Sarcocrassocolides M–O, Bioactive Cembranoids from the Dongsha Atoll Soft Coral Sarcophyton crassocaule
Abstract
:1. Introduction
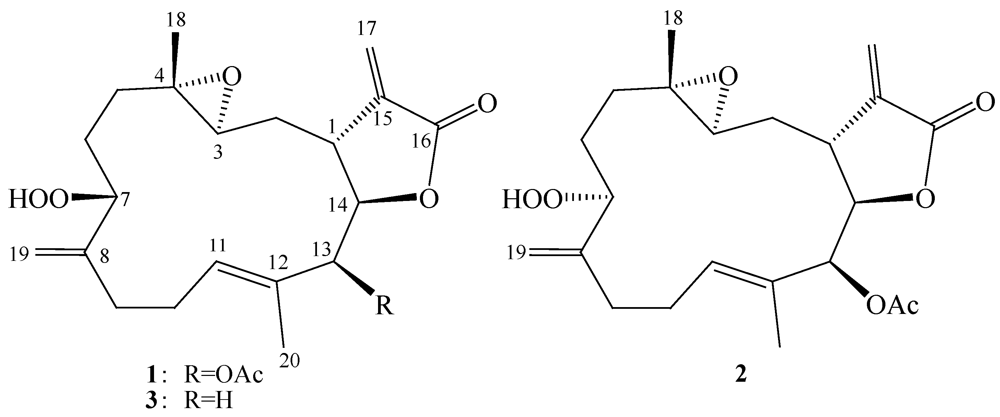
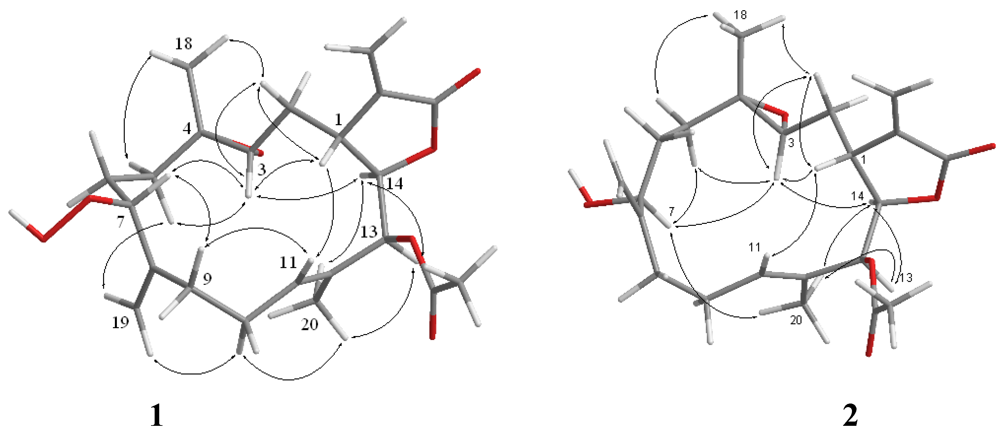
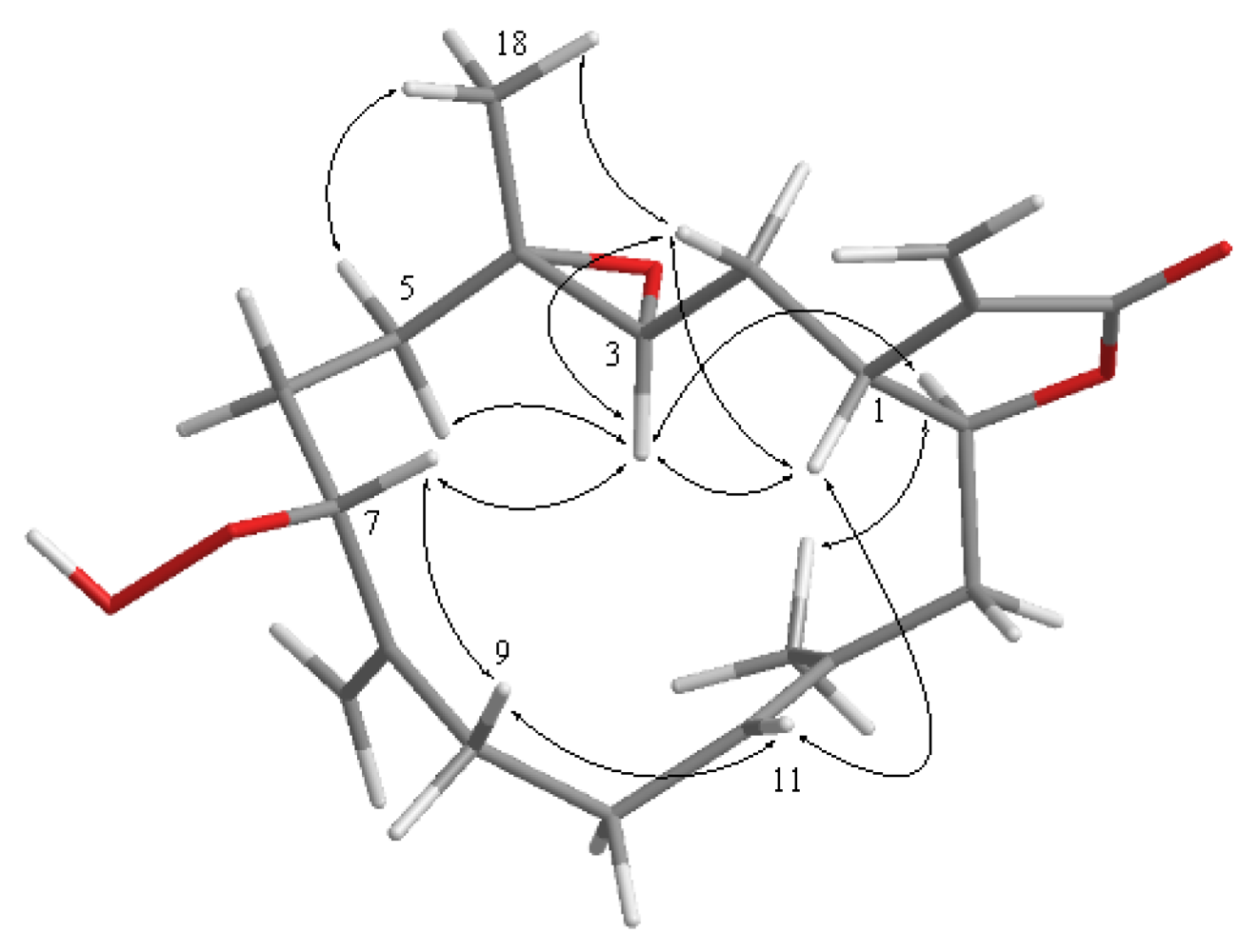
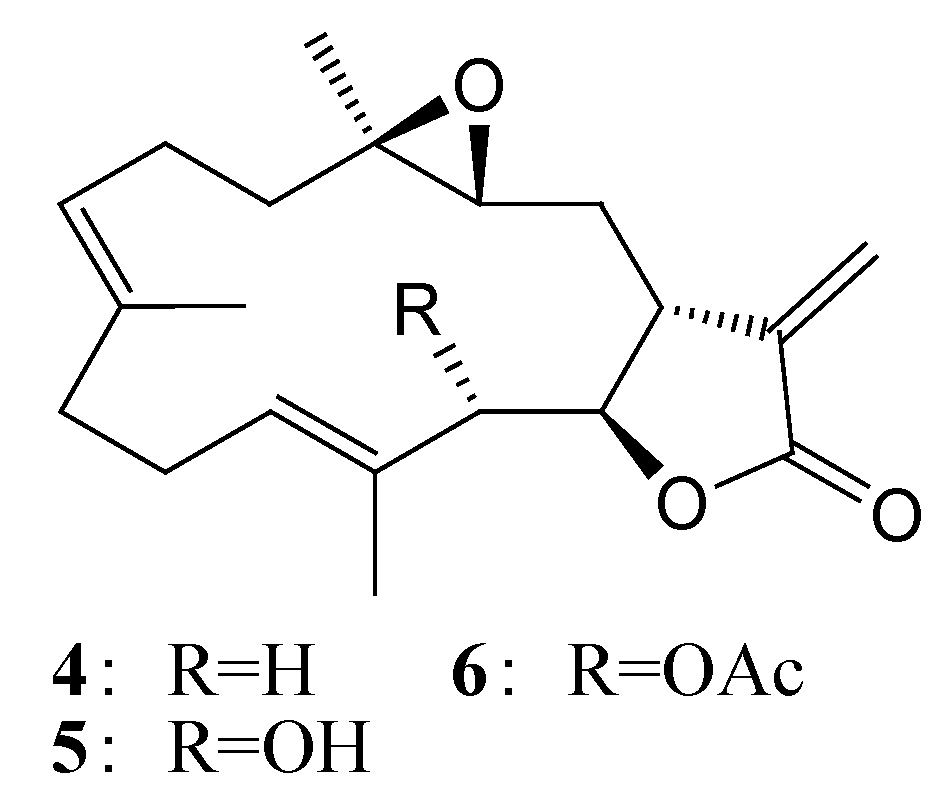
2. Results and Discussion

| Position | Sarcrocrassocolide M (1) | Sarcrocrassocolide N (2) | Sarcrocrassocolide O (3) | |||
|---|---|---|---|---|---|---|
| δC, mult.a | δH (J in Hz) b | δC, mult.a | δH (J in Hz)b | δC, mult.c | δH (J in Hz) d | |
| 1 | 37.4, CH | 3.07, dt (11.5, 2.5) | 37.5, CH | 3.07, brd (11.5) | 41.2, CH | 2.78, ddd (11.2, 4.8, 2.8) |
| 2 | 34.8, CH2 | 1.87, ddd (14.5, 11.5, 4.0) | 35.1, CH2 | 1.86, ddd (15.5, 11.5, 6.0) | 33.2, CH2 | 1.97, m |
| 1.71, m | 1.71, ddd (15.5, 6.0, 2.0) | 1.64, m | ||||
| 3 | 60.0, CH | 2.53, dd (7.0, 4.0) | 59.9, CH | 2.59, t (6.0) | 59.8, CH | 2.63, dd (8.8, 4.6) |
| 4 | 60.1, C | 60.9, C | 60.8, C | |||
| 5 | 32.8, CH2 | 1.98, ddd (14.0, 7.0, 3.5) | 32.9, CH2 | 1.96, m | 32.6, CH2 | 1.96, m |
| 1.46, dt (14.0, 7.0) | 1.42, m | 1.39, m | ||||
| 6 | 26.2, CH2 | 1.72, m | 28.6, CH2 | 1.62, m | 26.4, CH2 | 1.78, m |
| 1.58, m | 1.43, m | 1.52, m | ||||
| 7 | 87.1, CH | 4.30, t (5.5) | 86.1, CH | 4.38, t (5.0) | 86.6, CH | 4.38, t (5.2) |
| 8 | 146.6, C | 147.9, C | 146.3, C | |||
| 9 | 32.5, CH2 | 2.43, m | 31.9, CH2 | 2.47, m | 32.5, CH2 | 2.29, m |
| 2.14, ddd (14.5, 8.0, 4.0) | 2.01, m | 2.21, m | ||||
| 10 | 26.1, CH2 | 2.40, m | 25.5, CH2 | 2.33, m | 26.0, CH2 | 2.37, m |
| 2.29, m | 2.23, m | |||||
| 11 | 128.1, CH | 5.46, dd (7,0, 5.5) | 127.4, CH | 5.41, t (7.5) | 128.8, CH | 5.26, t (6.8) |
| 12 | 129.4, C | 129.4, C | 130.5, C | |||
