Isolation and Structural Determination of the First 8-epi-type Tetrodotoxin Analogs from the Newt, Cynops ensicauda popei, and Comparison of Tetrodotoxin Analogs Profiles of This Newt and the Puffer Fish, Fugu poecilonotus
Abstract
:1. Introduction
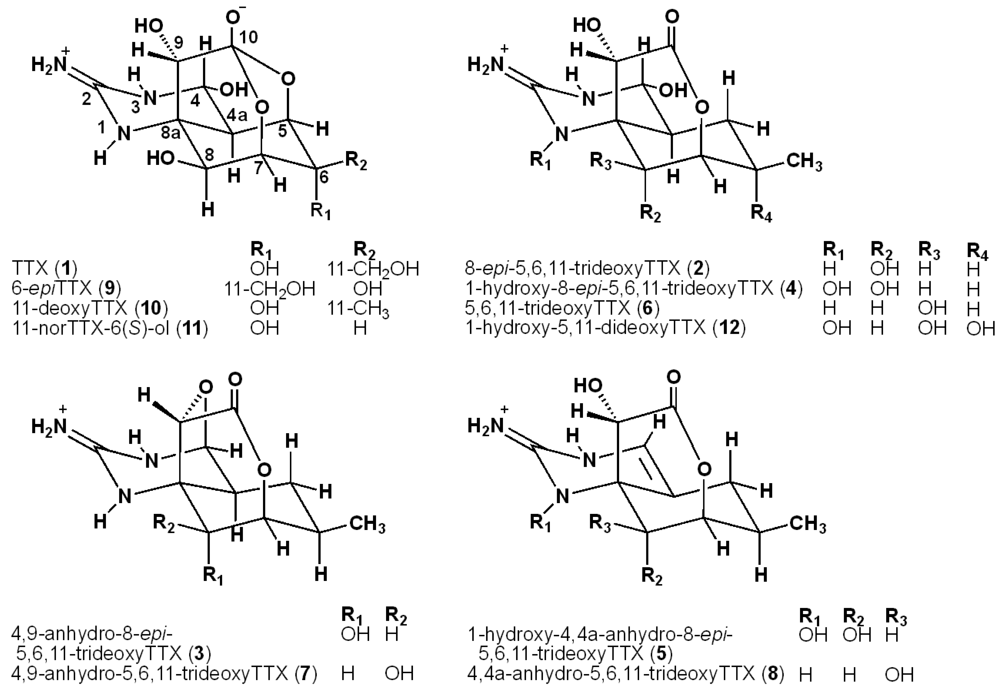
2. Results and Discussion
2.1. Purification of New TTX Analogs and Their Molecular Formulas
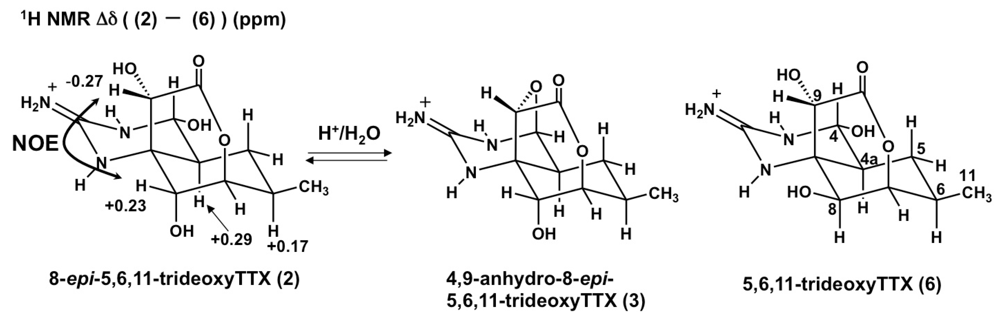
2.2. The Structures of 8-epi-5,6,11-trideoxyTTX (2) and 4,9-Anhydro-8-epi-5,6,11-trideoxyTTX (3)
| 8-epi-5,6,11-trideoxyTTX (2) | 5,6,11-trideoxyTTX (6) | Δδ (2–6) | ||||
|---|---|---|---|---|---|---|
| Position | δC | δH (J in Hz) | δC | δH (J in Hz) | ΔδC | ΔδH |
| 2 | ND | 155.7 | ||||
| 4 | 77.6 | 5.17, d (8.8) | 77.3 | 5.17, d (10.0) | 0.3 | 0.00 |
| 4a | 40.6 | 2.28, m | 46.2 | 1.99, ddd (13.3, 10.0, 3.9) | −5.6 | 0.29 |
| 5eq | 27.5 | 2.05, m | 27.8 | 2.07, dt (13.3, 4.0) | −0.3 | −0.02 |
| 5ax | 0.87, q (13.3) | 0.92, q (13.3) | −0.05 | |||
| 6 | 32.1 | 2.30, m | 36.9 | 2.13, m | −4.8 | 0.17 |
| 7 | 83.9 | 4.55, br s | 87.2 | 4.61, br t | −3.3 | −0.06 |
| 8 | 67.9 | 4.33, d (4.1) | 74.5 | 4.10, d (2.3) | −6.6 | 0.23 |
| 8a | 61.4 | 61.2 | 0.2 | |||
| 9 | 75.0 | 4.36, s | 72.4 | 4.63, s | 2.6 | −0.27 |
| 10 | 176.6 | 177.4 | −0.8 | |||
| 11 | 17.8 | 1.06, d (7.0) | 18.3 | 1.08, d (6.7) | −0.5 | −0.02 |
| 4,9-Anhydro-8- epi-5,6,11-trideoxyTTX (3) | 4,9-Anhydro-5,6,11-trideoxyTTX (7) | Δδ (3–7) | ||||
|---|---|---|---|---|---|---|
| Position | δC | δH (J in Hz) | δC | δH (J in Hz) | ΔδC | ΔδH |
| 2 | ND | 156.0 | ||||
| 4 | 86.6 | 5.24, s | 85.7 | 5.21, s | 0.9 | 0.03 |
| 4a | 40.7 | 2.72, dt (11.4, 6.5) | 43.7 | 2.67, dd (11.2, 7.0) | −3.0 | 0.05 |
| 5eq | 27.9 | 2.12, m | 26.6 | 2.12–2.04, m | 1.3 | 0.00–0.08 |
| 5ax | 0.82, dt (15.9, 12.4) | 0.82, q (11.2) | 0.00 | |||
| 6 | ND | 2.42, m | 33.8 | 2.12–2.04, m | 0.30–0.38 | |
| 7 | 84.0 | 4.60, br s | 86.7 | 4.70, br s | −2.7 | −0.10 |
| 8 | 64.3 | 4.60, br s | 68.0 | 4.43, d (2.1) | −3.7 | 0.17 |
| 8a | ND | 62.6 | ||||
| 9 | 85.6 | 5.04, s | 83.5 | 4.98, s | 2.1 | 0.06 |
| 10 | ND | 178.2 | ||||
| 11 | 18.7 | 1.03, d (6.8) | 18.2 | 1.02, d (6.6) | 0.5 | 0.01 |
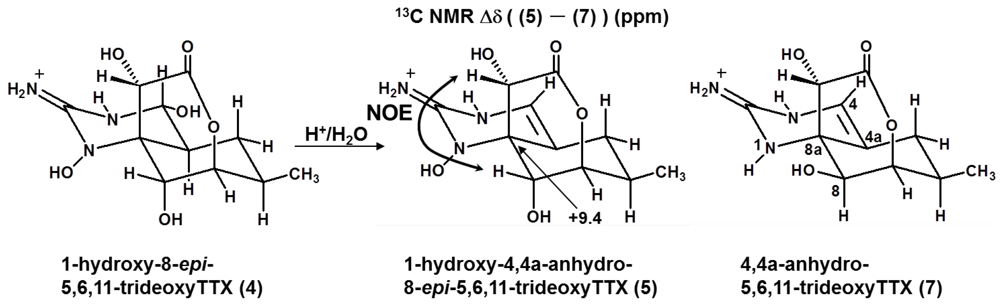
