Abstract
Three new polyketides, woodylides A–C (1–3), were isolated from the ethanol extract of the South China Sea sponge Plakortis simplex. The structures were elucidated by spectroscopic data (IR, 1D and 2D NMR, and HRESIMS). The absolute configurations at C-3 of 1 and 3 were determined by the modified Mosher’s method. Antifungal, cytotoxic, and PTP1B inhibitory activities of these polyketides were evaluated. Compounds 1 and 3 showed antifungal activity against fungi Cryptococcus neoformans with IC50 values of 3.67 and 10.85 µg/mL, respectively. In the cytotoxicity test, compound 1 exhibited a moderate effect against the HeLa cell line with an IC50 value of 11.2 µg/mL, and compound 3 showed cytotoxic activity against the HCT-116 human colon tumor cell line and PTP1B inhibitory activity with IC50 values of 9.4 and 4.7 µg/mL, respectively.
1. Introduction
Polyketides are a structurally diverse family of natural products with various biological activities and pharmacological properties, biogenetically derived from acetate, propionate and butyrate units [1,2,3]. Marine sponges provide a wide range of polyketides with antibacterial [4], antiviral [5], antitumor [6], antimalarial [7], and taxol-like microtubule-stabilizing activities [8]. The sponge-derived polyketides often contain cyclic peroxides and lactone functionalities, linear and bicyclic carbon frameworks [1,9], and macrolide and aromatic groups in some cases [8,10]. A prolific source of new and bioactive polyketides derived from sponges of the genus Plakortis attracted our attention. As part of our ongoing search for new pharmacologically active lead compounds from the marine sponges collected off Xisha Islands in the South China Sea [11,12], we investigated polyketides from the marine sponge Plakortis simplex. A preliminary study led to the isolation of two new polyketides named simplextones A and B with an unusual cyclopentane skeleton [6]. The interesting chemical and bioactive significance of P. simplex prompted us to continue the study of this sponge, which has led to the isolation of three new linear polyketides, named as woodylide A (1), B (2) and C (3) (Figure 1) [13], which are the acyclic diol analogues of the cyclic polyketide peroxides isolated from the genus of Plakortis [14,15]. This article describes the isolation, identification and bioactivity of the new compounds.
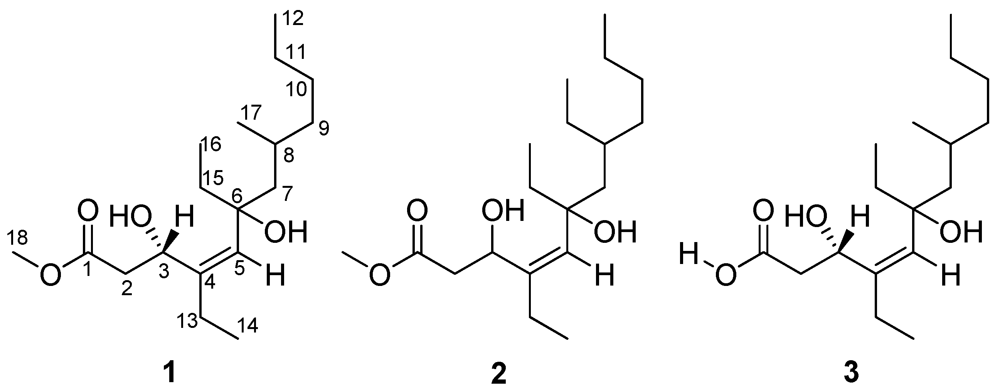
Figure 1.
Structures of woodylides A (1), B (2) and C (3).
