Bioactive Peptides and Depsipeptides with Anticancer Potential: Sources from Marine Animals
Abstract
:1. Introduction
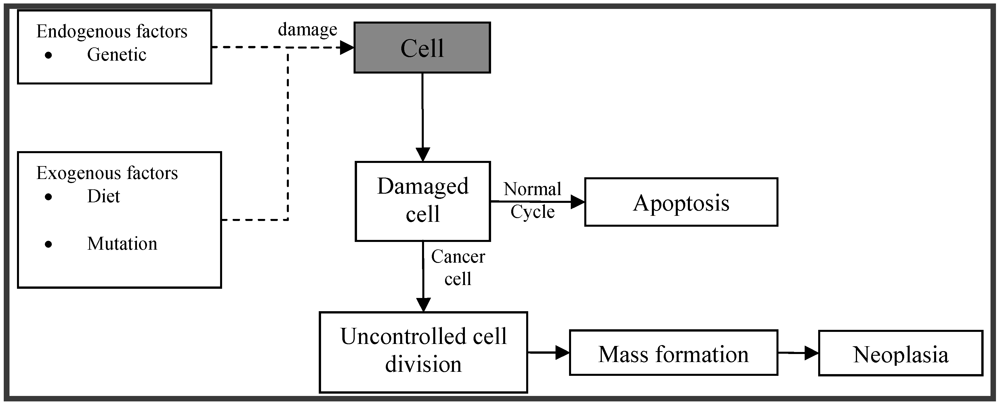
2. Sources of Bioactive Marine Peptides
| Compound | Source | Organism | Bioactivity | Reference |
|---|---|---|---|---|
| Aplidine | Ascidian | Aplidium albicans | Antitumor Anti leukemic | [24,25] |
| Arenastatin A | Sponge | Dysidea arenaria | Antitubulin | [26,27,28] |
| Aurilide | Tunicate | Dolabella auricularia | Antitumor | [29,30] |
| Didemnin | Tunicate | Trididemnum sp. | Antitumor | [3,31] |
| Dolastatin | Mollusk | Dolabella auricularia | Antineoplastic | [32] |
| Geodiamolide H | Sponge | Geodia sp. | Antiprolfierative | [28,33] |
| Homophymines | Sponge | Homophymia sp. | Antitumor | [34] |
| Jaspamide | Sponge | Jaspis sp. Hemiastrrella sp. | Antiproliferative | [35,36] |
| Kahalalide F | Mollusk | Elysia rufescens, Spisula polynyma | Antitubulin | [28] |
| Keenamide A | Mollusk | Pleurobranchus forskalii | Antitumor | [37] |
| Mollamide | Ascidian | Didemnum molle | Antiproliferative | [30,38] |
| Phakellistatins | Sponge | Phakellia carteri | Antiproliferative | [30,39] |
| Tamandarins A and B | Ascidian | Didemnum sp. | Antitumor | [30,40] |
| Trunkamide A | Ascidian | Lissoclinum sp. | Antitumor | [30,41] |
2.1. Sponges
2.2. Tunicates and Ascidians
2.3. Mollusks
2.4. Marine Protein Hydrolysates
| Source | Enzyme | Amino Acid Sequence | Bioactivity | Reference |
|---|---|---|---|---|
| Alaska pollack collagen (Theragra chalcogramma) | Trypsin and Flavourzyme | nd | Antioxidant in vitro | [88] |
| Croaker muscle (Otolithes ruber) | Pepsin, followed by Trypsin + αChymotrypsin | GNRGFACRHA | Antioxidant in vitro | [89] |
| Flyingfish (Exocoetus volitans) | Trypsin | nd | Antioxidant Antiproliferative for Hep G2 | [14] |
| Flying squid skin gelatin (Ommastrephes batramii) | Pepsin, followed by Trypsin + αChymotrypsin | nd | Antioxidant in vitro | [90] |
| Horse mackerel muscle (Magalapsis cordyla) | Pepsin, followed by Trypsin + αChymotrypsin | NHRYDR | Antioxidant in vitro | [89] |
| Jellyfish umbrella collagen (Rhopilema esculentum) | Trypsin and Flavourzyme | nd | Antioxidant | [88] |
| Jumbo flying squid skin gelatin (Dosidicus gigas) | Esperase and Alcalase | nd | Antioxidant in vitro Antiproliferative/Cytotoxic on MCF-7 and U87 cells | [82,91] |
| Oyster (Crassostrea gigas) | Protease from Bacillus sp. SM98011 | nd | Antitumor in BALB/c mice | [4] |
| Smooth hound (Mustelus mustelus) | LMW alkaline protease | nd | Antioxidant in vitro | [92] |
| Solitary tunicate (Styela clava) | Alcalase | nd | Antioxidant in vitro Antiproliferative on AGS, DLD-1, and HeLa cells | [13] |
| Threadfin bream (Nemipterus japonicas) | Trypsin | nd | Antioxidant Antiproliferative on HepG2 | [14] |
| Tilapia (Oreochromis niloticus) | Cryotin, Flavourzyme, Alcalase | nd | Antioxidant in vitro | [93,94] |
| Tuna dark muscle byproduct (Thunnus tonggol) | Papain and Protease XXIII | LPHVLTPEAGAT PTAEGGVYMVT | Antiproliferative on MCF7 cells | [83] |
| Tuna skin gelatin (Thunnus spp.) | Alcalase | nd | Antioxidant in vitro | [82] |
By-Products from Processing Hydrolysates
3. Anticancer Activities of Marine Animal Peptides
3.1. Antioxidative Activity
3.2. Antiproliferative Activity
4. Pharmacological Application and New Perspectives of Bioactive Peptides
5. Conclusions
Acknowledgments
References
- Fearon, E.R.; Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar]
- Vermeirssen, V.; Camp, J.V.; Verstraete, W. Bioavailability of angiotensin I converting enzyme inhibitory peptides. Br. J. Nutr. 2007, 92, 357–366. [Google Scholar]
- Aneiros, A.; Garateix, A. Bioactive peptides from marine sources: Pharmacological properties and isolation procedures. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 803, 41–53. [Google Scholar]
- Wang, Y.; He, H.; Wang, G.; Wu, H.; Zhou, B.; Chen, X.; Zhang, Y. Oyster (Crassostrea gigas) hydrolysates produced on a plant scale have antitumor activity and immunostimulating effects in BALB/c Mice. Mar. Drugs 2010, 8, 255–268. [Google Scholar] [CrossRef]