2.3. The Structures of 1-Hydroxy-8-epi-5,6,11-trideoxyTTX (4) and 1-Hydroxy-4,4a-anhydro-8-epi-5,6,11-trideoxyTTX (5)
| 1-Hydroxy-8- epi-5,6,11-trideoxyTTX (4) | Δδ (4–6) | |||
|---|---|---|---|---|
| Position | δC | δH (J in Hz) | ΔδC | ΔδH |
| 2 | ND | |||
| 4 | 77.3 | 5.08, d (9.4) | 0.0 | −0.09 |
| 4a | 40.3 | 2.54, dd (9.4, 2.3) | −5.9 | 0.55 |
| 5eq | 28.2 | 2.05, m | 0.4 | −0.02 |
| 5ax | 0.90, q (13.5) | −0.02 | ||
| 6 | 31.8 | 2.32, m | −5.1 | 0.19 |
| 7 | 84.2 | 4.67, d (4.4) | −3.0 | 0.06 |
| 8 | 65.3 | 4.53, d (4.1) | −9.2 | 0.43 |
| 8a | 68.0 | 6.8 | ||
| 9 | 69.0 | 4.79, s | −3.4 | 0.16 |
| 10 | 177.9 | 0.5 | ||
| 11 | 17.5 | 1.06, d (7.0) | −0.8 | −0.02 |
| 1-Hydroxy-4,4a-Anhydro-8-epi-5,6,11-trideoxyTTX (5) | 4,4a-Anhydro-5,6,11-trideoxyTTX (8) | Δδ (5–8) | ||||
|---|---|---|---|---|---|---|
| Position | δC | δH (J in Hz) | δC | δH (J in Hz) | ΔδC | ΔδH |
| 2 | 154.6 | 152.4 | 2.2 | |||
| 4 | 122.2 | 6.30, s | 120.4 | 6.24, s | 1.8 | 0.06 |
| 4a | 110.3 | 110.1 | 0.2 | |||
| 5eq | 30.9 | 2.38, dd (15.8, 5.3) | 29.9 | 2.37, d (14.1) | 1.0 | 0.01 |
| 5ax | 1.65, dd (15.0, 12.0) | 1.65, t (13.6) | 0.00 | |||
| 6 | 31.6 | 2.28, m | 35.6 | 2.03, m | −4.0 | 0.25 |
| 7 | 84.1 | 4.67, d (4.1) | 86.6 | 4.66, br s | −2.5 | 0.01 |
| 8 | 65.5 | 4.52, d (4.4) | 73.3 | 4.27, br s | −7.8 | 0.25 |
| 8a | 72.2 | 62.8 | 9.4 | |||
| 9 | 68.8 | 4.80, s | 71.2 | 4.48, s | −2.4 | 0.32 |
| 10 | 176.7 | 175.5 | 1.2 | |||
| 11 | 17.9 | 1.04, d (6.8) | 17.3 | 1.05, d (5.3) | 0.6 | −0.01 |
2.4. Comparison of TTX Analogs Profiles of the Newt, C. e. popei and the Puffer Fish, Fugu poecilonotus
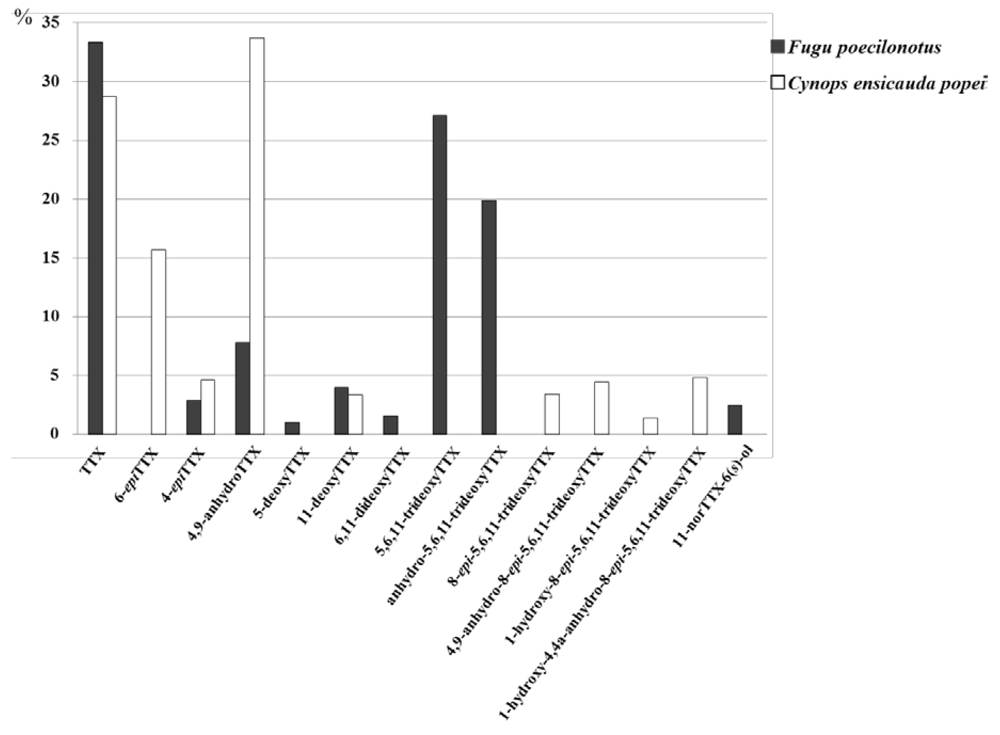
| Toxin | LC/MS Results (μg/g) | |
|---|---|---|
| F. poecilonotos | C. e. popei | |
| TTX | 134.0 | 49.6 |
| 6-epiTTX | <0.3 | 27.0 |
| 4-epiTTX | 11.5 | 8.0 |
| 4,9-anhydroTTX | 31.2 | 58.1 |
| 5-deoxyTTX | 4.2 | <0.3 |
| 11-deoxyTTX | 15.9 | 5.7 |
| 6,11-dideoxyTTX | 6.3 | <0.3 |
| 5,6,11-trideoxyTTX | 108.8 | ND * |
| anhydro-5,6,11-trideoxyTTX | 79.9 | ND * |
| 8-epi-5,6,11-trideoxyTTX | ND * | 5.8 |
| 4,9-anhydro-8-epi-5,6,11-trideoxyTTX | ND * | 7.7 |
| 1-hydroxy-8-epi-trideoxyTTX | <0.3 | 2.4 |
| 1-hydroxy-4,4a-anhydro-8-epi-5,6,11-trideoxyTTX | <0.3 | 8.3 |