2. Results and Discussion
Compound 1 was obtained as a colorless oil. The positive HRESIMS exhibited a pseudomolecular ion peak at m/z 337.2354, [M + Na]+ (calcd 337.2355 [16]), consistent with a molecular formula of C18H34O4, indicating two double bond equivalents. The IR absorption bands supported the existence of hydroxyl (3275 cm−1), carbonyl (1742 cm−1), and olefinic (1650 cm−1) functional groups. The 13C NMR and DEPT spectra indicated the presence of 18 carbon atoms, corresponding to a total of one carbonyl (δC 173.6), one olefinic quaternary carbon (δC 140.5), one olefinic methine (δC 132.4), one oxygenated quaternary carbon (δC 77.0), one oxymethine (δC 68.8), one methoxyl (δC 51.8), one aliphatic methine (δC 29.2), seven aliphatic methylenes (δC 23.0, 29.4, 29.4, 36.5, 38.5, 40.7, and 48.9), and four methyl carbons (δC 8.3, 13.7, 14.2, and 22.2) (Table 1). The 1H NMR spectrum displayed resonances for one methyl group attached to a tertiary carbon at δH 0.98 (3H, d, J = 6.5 Hz), three methyl groups attached to secondary carbons at δH 0.88 (3H, t, J = 7.0 Hz), δH 0.88 (3H, t, J = 7.0 Hz overlapped), and δH 1.05 (3H, t, J = 7.0 Hz), one methoxyl group at δH 3.71 (3H, s), and one olefinic proton at δH 5.11 (1H, s). The two double bond equivalents of 1 were accounted for one double bond and one carbonyl group, revealing the linearity of its carbon scaffold. Analysis of the COSY and HSQC spectra revealed the presence of five spin systems in the structure: H2-2/H-3, H-8/H3-17, H2-11/H3-12, H2-13/H3-14, and H2-15/H3-16 (Figure 2). The HMBC correlation from H3-18 (δH 3.71) to C-1 (δC 173.6) positioned the methoxyl group at C-1. The olefinic proton H-5 (δH 5.11) afforded HMBC correlations to C-3 (δC 68.8), C-4 (δC 140.5), and C-6 (δC 77.0), whereas H-7a (δH 1.34) showed HMBC correlations to C-5 (δC 132.4) and C-6, which established the connectivity of the partial structure C-3 to C-7 (δC 48.9). Obviously, the double bond was located between C-4 and C-5 on the linear carbon scaffold based on the carbon resonances of C-4 and C-5. Accordingly, the methyl acetate group was tethered to C-4 via C-3 by HMBC correlations from H-2b (δH 2.92) to C-1 and C-4, from H2-13 (δH 2.02) to C-3, and from H-5 to C-3. The HMBC correlations from H3-14 (δH 1.05) to C-4, and from H3-16 (δH 0.88) to C-6, unambiguously assigned the ethyl groups to C-4 and C-6, respectively. Moreover, the HMBC correlations from H3-17 (δH 0.98) to C-7 and C-9 (δC 38.5), and from H-7b (δH 1.55) to C-9 demonstrated the linkage of C-7, C-9 and C-17 (δC 22.2) via C-8. Even though no COSY correlation was observed between H2-10 (δH 1.25) and H2-11 (δH 1.25), the connectivity of the partial structure C-9 to C-12 (δC 14.2) was secured by the HMBC correlations of H-9b (δH 1.30)/C-10 (δC 29.4), and H3-12 (δH 0.88)/C-10, and by comparison of the NMR date with the known derivatives [17]. With this assignment secured, the final methine (C-3) and the oxygenated quaternary carbon (C-6) had to be substituted with hydroxyl groups to satisfy the molecular formula and shifts.

Table 1.
NMR data for woodylides A-C (1–3) in CDCl3.
| Position | 1 a | 2 b | 3 b | |||
|---|---|---|---|---|---|---|
| δC | δH, mult. (J in Hz) | δC | δH, mult. (J in Hz) | δC | δH, mult. (J in Hz) | |
| 1 | 173.6 qC | 173.6 qC | 176.4 qC | |||
| 2a | 40.7 CH2 | 2.57, dd (16.5, 3.0) | 40.6 CH2 | 2.57, dd (16.8, 3.0) | 41.0 CH2 | 2.53, d (15.6) |
| 2b | 2.92, dd (16.5, 10.0) | 2.99, dd (16.8, 10.2) | 2.90, dd (15.6, 4.8) | |||
| 3 | 68.8 CH | 4.83, dd (10.0, 3.0) | 68.8 CH | 4.81, dd (10.2, 3.0) | 68.9 CH | 4.67,d (9.6) |
| 4 | 140.5 qC | 140.4 qC | 140.9 qC | |||
| 5 | 132.4 CH | 5.11, s | 132.5 CH | 5.11, s | 131.3 CH | 5.05, s |
| 6 | 77.0 qC | 77.0 qC | 77.8 qC | |||
| 7a | 48.9 CH2 | 1.34, dd (14.0, 7.0) | 45.8 CH2 | 1.44, m | 49.0 CH2 | 1.35, dd (13.8, 6.6) |
| 7b | 1.55, dd (15.0, 7.0) | 1.47, m | 1.56, dd (12.0, 4.8) | |||
| 8 | 29.2 CH | 1.61, m | 35.0 CH | 1.55, m | 29.1 CH | 1.60, m |
| 9a | 38.5 CH2 | 1.14, m | 34.3 CH2 | 1.26, m | 38.5 CH2 | 1.13, m |
| 9b | 1.30, m | 1.33, m | 1.30, m | |||
| 10 | 29.4 CH2 | 1.25, m | 29.0 CH2 | 1.22, m | 29.3 CH2 | 1.25, m |
| 11 | 23.0 CH2 | 1.25, m | 23.2 CH2 | 1.26, m | 23.0 CH2 | 1.25,m |
| 12 | 14.2 CH3 | 0.88, t (7.0) | 14.2 CH3 | 0.88, t (7.2) | 14.1 CH3 | 0.86, t (7.2) |
| 13 | 29.4 CH2 | 2.02, m | 29.6 CH2 | 2.04, m | 29.8 CH2 | 2.02, m |
| 14 | 13.7 CH3 | 1.05, t (7.0) | 13.6 CH3 | 1.05, t (7.8) | 13.5 CH3 | 1.04, t (7.2) |
| 15 | 36.5 CH2 | 1.55, m | 36.6 CH2 | 1.55, m | 36.5 CH2 | 1.56, m |
| 16 | 8.3 CH3 | 0.88, t (7.0) | 8.4 CH3 | 0.88, t (7.2) | 8.3 CH3 | 0.86, t (7.2) |
| 17 | 22.2 CH3 | 0.98, d (6.5) | 27.7 CH2 | 1.45, m | 22.1 CH3 | 0.96, d (6.6) |
| 18 | 51.8 -OCH3 | 3.71, s | 10.7 CH3 | 0.85, t (7.2) | ||
| 19 | 51.8-OCH3 | 3.71, s | ||||
a Measured at 500 MHz (1H) and 125 MHz (13C); b Measured at 600 MHz (1H) and 150 MHz (13C).