| 13 | 77.4, CH | 5.40, s | 77.4, CH | 5.39, s | 44.9, CH2 | 2.58, brd (14.4) |
| 2.32, dd (14.4, 8.0) | ||||||
| 14 | 81.4, CH | 4.61, t (2.5) | 81.7, CH | 4.62, s | 82.1, CH | 4.47, dt (8.0, 4.0) |
| 15 | 139.3, C | 139.3, C | 138.8, C | |||
| 16 | 169.1, C | 169.1, C | 169.4, C | |||
| 17 | 121.6, CH2 | 6.28, d (2.0) | 122.0, CH2 | 6.30, d (2.0) | 122.8, CH2 | 6.32, d (2.4) |
| 5.63, d (2.0) | 5.64, d (2.0) | 5.62, d (2.4) | ||||
| 18 | 17.5, CH3 | 1.32, s | 17.7, CH3 | 1.29, s | 17.0, CH3 | 1.33, s |
| 19 | 113.5, CH2 | 5.16, s | 111.9, CH2 | 5.09, s | 113.8, CH2 | 5.17, s |
| 5.12, s | 5.08, s | 5.12, s | ||||
| 20 | 14.8, CH3 | 1.76, s | 15.1, CH3 | 1.78, s | 17.3, CH3 | 1.70, s |
| OAc | 20.7, CH3 | 2.02, s | 20.7, CH3 | 2.03, s | ||
| 169.2, C | 169.2, C | |||||

| Compound | Daoy | HEp-2 | MCF-7 | WiDr |
|---|---|---|---|---|
| 1 | 6.6 ± 0.8 | 10.4 ±1.1 | 10.6 ± 0.5 | >40 |
| 2 | 5.2 ± 0.6 | 12.3 ± 1.6 | 10.1 ± 2.3 | 30.1 ± 2.8 |
| 3 | 5.0 ± 0.7 | 12.4 ± 2.1 | 6.4 ± 0.5 | >40 |
| Mitomycin-C | 0.44 ± 0.06 | 0.30 ± 0.06 | 0.30 ± 0.12 | 0.47 ± 0.12 |
3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Separation
3.4. Cytotoxicity Testing
3.5. In Vitro Anti-Inflammatory Assay
4. Conclusions
Acknowledgements
References
- Bishara, A.; Rudi, A.; Benayahu, Y.; Kashman, Y. Three biscembranoids and their monomeric counterpart cembranoid, a biogenetic diels-alder precursor, from the soft coral Sarcophyton elegans. J. Nat. Prod. 2007, 70, 1951–1954. [Google Scholar] [CrossRef]
- Bensemhoun, J.; Rudi, A.; Bombarda, I.; Gaydou, E.M.; Kashman, Y.; Aknin, M. Flexusines A and B and epimukulol from the soft coral Sarcophyton flexuosum. J. Nat. Prod. 2008, 71, 1262–1264. [Google Scholar] [CrossRef]