| 11-norTTX-6(S)-ol | 9.9 | <0.3 |
2.5. Discussion
3. Experimental Section
3.1. Purification of 8-epi-5,6,11-trideoxyTTX (2), 4,9-Anhydro-8-epi-5,6,11-trideoxyTTX (3), 1-Hydroxy-8-epi-5,6,11-trideoxyTTX (4), and 1-Hydroxy-4,4a-Anhydro-8-epi-5,6,11-trideoxyTTX (5)
3.2. LC/MS and LC/MS/MS
3.3. NMR Spectroscopy and HR-FAB-MS
3.4. Quantitative/Qualitative Analysis of TTX Analogs in the Newt and Puffer Fish
4. Conclusions
Acknowledgments
References
- Tsuda, K.; Ikuma, S.; Kawamura, M.; Tachikawa, R.; Sakai, K.; Tamura, C.; Amalasu, O. Tetrodotoxin. VII. On the structure of tetrodotoxin and its derivatives. Chem. Pharm. Bull. 1964, 12, 1357–1374. [Google Scholar] [CrossRef]
- Woodward, R.B. The structure of tetrodotoxin. Pure Appl. Chem. 1964, 9, 49–74. [Google Scholar]
- Goto, T.; Kishi, Y.; Takahashi, S.; Hirata, Y. Tetrodotoxin. Tetrahedron 1965, 21, 2059–2088. [Google Scholar]
- Buchwald, H.D.; Durham, L.; Fischer, H.G.; Harada, R.; Mosher, H.S.; Kao, C.Y.; Fuhrman, F.A. Identity of tarichatoxin and tetrodotoxin. Science 1964, 143, 474–475. [Google Scholar]
- Brodie, E.D., Jr.; Hensel, J.L.; Johnson, J.A. Toxicity of the Urodele amphibians Taricha, Notophthalmus, Cynops, and Paramesotriton (Salamandrid). Copeia 1974, 1974, 506–511. [Google Scholar] [CrossRef]
- Yotsu, M.; Iorizzi, M.; Yasumoto, T. Distribution of tetrodotoxin, 6-epitetrodotoxin, and 11-deoxytetrodotoxin in newts. Toxicon 1990, 28, 238–241. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Mebs, D.; Kwet, A.; Schneider, M. Tetrodotoxin and its analogue 6-epitetrodotoxin in newts (Triturus spp.; Urodela, Salamandridae) from southern Germany. Toxicon 2007, 50, 306–309. [Google Scholar] [CrossRef]
- Kim, Y.H.; Brown, G.B.; Mosher, H.S.; Fuhrman, F.A. Tetrodotoxin: Occurrence in atelopid frogs of Costa Rica. Science 1975, 189, 151–152. [Google Scholar]
- Daly, J.W.; Gusovsky, F.; Myers, C.W.; Yotsu-Yamashita, M.; Yasumoto, T. First occurrence of tetrodotoxin in a dendrobatid frog (Colostethus inguinalis), with further reports for the bufonid genus Atelopus. Toxicon 1994, 32, 279–285. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Tateki, E. First report on toxins in the Panamanian toads Atelopus limosus, A. glyphus and A. certus. Toxicon 2010, 55, 153–156. [Google Scholar] [CrossRef]