- Wilson-Sanchez, G.; Moreno-Félix, C.; Velazquez, C.; Plascencia-Jatomea, M.; Acosta, A.; Machi-Lara, L.; Aldana-Madrid, M.L.; Ezquerra-Brauer, J.M.; Robles-Zepeda, R.; Burgos-Hernandez, A. Antimutagenicity and antiproliferative studies of lipidic extracts from white shrimp (Litopenaeus vannamei). Mar. Drugs 2010, 8, 2795–2809. [Google Scholar] [CrossRef]
- Bhatnagar, I.; Kim, S. Immense essence of excellence: Marine microbial bioactive compounds. Mar. Drugs 2010, 8, 2673–2701. [Google Scholar]
- Mayer, F.; Mueller, S.; Malenke, E.; Kuczyk, M.; Hartmann, J.T.; Bokemeyer, C. Induction of apoptosis by flavopiridol unrelated to cell cycle arrest in germ cell tumour derived cell lines. Invest. New Drugs 2005, 23, 205–211. [Google Scholar]
- Wijesekara, I.; Kim, S. Angiotensin-I-converting enzyme (ACE) inhibitors from marine resources: Prospects in the pharmaceutical industry. Mar. Drugs 2010, 8, 1080–1093. [Google Scholar]
- Jimeno, J.; Faircloth, G.; Soussa-Faro, J.F.; Scheuer, P.; Rinehart, K. New marine derived anticancer therapeutics—A journey from the sea to clinical trials. Mar. drugs 2004, 2, 14–29. [Google Scholar]
- Chakraborty, S.; Ghosh, U. Oceans: a store of house of drugs—A review. J. Pharm. Res. 2010, 3, 1293–1296. [Google Scholar]
- Ryan, J.T.; Ross, R.P.; Bolton, D.; Fitzgerald, G.F.; Stanton, C. Bioactive peptides from muscle sources: Meat and fish. Nutrients 2011, 3, 765–791. [Google Scholar]
- Haefner, B. Drugs from the deep: Marine natural products as drug candidates. Drug Discovery Today 2003, 8, 536–544. [Google Scholar]
- Jumeri; Kim, S.M. Antioxidant and anticancer activities of enzymatic hydrolysates of solitary tunicate (Styela clava). Food Sci. Biotechnol. 2011, 20, 1075–1085. [Google Scholar] [CrossRef]
- Naqash, S.Y.; Nazeer, R.A. Antioxidant activity of hydrolysates and peptide fractions of Nemipterus japonicus and Exocoetus volitans muscle. J. Aquat. Food Prod. Technol. 2010, 19, 180–192. [Google Scholar] [CrossRef]
- Holzinger, A.; Meindl, U. Jasplakinolide, a novel actin targeting peptide, inhibits cell growth and induces actin filament polymerization in the green alga Micrasteria. Cell Motil. Cytoskeleton 1997, 38, 365–372. [Google Scholar] [CrossRef]
- Kim, S.; Wijesekara, I. Development and biological activities of marine-derived bioactive peptides: A review. J. Funct. Foods 2010, 2, 1–9. [Google Scholar]
- Libes, S.M. Organic Product from the Sea: Pharmaceuticals, Nutraceuticals, Food Additives, and Cosmoceuticals. In Introduction to Marine Biogeochemistry, 2nd; Libes, S.M., Ed.; Academic Press: Conway, SC, USA, 2009. [Google Scholar]
- Mohammed, R.; Peng, J.N.; Kelly, M.; Hamann, M.T. Cyclic heptapeptides from the Jamaican sponge Stylissa caribica. J. Nat. Prod. 2006, 69, 1739–1744. [Google Scholar] [CrossRef]
- Prasad, P.; Aalbersberg, W.; Feussner, K.D.; van Wagoner, R.M. Papuamides E and F, cytotoxic depsipeptides from the marine sponge Melophlus sp. Tetrahedron 2011, 67, 8529–8531. [Google Scholar]
- Lee, J.; Currano, J.N.; Carroll, P.J.; Joullie, M.M. Didemnins, tamandarins and related natural products. Nat. Prod. Rep. 2012, 29, 404–424. [Google Scholar]
- Shilabin, A.G.; Hamann, M.T. In vitro and in vivo evaluation of select kahalalide F analogs with antitumor and antifungal activities. Bioorg. Med. Chem. 2011, 19, 6628–6632. [Google Scholar] [CrossRef]
- Adrio, J.; Cuevas, C.; Manzanares, I.; Joullie, M.M. Total synthesis and biological evaluation of tamandarin B analogues. J. Org. Chem. 2007, 72, 5129–5138. [Google Scholar]
- Simmons, T.; Andrianasolo, E.; McPhail, K.; Flatt, P.; Gerwick, W. Marine natural products as anticancer drugs. Mol. Cancer Ther. 2005, 4, 333–342. [Google Scholar]
- Erba, E.; Bassano, L.; di Liberti, G.; Muradore, I.; Chiorino, G.; Ubezio, P.; Vignati, S.; Codegoni, A.; Desiderio, M.A.; Faircloth, G.; et al. Cell cycle phase perturbations and apoptosis in tumour cells induced by aplidine. Br. J. Cancer 2002, 86, 1510–1517. [Google Scholar]
- Broggini, M.; Marchini, S.V.; Galliera, E.; Borsotti, P.; Taraboletti, G.; Erba, E.; Sironi, M.; Jimeno, J.; Faircloth, G.T.; Giavazzi, R.; et al. Aplidine, a new anticancer agent of marine origin, inhibits vascular endothelial growth factor (VEGF) secretion and blocks VEGF-VEGFR-1 (flt-1) autocrine loop in human leukemia cells MOLT-4. Leukemia 2003, 17, 52–59. [Google Scholar]