- Marrero, J.; Benítez, J.; Rodríguez, A.D.; Zhao, H.; Raptis, R.G. Bipinnatins K-Q, Minor cembrane-type diterpenes from the west Indian gorgonian Pseudopterogorgia kallos: Isolation, structure assignment, and evaluation of biological activities. J. Nat. Prod. 2008, 71, 381–389. [Google Scholar] [CrossRef]
- Shi, Y.-P.; Rodríguez, A.D.; Barnes, C.L.; Sánchez, J.A.; Raptis, R.G.; Baran, P. New terpenoid constituents from Eunicea pinta. J. Nat. Prod. 2002, 65, 1232–1241. [Google Scholar] [CrossRef]
- Rashid, M.A.; Gustafson, K.R.; Boyd, M.R. HIV-inhibitory cembrane derivatives from a Philippines collection of the soft coral Lobophytum species. J. Nat. Prod. 2000, 63, 531–533. [Google Scholar] [CrossRef]
- König, G.M.; Wright, A.D. New cembranoidditerpenes from the soft coral Sarcophyton ehrenbergi. J. Nat. Prod. 1998, 61, 494–496. [Google Scholar] [CrossRef]
- Iwashima, M.; Matsumoto, Y.; Takahashi, H.; Iguchi, K. New marine cembrane-type diterpenoids from the Okinawan soft coral Clavularia koellikeri. J. Nat. Prod. 2000, 63, 1647–1652. [Google Scholar] [CrossRef]
- Iguchi, K.; Fukaya, T.; Takahashi, H.; Watanabe, K. Stolonilactone, a novel terpenoid-related compound, isolated from the Okinawan soft coral Clavularia koellikeri. J. Org. Chem. 2004, 69, 4351–4355. [Google Scholar] [CrossRef]
- Su, J.-H.; Ahmed, A.F.; Sung, P.-J.; Chao, C.-H.; Kuo, Y.-H.; Sheu, J.-H. ManaarenolidesA-I, new diterpenoids from the soft coral Sinularia manaarensis. J. Nat. Prod. 2006, 69, 1134–1139. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, C.-Y.; Lin, Y.-F.; Wen, Z.-H.; Su, J.-H.; Kuo, Y.-H.; Chiang, M. Y.; Sheu, J.-H. Anti-inflammatory cembranoids from the soft corals Sinularia querciformis and Sinularia granosa. J. Nat. Prod. 2008, 71, 1754–1759. