Scopararanes C–G: New Oxygenated Pimarane Diterpenes from the Marine Sediment-Derived Fungus Eutypella scoparia FS26
Abstract
:1. Introduction
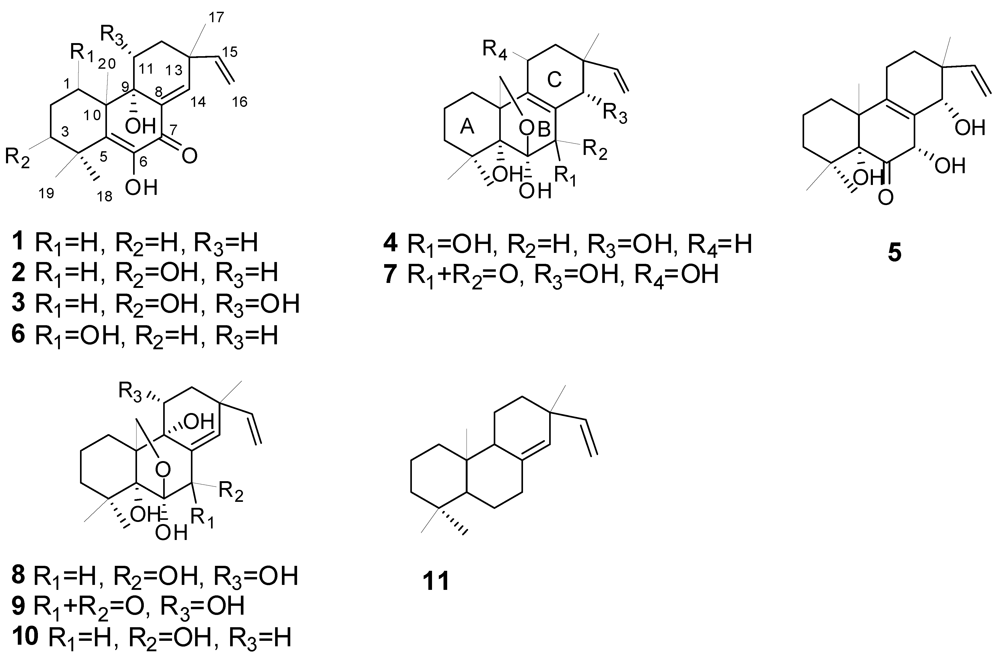
2. Results and Discussion
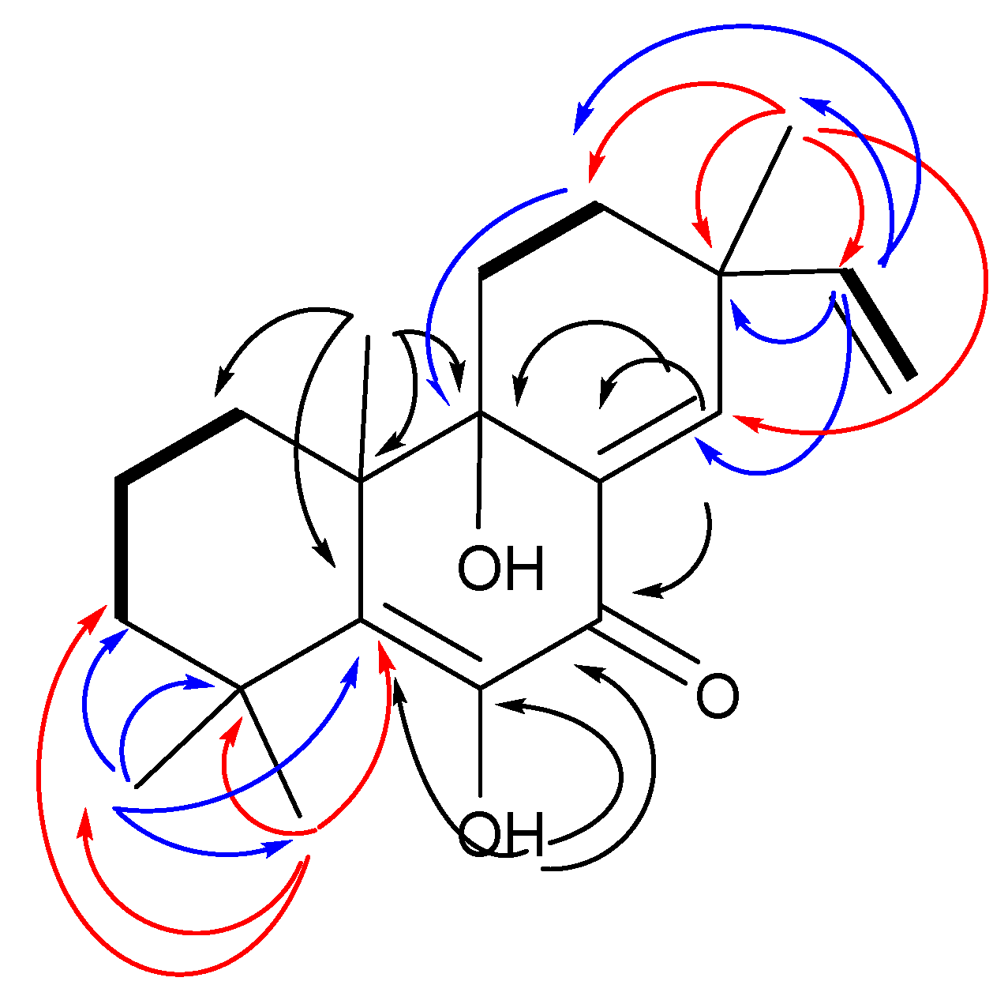
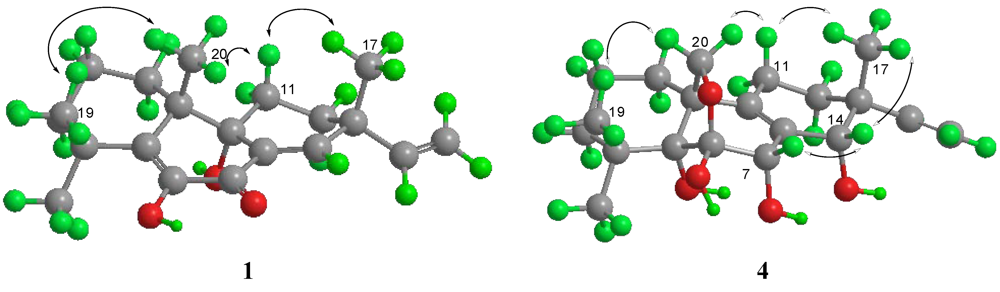
2.1. Structural Elucidation
| Position | 1 a | 2 a | 3 b | |||
|---|---|---|---|---|---|---|
| δC, mult. | δH ( J in Hz) | δC, mult. | δH ( J in Hz) | δC, mult. | δH ( J in Hz) | |
| 1α | 30.6, CH2 | 1.98, m | 28.8, CH2 | 2.12, ddd (13.0, 13.0, 4.1) | 32.0, CH2 | 2.18, ddd (13.5, 13.5, 4.7) |
| 1β | 1.53, m | 1.59, m | 1.85, ddd (13.5, 4.2, 1.6) | |||
| 2 | 17.7, CH2 | 1.65, 2m | 25.7, CH2 | 1.78, 2m | 26.7, CH2 | 1.74, 2m |
| 3 | 41.4, CH2 | 1.55, m; 1.45, m | 76.8, CH | 3.42, dd (11.4, 4.5) | 77.7, CH | 3.40, dd (11.1, 5.0) |
| 4 | 35.4, C | 40.7, C | 41.8, C | |||
| 5 | 142.8, C | 143.3, C | 145.6, C | |||
| 6 | 144.4, C | 6.73, s (OH) | 145.0, C | 144.9, C | ||
| 7 | 181.6, C | 182.1, C | 183.3, C | |||
| 8 | 133.7, C | 133.8, C | 134.9, C | |||
| 9 | 74.1, C | 73.6, C | 76.1, C | |||
| 10 | 45.1, C | 44.6, C | 46.7, C | |||
| 11α | 25.2, CH2 | 1.81, ddd (12.4, 3.2, 2.4) | 25.5, CH2 | 1.74, m | 65.8, CH | |
| 11β | 1.93, ddd (12.4, 12.4, 3.2) | 2.03, ddd (13.6, 13.6, 3.0) | 4.23, dd (12.1, 4.2) | |||
| 12α | 29.5, CH2 | 1.88, ddd (12.4, 12.4, 3.2) | 29.1, CH2 | 1.92, ddd (13.6, 13.6, 3.0) | 39.9, CH2 | 1.79, m |
| 12β | 1.58, m | 1.59, m | 1.65, ddd (12.1, 4.2, 1.9) | |||
| 13 | 38.8, C | 38.4, C | 40.1, C | |||
| 14 | 147.9, CH | 7.04, d (1.8) | 147.9, CH | 6.95, d (1.8) | 147.0, CH | 6.94, d (1.8) |
| 15 | 145.4, CH | 5.87, dd (17.5, 10.7) | 145.4, CH | 5.94, dd (17.5, 10.7) | 146.1, CH | 5.89, dd (17.5, 10.7) |