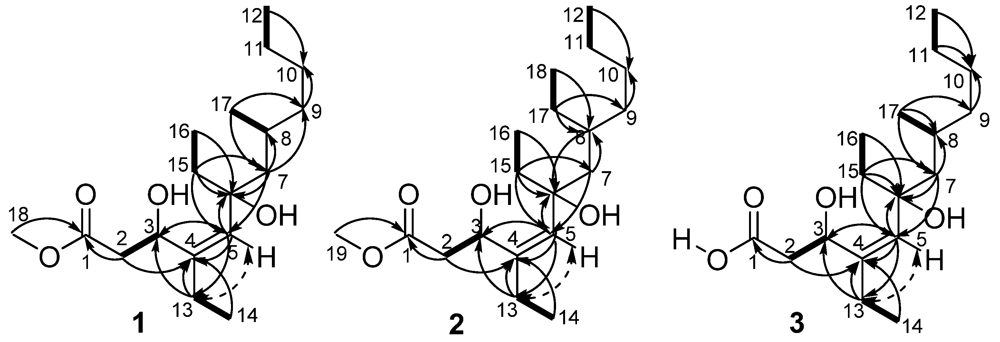
Figure 2.
COSY (▬), Key HMBC (→), and selected NOE (  ) correlations of 1, 2, and 3.
) correlations of 1, 2, and 3.
The configuration of double bond in 1 was established on the basis of NOESY data. The Z-geometry of the Δ4,5 double bond was deduced from a NOESY correlation between H-5 and H2-13, as well as derived from devoid of NOESY correlation between H-5 and H-3 (δH 4.83) (Figure 2). The absolute configuration of C-3 was determined by applying the modified Mosher’s method to the secondary hydroxyl group [18]. The (S)- and (R)-MTPA esters of 1 were prepared by reaction with (R)- and (S)-MTPA chlorides, respectively. The ΔδS−R values observed for the protons near the secondary C-3 hydroxyl group for the esters indicated the S-configuration for the secondary alcohol stereogenic center in 1 (Figure 3).
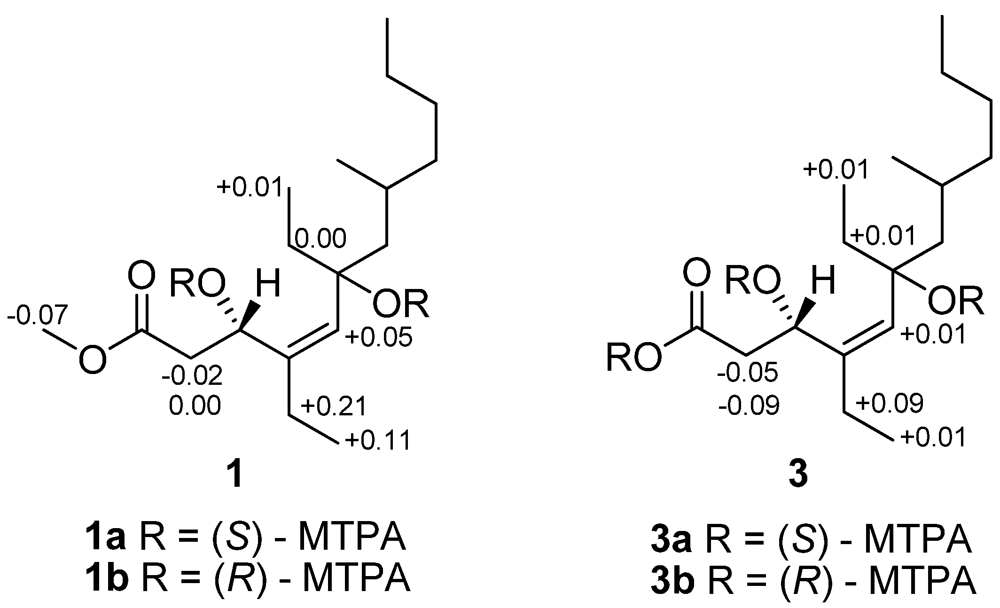
Figure 3.