- Hanifin, C.T. The chemical and evolutionary ecology of tetrodotoxin (TTX) toxicity in terrestrial vertebrates. Mar. Drugs 2010, 8, 577–593. [Google Scholar]
- Yasumoto, T.; Yotsu, M.; Murata, M.; Naoki, H. New tetrodotoxin analogue from the newt Cynops ensicauda. J. Am. Chem. Soc. 1988, 110, 2344–2345. [Google Scholar]
- Kotaki, Y.; Shimizu, Y. 1-Hydroxy-5,11-dideoxytetrodotoxin, the first N-hydroxy and ringdeoxy derivative of tetrodotoxin found in the newt Taricha granulosa. J. Am. Chem. Soc. 1993, 115, 827–830. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Schimmele, B.; Yasumoto, T. Isolation and structural assignment of 5-deoxytetrodotoxin from the puffer fish Fugu poecilonotus. Biosci. Biotechnol. Biochem. 1999, 63, 961–963. [Google Scholar] [CrossRef]
- Jang, J.H.; Yotsu-Yamashita, M. 6,11-DideoxyTTX from the puffer fish, Fugu pardalis. Toxicon 2007, 50, 947–951. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Yamagishi, Y; Yasumoto, T. 5,6,11-Trideoxytetrodotoxin from the puffer fish, Fugu poecilonotus. Tetrahedron Lett. 1995, 36, 9329–9332. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Hayashi, Y.; Khora, S.S.; Sato, S.; Yasumoto, T. Isolation and structural assignment of 11-nortetrodotoxin-6(S)-ol from the puffer Arothron nigropunctatus. Biosci. Biotechnol. Biochem. 1992, 56, 370–371. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Goto, A.; Nakagawa, T. Isolation of 4-S-cysteinyltetrodotoxin from the liver of the puffer fish Fugu pardalis, and formation of the adducts of 4,9-anhydrotetrodotoxin with thiols. Chem. Res. Toxicol. 2005, 18, 865–871. [Google Scholar] [CrossRef]
- Shoji, Y.; Yotsu-Yamashita, M.; Miyazawa, T.; Yasumoto, T. Electrospray ionization mass spectrometry of tetrodotoxin and its analogs: Liquid mass spectrometry, and liquid chromatography/tandem mass spectrometry. Anal. Biochem. 2001, 290, 10–17. [Google Scholar]
- Nakagawa, T.; Jang, J.; Yotsu-Yamashita, M. Hydrophilic interaction liquid chromatography-electrospray ionization mass spectrometry of tetrodotoxin and its analogs. Anal. Biochem. 2006, 352, 142–144. [Google Scholar]
