Polymethoxy-1-alkenes from Aphanizomenon ovalisporum Inhibit Vertebrate Development in the Zebrafish (Danio rerio) Embryo Model
Abstract
:1. Introduction
2. Results and Discussion
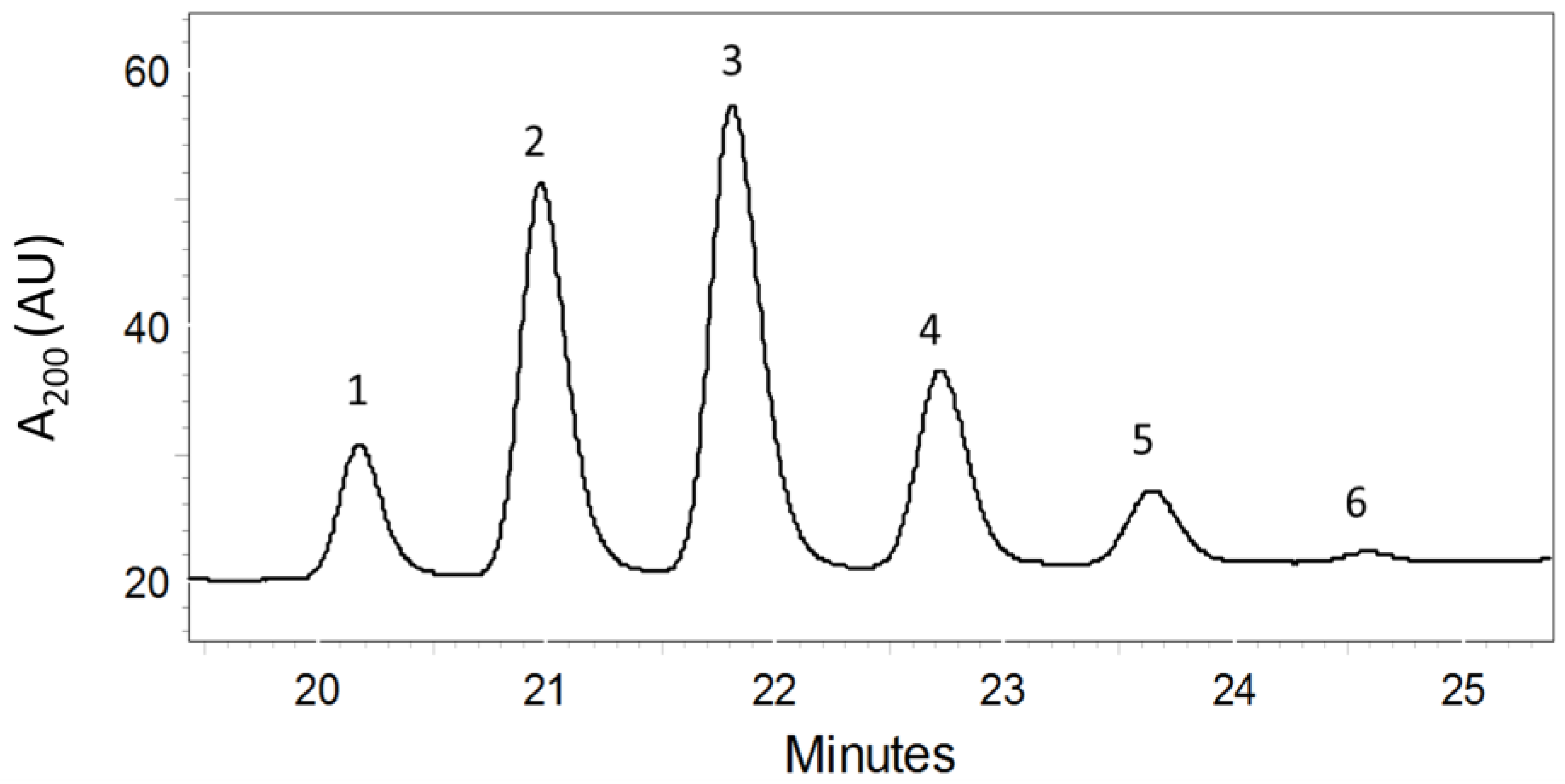
2.1. Purification of PMAs by Bioassay-Guided Fractionation
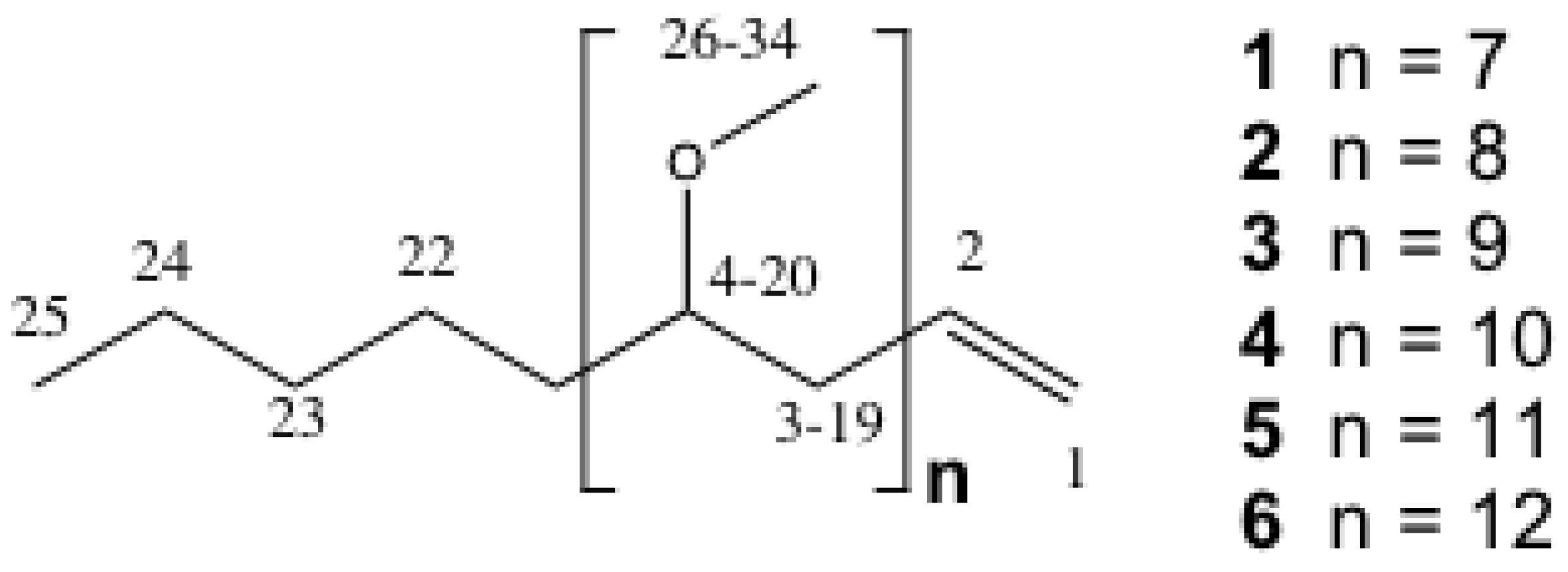
2.2. Structure Elucidation of the PMAs