- Morita, K.; Koiso, Y.; Hashimoto, Y.; Kobayashi, M.; Wang, W.; Ohyabu, N.; Iwasaki, S. Interaction of arenastatin A with porcine brain tubulin. Biol. Pharm. Bull. 1997, 20, 171–174. [Google Scholar]
- Kotoku, N.; Kato, T.; Narumi, F.; Ohtani, E.; Kamada, S.; Aoki, S.; Okada, N.; Nakagawa, S.; Kobayashi, M. Synthesis of 15,20-triamide analogue with polar substituent on the phenyl ring of arenastatin A, an extremely potent cytotoxic spongean depsipeptide. Bioorg. Med. Chem. 2006, 14, 7446–7457. [Google Scholar]
- Andavan, G.; Lemmens-Gruber, R. Cyclodepsipeptides from marine sponges: Natural agents for drug research. Mar. Drugs 2010, 8, 810–834. [Google Scholar]
- Suenaga, K.; Mutou, T.; Shibata, T.; Itoh, T.; Kigoshi, H.; Yamada, K. Isolation and stereostructure of aurilide, a novel cyclodepsipeptide from the Japanese sea hare Dolabella auricularia. Tetrahedron Lett. 1996, 37, 6771–6774. [Google Scholar]
- Hamada, Y.; Shioiri, T. Recent progress of the synthetic studies of biologically active marine cyclic peptides and depsipeptides. Chem. Rev. 2005, 105, 4441–4482. [Google Scholar]
- Geldof, A.; Mastbergen, S.; Henrar, R.; Faircloth, G. Cytotoxicity and neurocytotoxicity of new marine anticancer agents evaluated using in vitro assays. Cancer Chemother. Pharmacol. 1999, 44, 312–318. [Google Scholar] [CrossRef]
- Pettit, G.R.; Singh, S.B.; Hogan, F.; Lloyd-Williams, P.; Herald, C.L.; Burbett, D.D.; Clewlow, P.J. The absolute configuration and synthesis of natural (−)-dolostatin 10. J. Am. Chem. Soc. 1989, 70, 5463–5465. [Google Scholar]
- Freitas, V.; Rangel, M.; Bisson, L.; Jaeger, R.; Machado-Santelli, G. The geodiamolide H, derived from Brazilian sponge Geodia corticostylifera, regulates actin cytoskeleton, migration and invasion of breast cancer cells cultured in three-dimensional environment. J. Cell. Physiol. 2008, 216, 583–594. [Google Scholar] [CrossRef]
- Zampella, A.; Sepe, V.; Luciano, P.; Bellotta, F.; Monti, M.; D’Auria, M.; Jepsen, T.; Petek, S.; Adeline, M.; Laprevote, O.; et al. Homophymine A, an anti-HIV cyclodepsipeptide from the sponge Homophymia sp. J. Org. Chem. 2008, 73, 5319–5327. [Google Scholar]
- Nakazawa, H.; Kitano, K.; Cioca, D.; Ishikawa, M.; Ueno, M.; Ishida, F.; Kiyosawa, K. Induction of polyploidization by jaspamide in HL-60 cells. Acta Haematol. 2000, 104, 65–71. [Google Scholar]
- Gala, F.; D’Auria, M.; de Marino, S.; Sepe, V.; Zollo, F.; Smith, C.; Copper, J.; Zampella, A. Jaspamides H–L, new actin-targeting depsipeptides from the sponge Jaspis splendans. Tetrahedron 2008, 64, 7127–7130. [Google Scholar]
- Wesson, K.; Hamann, M. Keenamide A, a bioactive cyclic peptide from the marine mollusk Pleurobranchus forskalii. J. Nat. Prod. 1996, 59, 629–631. [Google Scholar] [CrossRef]
- Carroll, A.; Bowden, B.; Coll, J.; Hockless, D.; Skelton, B.; White, A. Studies of Australian ascidians. Mollamide, a cytotoxic cyclic heptapeptide from the compound ascidian Didemnum molle. Aust. J. Chem. 1994, 47, 61–69. [Google Scholar] [CrossRef]
- Li, W.-L.; Yi, Y.-H.; Wu, H.-M.; Xu, Q.-Z.; Tang, H.-F.; Zhou, D.-Z.; Lin, H.-W.; Wang, Z.-H. Isolation and structure of the cytotoxic cycloheptapeptide Phakellistatin 13. J. Nat. Prod. 2002, 66, 146–148. [Google Scholar]
- Vervoort, H.; Fenical, W.; Epifanio, R. Tamandarins A and B: New cytotoxic depsipeptides from a Brazilian ascidian of the family Didemnidae. J. Org. Chem. 2000, 65, 782–792. [Google Scholar]
- Wipf, P.; Miller, C.; Venkatraman, S.; Fritch, P. Thiolysis of oxazolines—A new, selective method for the direct conversion of peptide oxazolines into thiazolines. Tetrahedron Lett. 1995, 36, 6395–6398. [Google Scholar]
- Bergquist, R.M. The Porifera. In Invertebrate Zoology, 2nd; Anderson, D.T., Ed.; Oxford University Press: Oxford, UK, 2001; pp. 10–27. [Google Scholar]
- Demosponge Distribution Patterns. In Sponges in Time and Space; van Soest, R.W.M.; van Kempen, T.M.G.; Braekman, J.-C. (Eds.) Balkema: Rotterdam, The Netherlands, 1994; pp. 213–223.
