Scholar] [CrossRef]
- Sheu, J.-H.; Chen, Y.-P.; Hwang, T.-L.; Chiang, M.Y.; Fang, L.-S.; Sung, P.-J. Junceellolides J–L, 11,20-epoxybriaranes from the gorgonian coral Junceella fragilis. J. Nat. Prod. 2006, 69, 269–273. [Google Scholar] [CrossRef]
- Liaw, C.-C.; Shen, Y.-C.; Hwang, T.-L.; Kuo, Y.-H.; Khalil, A.T. Frajunolides E–K, briarane diterpenes from Junceella fragilis. J. Nat. Prod. 2008, 71, 1551–1556. [Google Scholar] [CrossRef]
- Samples Availability: Available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