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Tai, S.-H.; Wen, Z.-H.; Su, J.-H.; Wu, Y.-C.; Hu, W.-P.; Sheu, J.-H. A C-3 methylatedisocembranoid and 10-oxocembranoids from a Formosan soft coral Sinularia grandilobata. J. Nat. Prod. 2008, 71, 946–951. [Google Scholar] [CrossRef]
- Chen, B.-W.; Chao, C.-H.; Su, J.-H.; Huang, C.-Y.; Dai, C.-F.; Wen, Z.-H.; Sheu, J.-H. A novel symmetric sulfur-containing biscembranoid from the Formosan soft coral Sinularia flexibilis. Tetrahedron Lett. 2010, 44, 5764–5766. [Google Scholar]
- Cheng, S.-Y.; Wen, Z.-H.; Wang, S.-K.; Chiou, S.-F.; Hsu, C.-H.; Dai, C.-F.; Chiang, M.Y.; Duh, C.-Y. Unprecedented hemiketalcembranolides with anti-inflammatory activity from the soft coral Lobophytum durum. J. Nat. Prod. 2009, 72, 152–155. [Google Scholar] [CrossRef]
- Chao, C.-H.; Wen, Z.-H.; Wu, Y.-C.; Yeh, H.-C.; Sheu, J.-H. Cytotoxic and anti-inflammatory cembranoids from the soft coral Lobophytum crassum. J. Nat. Prod. 2008, 71, 1819–1824. [Google Scholar] [CrossRef]
- Huang, H.-C.; Ahmed, A.F.; Su, J.-H.; Wu, Y.-C.; Chiang, M.Y.; Sheu, J.-H. Crassocolides A–F, new cembranoids with a trans-fused lactone from the soft coral Sarcophyton crassocaule. J. Nat. Prod. 2006, 69, 1554–1559. [Google Scholar] [CrossRef]
- Huang, H.-C.; Chao, C.-H.; Kuo, Y.-H.; Sheu, J.-H. Crassocolides G-M, cembranoids from a Formosan soft coral Sarcophyton crassocaule. Chem. Biodivers. 2009, 6, 1232–1242. [Google Scholar] [CrossRef]
- Cheng, Y.-B.; Shen, Y.-C.; Kuo, Y.-H.; Khalil, A.T. Cembranediterpenoids from the Taiwanese soft coral Sarcophyton stolidotum. J. Nat. Prod. 2008, 71, 1141–1145. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Wang, S.-K.; Chiou, S.-F.; Hsu, C.-H.; Dai, C.-F.; Chiang, M.Y.; Duh, C.-Y. Cembranoids from the octocoral Sarcophyton ehrenbergi. J. Nat. Prod. 2010, 73, 197–203. [Google Scholar] [CrossRef]