- Blunt, J.; Copp, B.; Munro, M.; Northcote, P.; Prinsep, M. Marine natural products. Nat. Prod. Rep. 2004, 21, 1–49. [Google Scholar]
- Cioca, D.P.; Kitano, K. Induction of apoptosis and CD10/neutral endopeptidase expression by jaspamide in HL-60 line cells. Cell. Mol. Life Sci. 2002, 59, 1377–1387. [Google Scholar]
- Odaka, C.; Sanders, M.L.; Crews, P. Jasplakinolide induces apoptosis in various transformed cell lines by a caspase-3-like protease-dependent pathway. Clin. Diagn. Lab. Immunol. 2000, 7, 947–952. [Google Scholar]
- Ford, P.W; Gustafson, K.R.; McKee, T.C.; Shigematsu, N.; Maurizi, L.K.; Pannell, L.K.; Williams, D.E.; de Silva, E.P.; Lassota, P.; Allen, T.M.; et al. Papuamides A-D, HIV-inhibitory and cytotoxic depsipeptides from the sponges Theonella mirabilis and Theonella swinhoei collected in Papua New Guinea. J. Am. Chem. Soc. 1999, 121, 5899–5909. [Google Scholar]
- Napolitano, A.; Rodriquez, M.; Bruno, I.; Marzocco, S.; Autore, G.; Riccio, R.; Gomez-Paloma, L. Synthesis, structural aspects and cytotoxicity of the natural cyclopeptides yunnanins A, C and phakellistatins 1, 10. Tetrahedron 2003, 59, 10203–10211. [Google Scholar]
- Schmitz, F.J.; Bowden, B.F.; Toth, S. Antitumor and Cytotoxic Compounds from Marine Organism. In Marine Biotechnology: Pharmaceutical and Bioactive Natural Products; Attaway, D.H., Zaborsky, O.R., Eds.; Plenum Press: New York, NY, USA, 1993; Volume 1, pp. 197–308. [Google Scholar]
- Vera, M.D.; Joullié, M.M. Natural products as probes of cell biology: 20 years of didemnin research. Med. Res. Rev. 2002, 22, 102–145. [Google Scholar]
- Stewart, J.A.; Low, J.B.; Roberts, J.D.; Blow, A. A phase I clinical trial of didemnin B. Cancer 1991, 68, 2550–2554. [Google Scholar]
- Maroun, J.A.; Stewart, D.; Verma, S.; Eisenhauer, E. Phase I clinical study of didemnin B. A National Cancer Institute of Canada Clinical Trials Group study. Invest. New Drugs 1998, 16, 51–56. [Google Scholar] [CrossRef]
- Kucuk, O.; Young, M.L.; Habermann, T.M.; Wolf, B.C.; Jimeno, J.; Cassileth, P.A. Phase II trail of didemnin B in previously treated non-Hodgkin’s lymphoma: An Eastern Cooperative Oncology Group (ECOG) study. Am. J. Clin. Oncol. 2000, 23, 273–277. [Google Scholar]
- Benvenuto, J.A.; Newman, R.A.; Bignami, G.S.; Raybould, T.J.; Raber, M.N.; Esparza, L.; Walters, R.S. Phase II clinical and pharmacological study of didemnin B in patients with metastatic breast cancer. Invest. New Drugs 1992, 10, 113–117. [Google Scholar]
- Shin, D.M.; Holoye, P.Y.; Murphy, W.K.; Forman, A.; Papasozomenos, S.C.; Hong, W.K.; Raber, M. Phase I/II clinical trial of didemnin B in non-small-cell lung cancer: Neuromuscular toxicity is dose-limiting. Cancer Chemother. Pharmacol. 1991, 29, 145–149. [Google Scholar]
- Faivre, S.; Chieze, S.; Delbaldo, C.; Ady-Vago, N.; Guzman, C.; Lopez-Lazaro, L.; Lozahic, S.; Jimeno, J.; Pico, F.; Armand, J.; et al. Phase I and pharmacokinetic study of aplidine, a new marine cyclodepsipeptide in patients with advanced malignancies. J. Clin. Oncol. 2005, 23, 7871–7880. [Google Scholar]
- García-Fernández, L.F.; Losada, A.; Alcaide, V.; Alvarez, A.M.; Cuadrado, A.; González, L.; Nakayama, K.; Nakayama, K.I.; Fernández-Sousa, J.M.; Muñoz, A.; et al. Aplidin induces the mitochondrial apoptotic pathway via oxidative stress-mediated JNK and p38 activation and protein kinase C delta. Oncogene 2002, 21, 7533–7544. [Google Scholar]
- Maroun, J.A.; Belanger, K.; Seymour, L.; Matthews, S.; Roach, J.; Dionne, J.; Soulieres, D.; Stewart, D.; Goel, R.; Charpentier, D.; et al. Phase I study of Aplidine in a dailyx5 one-hour infusion every 3 weeks in patients with solid tumors refractory to standard therapy. A National Cancer Institute of Canada Clinical Trials Group study: NCIC CTG IND 115. Ann. Oncol. 2006, 17, 1371–1378. [Google Scholar]