∆δS−R values for the MTPA derivatives of 1 and 3 in CDCl3.
Compound 2 was also isolated as a colorless oil, with a molecular formula of C19H36O4 as determined by HRESIMS (m/z 351.2513, [M + Na]+, calcd 351.2511). Comparison of the 1H NMR data of 2 with those of 1, the obvious differences were the presence of an additional methyl triplet (δH 0.85, t, J = 7.2 Hz) and a methylene multiplet (δH 1.45, m), as well as the absence of the methyl doublet (δH 0.98, d, J = 6.5 Hz), indicating an overall structure similar to 1 except for an ethyl group C-17 (δC 27.7)/C-18 (δC 10.7) attached to C-8 (δC 35.0) in 2 (Table 1). This was also supported by the HMBC correlations from both H3-18 (δH 0.85) and H2-17 (δH 1.45) to C-8, and 1H–1H COSY correlations between H3-18 and H2-17. The geometry of the trisubstituted double bond was assigned as Z based on the NOESY correlation between H2-13 (δH 2.04, m) and H-5 (δH 5.11, m) (Figure 2).
Compound 3 was assigned a molecular formula of C17H32O4, implying two double bond equivalents, as deduced from the HRESIMS (m/z 323.2200, [M + Na]+, calcd 323.2198) and NMR data. The 13C NMR and DEPT spectra exhibited 17 carbon resonances corresponding to four methyl, seven methylene, three methine, and three quaternary carbons (Table 1). The overall appearance of the NMR spectrum showed close structural similarity between 3 and 1, except for the absence of a methoxyl resonance in 3 instead of H3-18 (δH 3.71)/C-18 (δC 51.8) in 1, indicating 3 was a free carboxylic acid. This was also confirmed by the observation of a Δδ ~3 downfield shift of the C-1 from δC 173.6 to δC 176.4. The NOESY correlations observed between H2-13 (δH 2.02) and H-5 (δH 5.05), confirmed the Z geometry of the double bond at C-4 (δC 140.9)/C-5 (δC 131.3). The absolute configuration of C-3 was determined by the modified Mosher’s method [18]. Analysis of the ΔδS−R values (Figure 3) according to Mosher’s model pointed to an S-configuration for C-3 in 3.
To confirm if compound 1 could be an artifact formed from 3 during the isolation processes, a solution of 3 was kept at room temperature for three days in the presence of Si-60 or RP-18 gel in MeOH, respectively. The formation of 1 was not observed, thus suggesting that compound 1 may be a natural product and not an artifact.
The three new polyketides 1–3 were evaluated for antifungal activity against Cryptococcus neoformans (ATCC 90113), Candida albicans (Y0109), Trichophyton rubrum (Cmccftla) and Microsporum gypseum (Cmccfmza) (Table 2), for in vitro cytotoxic activity against human cancer cell lines, HCT-116 (colon cancer), A549 (lung carcinoma), HeLa (cervical cancer), QGY-7703 (hepatocarcinoma), and MDA231 (breast adenocarcinoma) (Table 3), and protein tyrosine phosphatase 1B (PTP1B) inhibitory activity (Table 3). Compounds 1 and 3 showed moderate antifungal activity against the fungus C. neoformans with IC50 values of 3.67 and 10.85 µg/mL, respectively, while compound 2, bearing an ethyl group at C-8, was inactive even tested at a higher concentration. Compounds 1 and 3 showed weaker antifungal activity towards all the remaining assayed indicators. Furthermore, compound 1 showed moderate cytotoxicity (IC50, 11.22 µg/mL) against HeLa cell line, and compound 3 exhibited cytotoxic activity (IC50, 9.4 µg/mL) against HCT-116 cell line. Cytotoxicity of compound 3 against A549, HeLa, QGY-7703, and MDA231 cell lines was weaker when compared to that of 1. In addition, compound 3 was tested for PTP1B inhibitory activity in vitro, with an IC50 value of 4.7 µg/mL. The PTP1B inhibitors are recognized as potential therapeutic agents for the treatment of type II diabetes and obesity [19].

Table 2.
Antifungal activity of woodylides A–C (1–3).
| Compound | Antifungal Activity | |||
|---|---|---|---|---|
| C. neoformansa | C. albicansb | T. rubrum b | M. gypseumb | |
| 1 | 3.67 | 32 | 32 | 32 |
| 2 | NA | NT | NT | NT |
| 3 | 10.85 | NA | 32 | 32 |
| Amphotericin B | 0.35 | NT | NT | NT |
| Fluconazole | NT | 0.25 | 2 | 8 |
a Exhibited with IC50 value (μg/mL); b Exhibited with MIC (μg/mL); NT = Not tested; NA = Not active.