- Su, J.-H.; Lu, Y.; Lin, W.-Y.; Wang, W.-H.; Sung, P.-J.; Sheu, J.-H. A cembranoid, trocheliophorol, from the cultured soft coral Sarcophyton trocheliophorum. Chem. Lett. 2010, 39, 172–173. [Google Scholar] [CrossRef]
- Wang, G.-H.; Huang, H.-C.; Su, J.-H.; Huang, C.-Y.; Hsu, C.-H.; Kuo, Y.-H.; Sheu, J.-H. Crassocolides N–P, three cembranoids from the Formosan soft coral Sarcophyton crassocaule Bioorg. Med. Chem. Lett. 2011, 21, 7201–7204. [Google Scholar]
- Sheu, J.-H.; Wang, G.-H.; Duh, C.-Y.; Soong, K. Pachyclavulariolides M-R, six novel diterpenoids from a Taiwanese soft coral Pachyclavulariaviolacea. J. Nat. Prod. 2003, 66, 662–666. [Google Scholar] [CrossRef]
- Lin, W.-Y.; Lu, Y.; Su, J.-H.; Wen, Z.-H.; Dai, C.-F.; Kuo, Y.-H.; Sheu, J.-H. Bioactive cembranoids from the Dongsha Atoll soft coral Sarcophyton crassocaule. Mar. Drugs 2011, 9, 994–1006. [Google Scholar] [CrossRef]
- Lin, W.-Y.; Su, J.-H.; Lu, Y.; Wen, Z.-H.; Dai, C.-F.; Kuo, Y.-H.; Sheu, J.-H. Cytotoxic and anti-inflammatory cembranoids from the Dongsha Atoll soft coral Sarcophyton crassocaule. Bioorg. Med. Chem. 2010, 18, 1936–1941. [Google Scholar]
- Duh, C.-Y.; Wang, S.-K.; Chung, S.-G.; Chou, G.-C.; Dai, C.-F. Cytotoxiccembrenolides and steroids from the Formosan soft coral Sarcophyton crassocaule. J. Nat. Prod. 2000, 63, 1634–1637. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Y.; Deng, Z.; Ofwegen, L. V.; Proksch, P.; Lin, W. Cytotoxic cembranoid diterpenes from a soft coral Sinularia gibberosa. J. Nat. Prod. 2005, 68, 649–652. [Google Scholar] [CrossRef]