| 16a | 112.6, CH2 | 5.10, dd (17.5, 0.7) | 111.3, CH2 | 5.13, dd (17.5, 0.9) | 112.3, CH2 | 5.13, dd (17.5, 0.8) |
| 16b | 5.06, dd (10.7, 0.7) | 5.07, dd (10.7, 0.9) | 5.05, dd (10.7, 0.8) | |||
| 17 | 23.3, CH3 | 1.13, s | 22.3, CH3 | 1.15, s | 24.0, CH3 | 1.18, s |
| 18 | 30.1, CH3 | 1.41, s | 24.9, CH3 | 1.55, s | 25.9, CH3 | 1.54, s |
| 19 | 26.9, CH3 | 1.29, s | 18.1, CH3 | 1.25, s | 19.0, CH3 | 1.21, s |
| 20 | 29.4, CH3 | 1.21, s | 28.7, CH3 | 1.22, s | 29.2, CH3 | 1.24, s |
| Position | 4 b | 5 a | ||
|---|---|---|---|---|
| δC, mult. | δH ( J in Hz) | δC, mult. | δH ( J in Hz) | |
| 1 | 24.8, CH2 | 1.55, 2m | 29.1, CH2 | 1.63, 2m |
| 2 | 18.9, CH2 | 1.61, 2m | 17.9, CH2 | 1.67, 2m |
| 3 | 38.7, CH2 | 1.64, m; 1.11, m | 36.3, CH2 | 1.71, m; 1.53, m |
| 4 | 37.4, C | 36.7, C | ||
| 5 | 80.0, C | 82.6, C | ||
| 6 | 107.2, C | 210.9, C | ||
| 7 | 74.6, CH | 4.48, s | 74.6, CH | 5.23, s |
| 8 | 132.6, C | 131.8, C | ||
| 9 | 141.7, C | 139.8, C | ||
| 10 | 52.1, C | 50.7, C | ||
| 11α | 21.7, CH2 | 2.19, ddd (13.6, 5.8, 2.9) | 21.2, CH2 | 2.15, m |
| 11β | 1.92, m | 1.90, m | ||
| 12α | 28.0, CH2 | 1.80, m | 30.2, CH2 | 1.83, m |
| 12β | 1.41, m | 1.49, m | ||
| 13 | 39.6, C | 39.7, C | ||
| 14 | 70.6, CH | 4.01, s | 72.4, CH | 4.13, s |
| 15 | 146.2, CH | 6.07, dd (17.5, 11.1) | 142.0, CH | 6.03, dd (17.3, 1.4) |
| 16a | 112.1, CH2 | 5.07, dd (17.5, 1.5) | 114.8, CH2 | 5.23, dd (17.3, 1.4) |
| 16b | 5.05, dd (11.1, 1.5) | 5.21, dd (11.4, 1.4) | ||
| Position | 4 b | 5 a | ||
| δC, mult. | δH (J in Hz) | δC, mult. | ||
| 17 | 21.3, CH3 | 0.93, s | 22.8, CH3 | 1.08, s |
| 18 | 24.3, CH3 | 1.46, s | 23.8, CH3 | 1.46, s |
| 19 | 28.9, CH3 | 1.23, s | 27.8, CH3 | 1.07, s |
| 20 | 72.4, CH2 | 4.13, d (9.2) | 23.5, CH3 | 1.07, s |