Table 3.
Cytotoxic and PTP1B inhibitory activitives of woodylides A–C (1–3).
| Compound | Cytotoxicity (IC50, μg/mL) | PTP1B Inhibitory Activity (IC50, μg/mL) | ||||
|---|---|---|---|---|---|---|
| HCT-116 | A549 | HeLa | QGY-7703 | MDA231 | ||
| 1 | NT | 37.83 | 11.22 | 25.80 | NA | NT |
| 2 | NT | NT | NT | NT | NT | NT |
| 3 | 9.4 | NA | NA | NA | NT | 4.7 |
| Sodium orthovanadate | NT | NT | NT | NT | NT | 88.46 |
NT = Not tested; NA = Not active.
3. Experimental Section
3.1. General Experimental Procedures
Optical rotations were determined with a Perkin-Elmer 341 polarimeter with 1 mm cell. IR spectra were recorded on a Bruker vector 22 spectrometer with KBr pellets. The NMR experiments were conducted on Bruker AVANCE-600 and Bruker AMX-500 instruments. HRESIMS and ESIMS were obtained on a Q-Tof micro YA019 mass spectrometer. In antifungal evaluation, IC50 values were calculated on XLfit 4.2 software (IDBS: Alameda, CA, USA, 2005). Reversed-phase HPLC was performed on YMC-Pack Pro C18 RS (5 μm) columns with a Waters 1525/2998 liquid chromatograph. Column chromatographies were carried out on silica gel 60 (200–300 mesh; Yantai, China), Sephadex LH-20 (Amersham Biosciences). TLC was carried out using HSGF 254 plates and visualized by spraying with anisaldehyde-H2SO4 reagent.
3.2. Animal Material
The sponge, identified by Jin-He Li (Institute of Oceanology, Chinese Academy of Sciences, China), was collected off Woody (Yongxing) Island and seven connected islets in the South China Sea in June 2007. A voucher sample (No. B-3) was deposited in the Laboratory of Marine Drugs, Department of Pharmacy, Changzheng Hospital, Second Military Medical University, China.
3.3. Extraction and Isolation
The air-dried and powdered sponge (1.0 kg, dry weight) was extracted with 95% aqueous EtOH, and the combined extracts were concentrated under reduced pressure at 45 °C to yield the crude extract (100 g). This extract was suspended in H2O and extracted with EtOAc and n-BuOH to afford the EtOAc- and n-BuOH-soluble extracts. The EtOAc-soluble extract (80 g) was partitioned between 90% aqueous MeOH and n-hexane to afford the n-hexane-soluble extract (21 g), which was subjected to Vacuum Liquid Chromatography (VLC) on silica gel by gradient elution using n-hexane/acetone (100:1, 50:1, 20:1, 15:1, 10:1, 5:1, 1:1, 0:1) as solvents to give seven subfractions (A–G). Subfraction G was subjected to CC on Sephadex LH-20, ODS and further purified by reversed-phase preparative HPLC (YMC-Pack Pro C18 RS, 5 μm, 10 × 250 mm, 2.0 mL/min), to yield compound 1 (CH3OH/H2O 80:20, 2.0 mL/min, 208 nm, tR = 44.03 min, 10.2 mg), compound 2 (CH3OH/H2O 80:20, 2.0 mL/min, 201 nm, tR = 59.65 min, 2.5 mg), and compound 3 (CH3OH:H2O 80:20, 2.0 mL/min, 208 nm, tR = 33.08 min, 22.3 mg).
Preparation of MTPA esters 1a and 1b: Woodylide A (1; 1.2 mg (3.8 μmol) and 1.0 mg (3.2 μmol), respectively) was reacted with R-(−)- or S-(+)-MTPACl (59.4 μmol) in freshly distilled dry pyridine (500 µL) and stirred under N2 at room temperature for 18 h, respectively, and then the solvent was removed. The products were purified by mini-CC on silica gel (200 mesh, n-hexane:EtOAc, 3:1) to afford S-(−)- and R-(+)-MTPA esters 1a and 1b, respectively.
Preparation of MTPA esters 3a and 3b: Woodylide C (3; 1.2 mg (4.0 μmol) and 1.1 mg (3.7 μmol), respectively) was similarly processed to give S-(−)- and R-(+)- MTPA esters 3a and 3b, respectively.