- Armand, J.-V.; Ady-Vago, N.; Faivre, S. Phase I and Pharmacokinetic Study of Aplidine (apl) Given as a 24-Hour Continuous Infusion Every Other Week (q2w) in Patients (pts) with Solid Tumor (st) and Lymphoma (NHL). In Proceeding of 2001 ASCO Annual Meeting; American Society of Clinical Oncology: San Francisco, CA, USA, 2001. [Google Scholar]
- Moneo, V.; Serelde, B.G.; Leal, J.F.; Blanco-Aparicio, C.; Diaz-Uriarte, R.; Aracil, M.; Tercero, J.C.; Jimeno, J.; Carnero, A. Levels of p27(kip1) determine Aplidin sensitivity. Mol. Cancer Ther. 2007, 6, 1310–1316. [Google Scholar]
- Mitsiades, C.; Ocio, E.; Pandiella, A.; Maiso, P.; Gajate, C.; Garayoa, M.; Vilanova, D.; Montero, J.; Mitsiades, N.; McMullan, C.; et al. Aplidin, a marine organism-derived compound with potent antimyeloma activity in vitro and in vivo. Cancer Res. 2008, 68, 5216–5225. [Google Scholar]
- Bhatnagar, I.; Kim, S. Marine antitumor drugs: Status, shortfalls and strategies. Mar. Drugs 2010, 8, 2702–2720. [Google Scholar]
- Olivera, B.M. w-Conotoxin MVIIA: From Marine Snail Venom to Analgesic Drug. In Drugs from the Sea; Fusetani, N., Ed.; Karger: Basel, Switzerland, 2000; pp. 74–85. [Google Scholar]
- Shen, G.; Layer, R.; McCabe, R. Conopeptides: From deadly venoms to novel therapeutics. Drug Discovery Today 2000, 5, 98–106. [Google Scholar]
- Pettit, G.R.; Srirangam, J.K.; Barkoczy, J.; Williams, M.D.; Durkin, K.P.; Boyd, M.R.; Bai, R.; Hamel, E.; Schmidt, J.M.; Chapuis, J.C. Antineoplastic agents 337. Synthesis of dolastatin 10 structural modifications. Anticancer Drug Des. 1995, 10, 529–544. [Google Scholar]
- Pettit, G.R.; Flahive, E.J.; Boyd, M.R.; Bai, R.; Hamel, E.; Pettit, R.K.; Schmidt, J.M. Antineoplastic agents 360. Synthesis and cancer cell growth inhibitory studies of dolastatin 15 structural modifications. Anticancer Drug Des. 1998, 13, 47–66. [Google Scholar]
- Garteiz, D.A.; Madden, T.; Beck, D.E.; Huie, W.R.; McManus, K.T.; Abbruzzese, J.L.; Chen, W.; Newman, R.A. Quantitation of dolastatin-10 using HPLC/electrospray ionization mass spectrometry: application in a phase I clinical trial. Cancer Chemother. Pharmacol. 1998, 41, 299–306. [Google Scholar]
- Pitot, H.C.; McElroy, E.A.; Reid, J.M.; Windebank, A.J.; Sloan, J.A.; Erlichman, C.; Bagniewski, P.G.; Walker, D.L.; Rubin, J.; Goldberg, R.M.; et al. Phase I trial of dolastatin-10 (NSC 376128) in patients with advanced solid tumors. Clin. Cancer Res. 1999, 5, 525–531. [Google Scholar]
- Tamura, K.; Nakagawa, K.; Kurata, T.; Satoh, T.; Nogami, T.; Takeda, K.; Mitsuoka, S.; Yoshimura, N.; Kudoh, S.; Negoro, S.; et al. Phase I study of TZT-1027, a novel synthetic dolastatin 10 derivative and inhibitor of tubulin polymerization, which was administered to patients with advanced solid tumors on days 1 and 8 in 3-week courses. Cancer Chemother. Pharmacol. 2007, 60, 285–293. [Google Scholar] [CrossRef]
- de Arruda, M.; Cocchiaro, C.A.; Nelson, C.M.; Grinnell, C.M.; Janssen, B.; Haupt, A.; Barlozzari, T. LU103793 (NSC D-669356): A synthetic peptide that interacts with microtubules and inhibits mitosis. Cancer Res. 1995, 55, 3085–3092. [Google Scholar]
- Rajaganapathi, J.; Kathiresan, K.; Singh, T.P. Purification of anti-HIV protein from purple fluid of the sea hare Bursatella leachii de Blainville. Mar. Biotechnol. 2002, 4, 447–453. [Google Scholar] [CrossRef]
- García-Rocha, M.; Bonay, P.; Avila, J. The antitumoral compound Kahalalide F acts on cell lysosomes. Cancer Lett. 1996, 99, 43–50. [Google Scholar]
- Faircloth, G.T.; Smith, B.; Grant, W. Selective antitumor activity of Kahalalide F, a marine-derived cyclic depsipeptide. Proc. Am. Assoc. Cancer Res. 2001, 42, 1140. [Google Scholar]
- Rademaker-Lakhai, J.M.; Horenblas, S.; Meinhardt, W.; Stokvis, E.; de Reijke, T.M.; Jimeno, J.M.; Lopez-Lazaro, L.; Lopez Martin, J.A.; Beijnen, J.H.; Schellens, J.H. Phase I clinical and pharmacokinetic study of kahalalide F in patients with advanced androgen refractory prostate cancer. Clin. Cancer Res. 2005, 11, 1854–1862. [Google Scholar]