| 3.40, d (9.2) | ||||
2.2. Cytotoxic Activities
| Compounds | IC50 (μM) | ||
|---|---|---|---|
| SF-268 | MCF-7 | NCI-H460 | |
| 1 | >100 | 35.9 | >100 |
| 2 | 43.5 | 25.6 | 46.1 |
| 3 | >100 | 74.1 | >100 |
| 4 | >100 | >100 | >100 |
| 5 | >100 | 85.5 | >100 |
| 6 | 20.5 | 12.0 | 40.2 |
| 7 | 80.1 | 60.1 | >100 |
| 8 | >100 | >100 | >100 |
| 9 | 9.2 | 4.4 | 9.9 |
| 10 | >100 | >100 | >100 |
| 11 | >100 | >100 | >100 |
| Cisplatin | 4.0 | 9.2 | 1.5 |
3. Experimental Section
3.1. General Experimental Procedures
3.2. Fungal Material and Identification
3.3. Fermentation, Extraction and Isolation
3.4. Characterization Data
3.5. Cytotoxicity Assay
4. Conclusion
Acknowledgments
References
- Bugni, T.S.; Ireland, C.M. Marine-derived fungi: A chemically and biologically diverse group of microorganisms. Nat. Prod. Rep. 2004, 21, 143–163. [Google Scholar]
- Saleem, M.; Ali, M.S.; Hussain, S.; Jabbar, A.; Ashraf, M.; Lee, Y.S. Marine natural products of fungal origin. Nat. Prod. Rep. 2007, 24, 1142–1152. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2009, 26, 170–244. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2010, 27, 165–237. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2011, 28, 196–268. [Google Scholar]
- Rateb, M.E.; Ebel, R. Secondary metabolites of fungi from marine habitats. Nat. Prod. Rep. 2011, 28, 290–344. [Google Scholar]
- Ciavatta, M.L.; Lopez-Gresa, M.P.; Gavagnin, M.; Nicoletti, R.; Manzo, E.; Mollo, E.; Guo, Y.W.; Cimino, G. Cytosporin-related compounds from the marine-derived fungus Eutypella scoparia. Tetrahedron 2008, 64, 5365–5369. [Google Scholar]