Woodylide A (1): Colorless oil; [α]22D −15.0 (c 0.06, MeOH); IR (KBr) νmax 3275, 2961, 2928, 2874, 2858, 1742, 1650, 1460, 1438, 1412, 1376, 1356, 1286, 1252, 1213, 1169, 1108, 1066, 1036, 1016, 992, 966, 933, 870, 852, 806, 781, 706 cm−1; 1H NMR (CDCl3, 500 MHz) and 13C NMR (CDCl3, 125 MHz) data, see Table 1; HRESIMS m/z 337.2354 [M + Na]+ (calcd for C18H34O4Na, 337.2355). CD spectrum (c 1.91 × 10−3 M, CH3CN), 197 nm (Δε 3.27), 200 nm (Δε 3.54).
Woodylide B (2): Colorless oil; [α]22D +5.5 (c 0.06, MeOH); IR (KBr) νmax 3301, 2961, 2928, 2874, 2857, 1742, 1667, 1462, 1438, 1410, 1378, 1358, 1286, 1169, 1108, 1067, 1035, 1018, 994, 872, 852, 806, 781, 706 cm−1; 1H NMR (CDCl3, 600 MHz) and 13C NMR (CDCl3,150 MHz) data, see Table 1; HRESIMS m/z 351.2513 [M + Na]+ (calcd for C19H36O4Na, 351.2511). CD spectrum (c 2.13 × 10−3 M, CH3CN), 196 nm (Δε 3.02), 203 nm (Δε 2.36).
Woodylide C (3): Light yellow oil; [α]22D −11.4 (c 0.14, MeOH); IR (KBr) νmax 3422, 2961, 2928, 2874, 2858, 1757, 1655, 1462, 1401, 1379, 1342, 1285, 1252, 1209, 1169, 1109, 1063, 1021, 954, 915, 879, 801, 729, 666 cm−1; 1H NMR (CDCl3, 600 MHz) and 13C NMR (CDCl3, 150 MHz) data, see Table 1; HRESIMS m/z 323.2200 [M + Na]+ (calcd for C17H32O4Na, 323.2198). CD spectrum (c 2.32 × 10−3 M, CH3CN), 191 nm (Δε 3.23), 196 nm (Δε 4.72), 202 nm (Δε 3.61).
1H NMR data of 1a (CDCl3, 600 MHz): δ 2.59 (1H, dd, H-2a), 2.91 (1H, dd, H-2b), 5.27 (1H, s, H-5), 1.47 (1H, dd, H-7a), 1.53 (1H, dd, H-7b), 1.60 (1H, m, H-8), 1.14 (2H, m, H-9), 1.26 (2H, m, H-10), 1.26 (2H, m, H-11), 0.88 (3H, t, H-12), 2.05 (2H, m, H-13), 1.01 (3H, t, H-14), 1.56 (2H, m, H-15), 0.86 (3H, t, H-16), 0.94 (3H, d, H-17), 3.60 (3H, s, H-18).
1H NMR data of 1b (CDCl3, 600 MHz): δ 2.59 (1H, dd, H-2a), 2.93 (1H, dd, H-2b), 5.22 (1H, s, H-5), 1.49 (1H, dd, H-7a), 1.53 (1H, dd, H-7b), 1.61 (1H, m, H-8), 1.19 (2H, m, H-9), 1.26 (2H, m, H-10), 1.26 (2H, m, H-11), 0.88 (3H, t, H-12), 1.84 (2H, m, H-13), 0.90 (3H, t, H-14), 1.56 (2H, m, H-15), 0.85 (3H, t, H-16), 0.99 (3H, d, H-17), 3.67 (3H, s, H-18).
1H NMR data of 3a (CDCl3, 600 MHz): δ 2.41 (1H, dd, H-2a), 2.95 (1H, dd, H-2b), 5.04 (1H, s, H-5), 1.29 (1H, dd, H-7a), 1.73 (1H, dd, H-7b), 1.80 (1H, m, H-8), 1.11 (2H, m, H-9), 1.73 (2H, m, H-10), 1.27 (2H, m, H-11), 0.81 (3H, t, H-12), 2.04 (2H, m, H-13), 0.98 (3H, t, H-14), 1.27 (2H, m, H-15), 0.79 (3H, t, H-16), 0.90 (3H, d, H-17).
1H NMR data of 3b (CDCl3, 600 MHz): δ 2.50 (1H, dd, H-2a), 3.00 (1H, dd, H-2b), 5.03 (1H, s, H-5), 1.28 (1H, dd, H-7a), 1.76 (1H, dd, H-7b), 1.76 (1H, m, H-8), 1.11 (2H, m, H-9), 1.71 (2H, m, H-10), 1.26 (2H, m, H-11), 0.81 (3H, t, H-12), 1.95 (2H, m, H-13), 0.97 (3H, t, H-14), 1.26 (2H, m, H-15), 0.78 (3H, t, H-16), 0.89 (3H, d, H-17).