- Pardo, B.; Paz-Ares, L.; Tabernero, J.; Ciruelos, E.; García, M.; Salazar, R.; López, A.; Blanco, M.; Nieto, A.; Jimeno, J.; et al. Phase I clinical and pharmacokinetic study of kahalalide F administered weekly as a 1-hour infusion to patients with advanced solid tumors. Clin. Cancer Res. 2008, 14, 1116–1123. [Google Scholar]
- Martín-Algarra, S.; Espinosa, E.; Rubió, J.; López, J.J.L.; Manzano, J.L.; Carrión, L.A.; Plazaola, A.; Tanovic, A.; Paz-Ares, L. Phase II study of weekly Kahalalide F in patients with advanced malignant melanoma. Eur. J. Cancer 2009, 45, 732–735. [Google Scholar]
- Erdmann, K.; Cheung, B.W.Y.; Schröder, H. The possible roles of food-derived bioactive peptides in reducing the risk of cardiovascular disease. J. Nutr. Biochem. 2008, 19, 643–654. [Google Scholar]
- Vioque, J.; Pedroche, J.; Yust, M.M.; Millán, F.; Clemente, A. Obtención y aplicación de hidrolizados protéicos. Grasas Aceites 2001, 52, 132–136. [Google Scholar]
- Neklyudov, A.; Ivankin, A.; Berdutina, A. Properties and uses of protein hydrolysates (Review). Appl. Biochem. Microbiol. 2000, 36, 452–459. [Google Scholar]
- Walker, J.M.; Sweeney, P.J. Production of Protein Hydrolysates Using Enzymes. In The Protein Protocols Handbook, 2nd; Walker, J.M., Ed.; Humana Press: Hatfield, UK, 2002. [Google Scholar]
- Korhonen, H.; Pihlanto, A. Bioactive peptides: Production and functionality. Int. Dairy J. 2006, 16, 945–960. [Google Scholar]
- Aleman, A.; Gimenez, B.; Montero, P.; Gomez-Guillen, M. Antioxidant activity of several marine skin gelatins. LWT Food Sci. Technol. 2011, 44, 407–413. [Google Scholar]
- Hsu, K.; Li-Chan, E.; Jao, C. Antiproliferative activity of peptides prepared from enzymatic hydrolysates of tuna dark muscle on human breast cancer cell line MCF-7. Food Chem. 2011, 126, 617–622. [Google Scholar]
- Mendis, E.; Rajapakse, N.; Kim, S.K. Antioxidant properties of a radical-scavenging peptide purified from enzymatically prepared fish skin gelatin hydrolysate. J. Agric. Food Chem. 2005, 53, 581–587. [Google Scholar]
- Picot, L.; Bordenave, S.; Didelot, S.; Fruitier-Arnaudin, I.; Sannier, F.; Thorkelsson, G.; Berge, J.P.; Guerard, F.; Chabeaud, A.; Piot, J.M. Antiproliferative activity of fish protein hydrolysates on human breast cancer cell lines. Process Biochem. 2006, 41, 1217–1222. [Google Scholar]
- Kim, S.; Je, J.; Kim, S. Purification and characterization of antioxidant peptide from hoki (Johnius belengerii) frame protein by gastrointestinal digestion. J. Nutr. Biochem. 2007, 18, 31–38. [Google Scholar] [CrossRef]
- Aleman, A.; Gimenez, B.; Perez-Santin, E.; Gomez-Guillen, M.; Montero, P. Contribution of Leu and Hyp residues to antioxidant and ACE-inhibitory activities of peptide sequences isolated from squid gelatin hydrolysate. Food Chem. 2011, 125, 334–341. [Google Scholar]
- Zhuang, Y.L.; Li, B.F.; Zhao, X. The scavenging of free radical and oxygen species activities and hydration capacity of collagen hydrolysates from walleye pollock (Theragra chalcogramma) skin. J. Ocean Univ. China 2009, 8, 171–176. [Google Scholar] [CrossRef]
- Kumar, N.; Nazeer, R.; Jaiganesh, R. Purification and biochemical characterization of antioxidant peptide from horse mackerel (Magalaspis cordyla) viscera protein. Peptides 2011, 32, 1496–1501. [Google Scholar] [CrossRef]
- Chen, X.-E.; Xie, N.-N.; Fang, X.-B.; Yu, H.; Ya-mei, J.; Zhen-da, L. Antioxidant activity and molecular weight distribution of in vitro gastrointestinal digestive hydrolysate from Flying squid (Ommastrephes batramii) skin-gelatin. Food Sci. 2010, 31, 123–130. [Google Scholar]
- Aleman, A.; Perez-Santin, E.; Bordenave-Juchereau, S.; Arnaudin, I.; Gomez-Guillen, M.; Montero, P. Squid gelatin hydrolysates with antihypertensive, anticancer and antioxidant activity. Food Res. Int. 2011, 44, 1044–1051. [Google Scholar]