- Isaka, M.; Palasarn, S.; Lapanun, S.; Chanthaket, R.; Boonyuen, N.; Lumyong, S. γ-Lactones and ent-eudesmane sesquiterpenes from the endophytic fungus Eutypella sp. BCC 13199. J. Nat. Prod. 2009, 72, 1720–1722. [Google Scholar] [CrossRef]
- Pongcharoen, W.; Rukachaisirikul, V.; Phongpaichit, S.; Rungjindamai, N.; Sakayaroj, J. Pimarane diterpene and cytochalasin derivatives from the endophytic fungus Eutypella scoparia PSU-D44. J. Nat. Prod. 2006, 69, 856–858. [Google Scholar] [CrossRef]
- Isaka, M.; Palasarn, S.; Prathumpai, W.; Laksancharoen, P. Pimarane diterpene from the endophytic fungus Eutypella sp. BCC 13199. Chem. Pharm. Bull. 2011, 59, 1157–1159. [Google Scholar] [CrossRef]
- Sun, L.; Li, D.L.; Chen, Y.C.; Tao, M.H.; Zhang, W.M.; Dan, F.J. Purification and identification of secondary metabolites from marine fungus Eutypella scoparia and their antitumor activities. Mycosystema 2011, 30, 268–274. [Google Scholar]
- Sun, L.; Li, D.L.; Chen, Y.C.; Tao, M.H.; Zhang, W.M.; Dan, F.J. Secondary metabolites of marine fungus Eutypella scoparia from the South China Sea and their antitumor activities. Chin. Tradit. Herb. Drugs 2011, 42, 432–436. [Google Scholar]
- Sun, L.; Li, D.L.; Chen, Y.C.; Tao, M.H.; Zhang, W.M.; Dan, F.J. Two new sesquiterpenes from the marine fungus Eutypella scoparia from the South China Sea. Helv. Chim. Acta. 2011, 95, 157–162. [Google Scholar]
- Oh, D.C.; Jensen, P.R.; Kauffman, C.A.; Fenical, W. Libertellenones A-D: Induction of cytotoxic diterpenoid biosynthesis by marine microbial competition. Bioorg. Med. Chem. 2005, 13, 5267–5273. [Google Scholar]
- ChemBio3D, version Ultra 10.0, 2000; CambridgeSoft, Cambridge, MA, USA.
- Evidente, A.; Sparapano, L.; Bruno, G.; Motta, M. Sphaeropsidins D and E, two other pimarane diterpenes, produced in vitro by the plant pathogenic fungus Sphaeropsis sapinea f. sp. cupressi. Phytochemistry 2002, 59, 817–823. [Google Scholar]