- Jang, J.; Yotsu-Yamashita, M. Distribution of tetrodotoxin, saxitoxin, and their analogs among tissues of the puffer fish Fugu pardalis. Toxicon 2006, 48, 980–987. [Google Scholar] [CrossRef]
- Jang, J.; Lee, J.S.; Yotsu-Yamashita, M. LC/MS analysis of tetrodotoxin and its deoxy analogs in the marine puffer fish Fugu niphobles from the southern coast of Korea, and in the brackishwater puffer fishes Tetraodon nigroviridis and Tetraodon biocellatus from Southeast Asia. Mar. Drugs 2010, 8, 1049–1058. [Google Scholar] [CrossRef]
- Yasumoto, T.; Yasumura, D.; Yotsu, M.; Michishita, T.; Endo, A.; Kotaki, Y. Bacterial production of tetrodotoxin and anhydrotetrodotoxin. Agric. Biol. Chem. 1986, 50, 793–795. [Google Scholar]
- Noguchi, T.; Jeon, J.K.; Arakawa, O.; Sugita, H.; Deguchi, Y.; Shida, Y.; Hashimoto, K. Occurrence of tetrodotoxin and anhydrotetrodotoxin in Vibrio sp. isolated from the intestines of a xantihd crab, Atergatis floridus. J. Biochem. 1986, 99, 311–314. [Google Scholar]
- Chau, R.; Kalaitzis, J.A.; Neilan, B.A. On the origins and biosynthesis of tetrodotoxin. Aquat. Toxicol. 2011, 104, 61–72. [Google Scholar]
- Lehman, E.M.; Brodie, E.D., Jr.; Brodie, E.D., III. No evidence for an endosymbiotic bacterial origin of tetrodotoxin in the newt Taricha granulosa. Toxicon 2004, 44, 243–249. [Google Scholar] [CrossRef]
- Shimizu, Y.; Kobayashi, M. Apparent lack of tetrodotoxin biosynthesis in captured Taricha torosa and Taricha granulosa. Chem. Pharm. Bull. (Tokyo) 1983, 31, 3625–3631. [Google Scholar] [CrossRef]
- Nakamura, M.; Yasumoto, T. Tetrodotoxin derivatives in puffer fish. Toxicon 1985, 23, 271–276. [Google Scholar]
- Umezawa, T.; Hayashi, T.; Sakai, H.; Teramoto, H.; Yoshikawa, T.; Izumida, M.; Tamatani, Y.; Hirose, T.; Ohfune, Y.; Shinada, T. Total synthesis of (−)-5,6,11-trideoxytetrodotoxin and its 4-epimer. Org. Lett. 2006, 8, 4971–4974. [Google Scholar]
- Shimizu, Y.; Hsu, C.P.; Fallon, W.E.; Oshima, Y.; Miura, I.; Nakakoshi, K. Structure of neosaxitoxin. J. Am. Chem. Soc. 1978, 100, 6791–6793. [Google Scholar]
- Nishikawa, T.; Asai, M.; Ohyabu, N.; Yamamoto, N.; Isobe, M. Stereocontrolled synthesis of (−)-5,11-dideoxytetrodotoxin. Angew. Chem. Int. Ed. 1999, 38, 3080–3084. [Google Scholar]