3.4. Antifungal Evaluation
Antifungal IC50 values of woodylides A–C against C. neoformans were calculated as described by Ikhlas A. Khan et al. [20]. Amphotericin B was used as the positive control. Minimal Inhibition Concentration (MIC) values of woodylides A–C were determined against three indicators (C. albicans, T. rubrum, and M. gypseum), following the National Center for Clinical Laboratory Standards (NCCLS) methods [21,22]. Fluconazole was used as the positive control. Briefly, samples (dissolved in DMSO) were serially diluted in 20% DMSO/saline and transferred (10 µL) in duplicate to 96 well flat bottom microplates. Bacterial strains were grown aerobically at 30 °C in SDA for 16–20 h. A set of different concentrations of compounds 1–3 prepared in RPMI 1640 were next inoculated with the microorganisms and incubated 70–74 h for C. neoformans at 35 °C, 46 h for C. albicans at 35 °C, and 4–7 days for T. rubrum and M. gypseum at 30 °C. The IC50 values were calculated by using the fit model 201 of XLfit 4.2 software. The MIC values were evaluated in triplicate for each compound (within the range 1.25–640 μg/mL).
3.5. Cytotoxicity Assay
The cytotoxicity of compounds 1–3 against HCT-116, A549, HeLa, QGY-7703, and MDA231 cell lines was evaluated by the MTT assay as described in a previous report [23]. Briefly, compounds were solubilized in DMSO with the working concentration of test substances ranging from 1 to 100 μg/mL. Cells at the exponential growth phase were harvested and seeded into 96-well plates. After incubation for 24 h, the cells were treated with various concentrations of test substances for 48 h and then incubated with 1 mg/mL MTT at 37 °C for 4 h, followed by dissolving in DMSO. The produced formazan was measured by the absorbance at 570 nm on a microplate reader. The IC50 values were calculated on the basis of percentage inhibition using the linear regression method.
3.6. PTP1B Inhibitory Assay
PTP1B inhibitory activity was determined using a PTP1B inhibitory assay as described previously [24]. The enzymatic activities of the PTP1B catalytic domain were determined at 30 °C by monitoring the hydrolysis of pNPP. Dephosphorylation of pNPP generated the product pNP, which was monitored at an absorbance of 405 nm. In a typical 100 μL assay mixture containing 50 mmol/L 3-[N-morpholino]propanesulfonic acid (MOPs), pH 6.5, 2 mmol/L pNPP, and 30 nmol/L recombinant PTP1B, activities were continuously monitored and the initial rate of the hydrolysis was determined using the early linear region of the enzymatic reaction kinetic curve.
4. Conclusions
In this paper we report the isolation and the structural determination of three new linear polyketides, woodylides A–C, endowed with antifungal, antineoplastic, and PTP1B inhibitory activities, from the South China Sea marine sponge P. simplex. Unfortunately, due to the lack of compound 2, the absolute configuration at C-3 as well as the bioactivity of woodylide B could not be determined. Woodylide C exhibited a good PTP1B inhibitory activity, and deserves further study for its therapeutic potential against type II diabetes and obesity diseases.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81172978, 81072573, 81001394, and 41106127) and the Major Program of Modernization of Chinese Medicine (STCSM, 09dZ1975800).
References and Notes
- John, J.P.; Jost, J.; Novikov, A.V. Synthesis of plakortethers F and G. J. Org. Chem. 2009, 74, 6083–6091. [Google Scholar] [CrossRef]
- Huang, X.H.; van Soest, R.; Roberge, M.; Andersen, R.J. Spiculoic acids A and B, new polyketides isolated from the caribbean marine sponge Plakortis angulospiculatus. Org. Lett. 2004, 6, 75–78. [Google Scholar]
- Kwan, D.H.; Schulz, F. The stereochemistry of complex polyketide biosynthesis by modular polyketide synthases. Molecules 2011, 16, 6092–6115. [Google Scholar] [CrossRef]
- Okada, Y.; Matsunaga, S.; van Soest, R.W.M.; Fusetani, N. Nagahamide A, an antibacterial depsipeptide from the marine sponge Theonella swinhoei. Org. Lett. 2002, 4, 3039–3042. [Google Scholar] [CrossRef]
- Plaza, A.; Bifulco, G.; Keffer, J.L.; Lloyd, J.R.; Baker, H.L.; Bewley, C.A. Celebesides A–C and Theopapuamides B-D, depsipeptides from an Indonesian sponge that inhibit HIV-1 entry. J. Org. Chem. 2009, 74, 504–512. [Google Scholar]