- Scudiero, D.A.; Shoemaker, R.H.; Paull, K.D.; Monks, A.; Tierney, S.; Nofziger, T.H.; Currens, M.J.; Seniff, D.; Boyd, M.R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988, 48, 4827–4833. [Google Scholar]
- Jean, Y.-H.; Chen, W.-F.; Duh, C.-Y.; Huang, S.-Y.; Hsu, C.-H.; Lin, C.-S.; Sung, C.-S.; Chen, I.-M.; Wen, Z.-H. Inducible nitric oxide synthase and cyclooxygenase-2 participate in anti-inflammatory and analgesic effects of the natural marine compound lemnalol from Formosan soft coral Lemnalia cervicorni. Eur. J. Pharmacol. 2008, 578, 323–331. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lin, W.-Y.; Lu, Y.; Chen, B.-W.; Huang, C.-Y.; Su, J.-H.; Wen, Z.-H.; Dai, C.-F.; Kuo, Y.-H.; Sheu, J.-H. Sarcocrassocolides M–O, Bioactive Cembranoids from the Dongsha Atoll Soft Coral Sarcophyton crassocaule. Mar. Drugs 2012, 10, 617-626. https://doi.org/10.3390/md10030617
Lin W-Y, Lu Y, Chen B-W, Huang C-Y, Su J-H, Wen Z-H, Dai C-F, Kuo Y-H, Sheu J-H. Sarcocrassocolides M–O, Bioactive Cembranoids from the Dongsha Atoll Soft Coral Sarcophyton crassocaule. Marine Drugs. 2012; 10(3):617-626. https://doi.org/10.3390/md10030617
Chicago/Turabian StyleLin, Wan-Yu, Yi Lu, Bo-Wei Chen, Chiung-Yao Huang, Jui-Hsin Su, Zhi-Hong Wen, Chang-Feng Dai, Yao-Haur Kuo, and Jyh-Horng Sheu. 2012. "Sarcocrassocolides M–O, Bioactive Cembranoids from the Dongsha Atoll Soft Coral Sarcophyton crassocaule" Marine Drugs 10, no. 3: 617-626. https://doi.org/10.3390/md10030617
APA StyleLin, W.-Y., Lu, Y., Chen, B.-W., Huang, C.-Y., Su, J.-H., Wen, Z.-H., Dai, C.-F., Kuo, Y.-H., & Sheu, J.-H. (2012). Sarcocrassocolides M–O, Bioactive Cembranoids from the Dongsha Atoll Soft Coral Sarcophyton crassocaule. Marine Drugs, 10(3), 617-626. https://doi.org/10.3390/md10030617