- Stuken, A.; Orr, J.S.R.; Kellmann, R.; Murray, S.A.; Neilan, B.A.; Jakobsen, K.S. Discovery of nuclear-encoded genes for the neurotoxin saxitoxin in dinoflagellates. PLoS One 2011, 6. [Google Scholar]
- Mihali, T.K.; Carmichael, W.W.; Neilan, B.A. A putative gene cluster from a Lyngbya wollei bloom that encodes paralytic shellfish toxin biosynthesis. PLoS One 2011, 6. [Google Scholar]
- Onodera, H.; Satake, M.; Oshima, Y.; Yasumoto, T.; Carmichael, W.W. New saxitoxin analogs from the freshwater filamentous cyanobacterium Lyngbya wollei. Nat. Toxins 1997, 5, 146–151. [Google Scholar]
- Sample Availability: Available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kudo, Y.; Yasumoto, T.; Konoki, K.; Cho, Y.; Yotsu-Yamashita, M. Isolation and Structural Determination of the First 8-epi-type Tetrodotoxin Analogs from the Newt, Cynops ensicauda popei, and Comparison of Tetrodotoxin Analogs Profiles of This Newt and the Puffer Fish, Fugu poecilonotus . Mar. Drugs 2012, 10, 655-667. https://doi.org/10.3390/md10030655
Kudo Y, Yasumoto T, Konoki K, Cho Y, Yotsu-Yamashita M. Isolation and Structural Determination of the First 8-epi-type Tetrodotoxin Analogs from the Newt, Cynops ensicauda popei, and Comparison of Tetrodotoxin Analogs Profiles of This Newt and the Puffer Fish, Fugu poecilonotus . Marine Drugs. 2012; 10(3):655-667. https://doi.org/10.3390/md10030655
Chicago/Turabian StyleKudo, Yuta, Takeshi Yasumoto, Keiichi Konoki, Yuko Cho, and Mari Yotsu-Yamashita. 2012. "Isolation and Structural Determination of the First 8-epi-type Tetrodotoxin Analogs from the Newt, Cynops ensicauda popei, and Comparison of Tetrodotoxin Analogs Profiles of This Newt and the Puffer Fish, Fugu poecilonotus " Marine Drugs 10, no. 3: 655-667. https://doi.org/10.3390/md10030655
APA StyleKudo, Y., Yasumoto, T., Konoki, K., Cho, Y., & Yotsu-Yamashita, M. (2012). Isolation and Structural Determination of the First 8-epi-type Tetrodotoxin Analogs from the Newt, Cynops ensicauda popei, and Comparison of Tetrodotoxin Analogs Profiles of This Newt and the Puffer Fish, Fugu poecilonotus . Marine Drugs, 10(3), 655-667. https://doi.org/10.3390/md10030655