- Dettrakul, S.; Kittakoop, P.; Isaka, M.; Nopichai, S.; Suyarnsestakorn, C.; Tanticharoen, M.; Thebtaranonth, M. Antimycobacterial pimarane diterpenes from the fungus Diaporthe sp. Bioorg. Med. Chem. 2003, 13, 1253–1255. [Google Scholar] [CrossRef]
- Alley, M.C.; Scudiero, D.A.; Monks, A.; Hursey, M.L.; Czerwinski, M.J.; Fine, D.L.; Abbott, B.J.; Mayo, J.G.; Shoemaker, R.H.; Boyd, M.R. Feasibility of drug screening with panels of humantumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988, 48, 589–601. [Google Scholar]
- Sample Availability: Available from the authors.
Supplementary Files
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sun, L.; Li, D.; Tao, M.; Chen, Y.; Dan, F.; Zhang, W. Scopararanes C–G: New Oxygenated Pimarane Diterpenes from the Marine Sediment-Derived Fungus Eutypella scoparia FS26. Mar. Drugs 2012, 10, 539-550. https://doi.org/10.3390/md10030539
Sun L, Li D, Tao M, Chen Y, Dan F, Zhang W. Scopararanes C–G: New Oxygenated Pimarane Diterpenes from the Marine Sediment-Derived Fungus Eutypella scoparia FS26. Marine Drugs. 2012; 10(3):539-550. https://doi.org/10.3390/md10030539
Chicago/Turabian StyleSun, Li, Dongli Li, Meihua Tao, Yuchan Chen, Feijun Dan, and Weimin Zhang. 2012. "Scopararanes C–G: New Oxygenated Pimarane Diterpenes from the Marine Sediment-Derived Fungus Eutypella scoparia FS26" Marine Drugs 10, no. 3: 539-550. https://doi.org/10.3390/md10030539
APA StyleSun, L., Li, D., Tao, M., Chen, Y., Dan, F., & Zhang, W. (2012). Scopararanes C–G: New Oxygenated Pimarane Diterpenes from the Marine Sediment-Derived Fungus Eutypella scoparia FS26. Marine Drugs, 10(3), 539-550. https://doi.org/10.3390/md10030539