- Bougatef, A.; Hajji, M.; Balti, R.; Lassoued, I.; Triki-Ellouz, Y.; Nasri, M. Antioxidant and free radical-scavenging activities of smooth hound (Mustelus mustelus) muscle protein hydrolysates obtained by gastrointestinal proteases. Food Chem. 2009, 114, 1198–1205. [Google Scholar] [CrossRef]
- Raghavan, S.; Kristinsson, H.G. Antioxidative efficacy of alkali-treated tilapia protein hydrolysates: A comparative study of five enzymes. J. Agric. Food Chem. 2008, 56, 1434–1441. [Google Scholar]
- Foh, M.B.K.; Amadou, I.; Foh, B.M.; Kamara, M.T.; Xia, W.S. Functionality and antioxidant properties of Tilapia (Oreochromis niloticus) as influenced by the degree of hydrolysis. Int. J. Mol. Sci. 2010, 11, 1851–1869. [Google Scholar] [CrossRef]
- Gildberg, A.; Arnesen, J.; Carlehog, M. Utilisation of cod backbone by biochemical fractionation. Process Biochem. 2002, 38, 475–480. [Google Scholar]
- Rustad, T.; Storrø, I.; Slizyte, R. Possibilities for the utilisation of marine by-products. Int. J. Food Sci. Technol. 2011, 46, 2001–2014. [Google Scholar]
- Centenaro, G.S.; Mellado, M.S.; Prentice-Hernández, C. Antioxidant activity of protein hydrolysates of fish and chicken bones. Adv. J. Food Sci. Technol. 2011, 3, 280–288. [Google Scholar]
- Dey, S.; Dora, K. Antioxidative activity of protein hydrolysate produced by alcalase hydrolysis from shrimp waste (Penaeus monodon and Penaeus indicus). J. Food Technol. 2012, 49, 1–9. [Google Scholar] [CrossRef]
- Ovissipour, M.; Abedian, A.; Motamedzadegan, A.; Rasco, B.; Safari, R.; Shahiri, H. The effect of enzymatic hydrolysis time and temperature on the properties of protein hydrolysates from Persian sturgeon (Acipenser persicus) viscera. Food Chem. 2009, 115, 238–242. [Google Scholar] [CrossRef]
- Bougatef, A.; Nedjar-Arroume, N.; Manni, L.; Ravallec, R.; Barkia, A.; Guillochon, D.; Nasri, M. Purification and identification of novel antioxidant peptides from enzymatic hydrolysates of sardinelle (Sardinella aurita) by-products proteins. Food Chem. 2010, 118, 559–565. [Google Scholar] [CrossRef]
- Elias, C.; Pereira, F.; Dias, F.; Silva, T.; Lopes, A.; d’Avila-Levy, C.; Branquinha, M.; Santos, A. Cysteine peptidases in the tomato trypanosomatid Phytomonas serpens: Influence of growth conditions, similarities with cruzipain and secretion to the extracellular environment. Exp. Parasitol. 2008, 120, 343–352. [Google Scholar] [CrossRef]
- Sheih, I.; Fang, T.; Wu, T.; Lin, P. Anticancer and antioxidant activities of the peptide fraction from algae protein waste. J. Agric. Food Chem. 2010, 58, 1202–1207. [Google Scholar]
- Kamau, S.M.; Lu, R.-R. The effect of enzymes and hydrolysis conditions on degree of hydrolysis and DPPH radical scavenging activity of whey protein hydrolysates. Curr. Res. Dairy Sci. 2011, 3, 25–35. [Google Scholar]
- Theodore, A.; Raghavan, S.; Kristinsson, H. Antioxidative activity of protein hydrolysates prepared from alkaline-aided channel catfish protein isolates. J. Agric. Food Chem. 2008, 56, 7459–7466. [Google Scholar]
- Zhuang, Y.; Zhao, X.; Li, B. Optimization of antioxidant activity by response surface methodology in hydrolysates of jellyfish (Rhopilema esculentum) umbrella collagen. J. Zhejiang Univ. Sci. B 2009, 10, 572–579. [Google Scholar] [CrossRef]
- Raghavan, S.; Kristinsson, H.G.; Leeuwenburgh, C. Radical scavenging and reducing ability of tilapia (Oreochromis niloticus) protein hydrolysates. J. Agric. Food Chem. 2008, 56, 10359–10367. [Google Scholar]
- Wu, H.; Chen, H.; Shiau, C. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus). Food Res. Int. 2003, 36, 949–957. [Google Scholar] [CrossRef]
- López-Exposito, I.; Quirós, A.; Amigo, L.; Recio, I. Casein hydrolysates as a source of antimicrobial, antioxidant and antihypertensive peptides. Lait 2007, 87, 241–249. [Google Scholar]
- Park, E.Y.; Morimae, M.; Matsumura, Y.; Nakamura, Y.; Sato, K. Antioxidant activity of some protein hydrolysates and their fractions with different isoelectric points. J. Agric. Food Chem. 2008, 56, 9246–9251. [Google Scholar]