- Liu, X.F.; Song, Y.L.; Zhang, H.J.; Yang, F.; Yu, H.B.; Jiao, W.H.; Piao, S.J.; Chen, W.S.; Lin, H.W. Simplextones A and B, unusual polyketides from the marine sponge Plakortis simplex. Org. Lett. 2011, 13, 3154–3157. [Google Scholar]
- Fattorusso, E.; Taglialatela-Scafati, O. Marine antimalarials. Mar. Drugs 2009, 7, 130–152. [Google Scholar] [CrossRef]
- Johnson, T.A.; Tenney, K.; Cichewicz, R.H.; Morinaka, B.I.; White, K.N.; Amagata, T.; Subramanian, B.; Media, J.; Mooberry, S.L.; Valeriote, F.A.; et al. Sponge-derived fijianolide polyketide class: Further evaluation of their structural and cytotoxicity properties. J. Med. Chem. 2007, 50, 3795–3803. [Google Scholar]
- Wang, X.D.; Fan, W.; Yu, H.B.; Jiao, W.H.; Lin, H.W.; Liu, X.F. Advances in studies on chemical constituents in marine sponges of genus Plakortis Schulze and their bioactivties. Chin. Tradit. Herb. Drugs 2010, 42, 1633–1645. [Google Scholar]
- Schneemann, I.; Kajahn, I.; Ohlendorf, B.; Zinecker, H.; Erhard, A.; Nagel, K.; Wiese, J.; Imhoff, J.F. Mayamycin, a cytotoxic polyketide from a streptomyces strain isolated from the marine sponge Halichondria panicea. J. Nat. Prod. 2010, 73, 1309–1312. [Google Scholar]
- Piao, S.J.; Zhang, H.J.; Lu, H.Y.; Yang, F.; Jiao, W.H.; Yi, Y.H.; Chen, W.S.; Lin, H.W. Hippolides A–H, acyclic manoalide derivatives from the marine sponge Hippospongia lachne. J. Nat. Prod. 2011, 74, 1248–1254. [Google Scholar]
- Jiao, W.H.; Huang, X.J.; Yang, J.S.; Yang, F.; Piao, S.J.; Gao, H.; Li, J.; Ye, W.C.; Yao, X.S.; Chen, W.S.; Lin, H.W. Dysidavarones A–D, new sesquiterpene quinones from the marine sponge Dysidea avara. Org. Lett. 2011, 14, 202–205. [Google Scholar]
- Lin, H.W.; Liu, X.F.; Yu, H.B.; Xu, Y.; Wang, R.P.; Jiao, W.H.; Chen, W.S. Chain polyketone-like compound and its application. China Patent 201110105074.8, 19 October 2011. [Google Scholar]
- Harrison, B.; Crews, P. Cyclic polyketide peroxides and acyclic diol analogues from the sponge Plakortis lita. J. Nat. Prod. 1998, 61, 1033–1037. [Google Scholar] [CrossRef]
- Fattorusso, C.; Campiani, G.; Catalanotti, B.; Persico, M.; Basilico, N.; Parapini, S.; Taramelli, D.; Campagnuolo, C.; Fattorusso, E.; Romano, A.; et al. Endoperoxide derivatives from marine organisms: 1,2-dioxanes of the plakortin family as novel antimalarial agents. J. Med. Chem. 2006, 49, 7088–7094. [Google Scholar]
- The pseudomolecular masses were calibrated on the masses of the respective neutral molecules.
- Braekman, J.C.; Daloze, D.; de Groote, S.; Fernandes, J.B.; van Soest, R.W.M. New polyketides from the sponge Plakortis sp. J. Nat. Prod. 1998, 61, 1038–1042. [Google Scholar]
- Ohtani, I.; Kusumi, T.; Kashman, Y.; Kakisawa, H. High-field FT NMR application of Mosher’s method. The absolute configurations of marine terpenoids. J. Am. Chem. Soc. 1991, 113, 4092–4096. [Google Scholar]
- Johnson, T.O.; Ermolieff, J.; Jirousek, M.R. Protein tyrosine phosphatase 1B inhibitors for diabetes. Nat. Rev. Drug Discov. 2002, 1, 696–709. [Google Scholar] [CrossRef]
- Ma, G.; Khan, S.I.; Jacob, M.R.; Tekwani, B.L.; Li, Z.; Pasco, D.S.; Walker, L.A.; Khan, I.A. Antimicrobial and antileishmanial activities of hypocrellins A and B. Antimicrob. Agents Chemother. 2004, 48, 4450–4452. [Google Scholar]
- Clinical and Laboratory Standards Institute, Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeast; Approved Standard, 3rd ed; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2009; Document M27-A3.
- Clinical and Laboratory Standards Institute, Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard, 2nd ed; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008; Document M38-A2.
- Carmichael, J.; DeGraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: Assessment of chemosensitivity testing. Cancer Res. 1987, 47, 936–942. [Google Scholar]
- Yang, X.; Li, J.; Zhou, Y.; Shen, Q.; Chen, J.; Li, J. Discovery of novel inhibitor of human leukocyte common antigen-related phosphatase. Biochim. Biophys. Acta 2005, 1726, 34–41. [Google Scholar] [CrossRef]
- Samples Availability: Available from the authors.
Supplementary Files
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).