- Gomez-Guillen, M.; Gimenez, B.; Lopez-Caballero, M.; Montero, M. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar]
- Suetsuna, K.; Ukeda, H.; Ochi, H. Isolation and characterization of free radical scavenging activities peptides derived from casein. J. Nutr. Biochem. 2000, 11, 128–131. [Google Scholar]
- Pena-Ramos, E.; Xiong, Y.; Arteaga, G. Fractionation and characterisation for antioxidant activity of hydrolysed whey protein. J. Sci. Food Agric. 2004, 84, 1908–1918. [Google Scholar]
- Chan, K.M.; Decker, E.A. Endogenous skeletal muscle antioxidants. Crit. Rev. Food Sci. Nutr. 1994, 34, 403–426. [Google Scholar]
- Chen, H.M.; Muramoto, K.; Yamauchi, F.; Fujimoto, K.; Nokihara, K. Antioxidative properties of histidine-containing peptides designed from peptide fragments found in the digests of a soybean protein. J. Agric. Food Chem. 1998, 46, 49–53. [Google Scholar]
- Ahuja, D.; Geiger, A.; Ramanjulu, J.; Vera, M.; SirDeshpande, B.; Pfizenmayer, A.; Abazeed, M.; Krosky, D.; Beidler, D.; Joullie, M.; et al. Inhibition of protein synthesis by didemnins: Cell potency and SAR. J. Med. Chem. 2000, 43, 4212–4218. [Google Scholar]
- Mayer, A.M.; Gustafson, K.R. Marine pharmacology in 2000: Antitumor and cytotoxic compounds. Int. J. Cancer 2003, 105, 291–299. [Google Scholar]
- Panda, D.; Ananthnarayan, V.; Larson, G.; Shih, C.; Jordan, M.; Wilson, L. Interaction of the antitumor compound cryptophycin-52 with tubulin. Biochemistry 2000, 39, 14121–14127. [Google Scholar]
- Armstrong, W.; Kennedy, A.; Wan, X.; Atiba, J.; McLaren, E.; Meyskens, F. Single-dose administration of Bowman-Birk inhibitor concentrate in patients with oral leukoplakia. Cancer Epidemiol. Biomark. Prev. 2000, 9, 43–47. [Google Scholar]
- Kobayashi, H.; Suzuki, M.; Kanayama, N.; Terao, T. A soybean Kunitz trypsin inhibitor suppresses ovarian cancer cell invasion by blocking urokinase upregulation. Clin. Exp. Metastasis 2004, 21, 159–166. [Google Scholar]
- Galvez, A.; Chen, N.; Macasieb, J.; de Lumen, B. Chemopreventive property of a soybean peptide (lunasin) that binds to deacetylated histones and inhibits acetylation. Cancer Res. 2001, 61, 7473–7478. [Google Scholar]
- Jeong, H.; Jeong, J.; Kim, D.; de Lumen, B. Inhibition of core histone acetylation by the cancer preventive peptide lunasin. J. Agric. Food Chem. 2007, 55, 632–637. [Google Scholar]
- Li, X.; Jiao, L.L.; Zhang, X.; Tian, W.M.; Chen, S.; Zhang, L.P. Anti-tumor and immunomodulating activities of proteoglycans from mycelium of Phellinus nigricans and culture medium. Int. Immunopharmacol. 2008, 8, 909–915. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, X.; Wang, H.; Liu, Y.; Chen, Y. Studies on mechanism of action of anticancer peptides by modulation of hydrophobicity within a defined structural framework. Mol. Cancer Ther. 2011, 10, 416–426. [Google Scholar]
- Samples Availability: Available from the authors.
Supplementary Files
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Suarez-Jimenez, G.-M.; Burgos-Hernandez, A.; Ezquerra-Brauer, J.-M. Bioactive Peptides and Depsipeptides with Anticancer Potential: Sources from Marine Animals. Mar. Drugs 2012, 10, 963-986. https://doi.org/10.3390/md10050963
Suarez-Jimenez G-M, Burgos-Hernandez A, Ezquerra-Brauer J-M. Bioactive Peptides and Depsipeptides with Anticancer Potential: Sources from Marine Animals. Marine Drugs. 2012; 10(5):963-986. https://doi.org/10.3390/md10050963
Chicago/Turabian StyleSuarez-Jimenez, Guadalupe-Miroslava, Armando Burgos-Hernandez, and Josafat-Marina Ezquerra-Brauer. 2012. "Bioactive Peptides and Depsipeptides with Anticancer Potential: Sources from Marine Animals" Marine Drugs 10, no. 5: 963-986. https://doi.org/10.3390/md10050963
APA StyleSuarez-Jimenez, G.-M., Burgos-Hernandez, A., & Ezquerra-Brauer, J.-M. (2012). Bioactive Peptides and Depsipeptides with Anticancer Potential: Sources from Marine Animals. Marine Drugs, 10(5), 963-986. https://doi.org/10.3390/md10050963