| C# | δC | δH (mult, J in Hz; #H) | COSY |
|---|---|---|---|
| 1 | 117.01 | 5.13 (dd, 17, 2; 1H) | H-2 |
| 5.09 (dd, 10, 2; 1H) | H-2 | ||
| 2 | 135.26 | 5.93 (ddt, 17, 10, 7; 1H) | H-1, H2-3 |
| 3 | 38.66 | 2.30 (m; 2H) | H-2, H-4 |
| 4 | 77.61 | 3.37 (m; 1H) | H-3, H2-5 |
| 5, 7, 9…19 | 38.35–38.97 | 1.67–2.04 (m; 16H) | See text |
| 6, 8, 10…18 | 75.92–76.11 | 3.65 (m; 7H) | See text |
| 20 | 78.13 | 3.37 (m; 1H) | H2-21, H2-19 |
| 21 | 33.96 | 1.56 (m; 2H) | H2-20, H2-22 |
| 22 | 25.09 | 1.43 (m; 2H) | H2-21, H2-23 |
| 23 | 32.51 | 1.30 (m; 2H) | H2-22, H2-24 |
| 24 | 23.08 | 1.30 (m; 2H) | H2-23, H2-25 |
| 25 | 14.27 | 0.91 (t, 7.1; 3H) | H2-24 |
| 26–34 | 55.93–56.16 | See text |
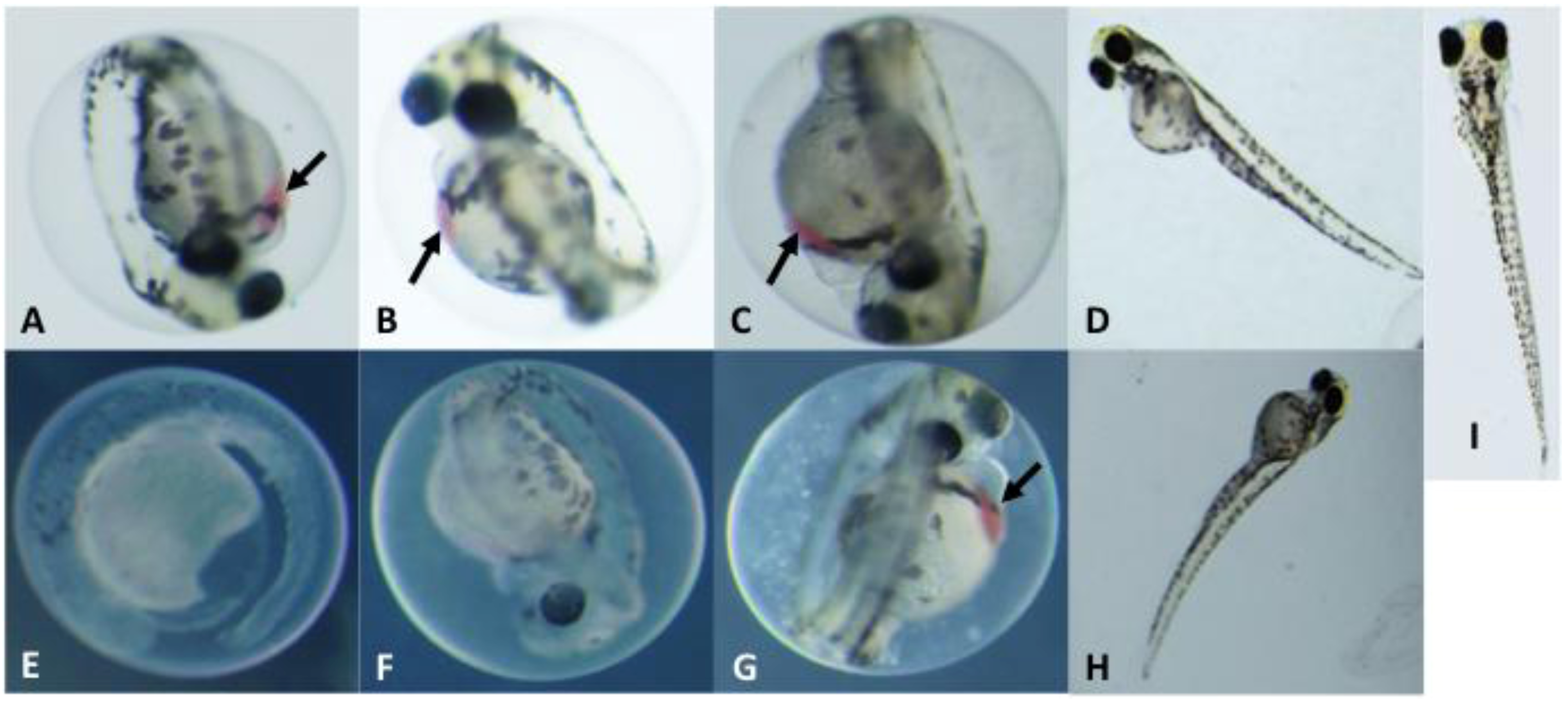
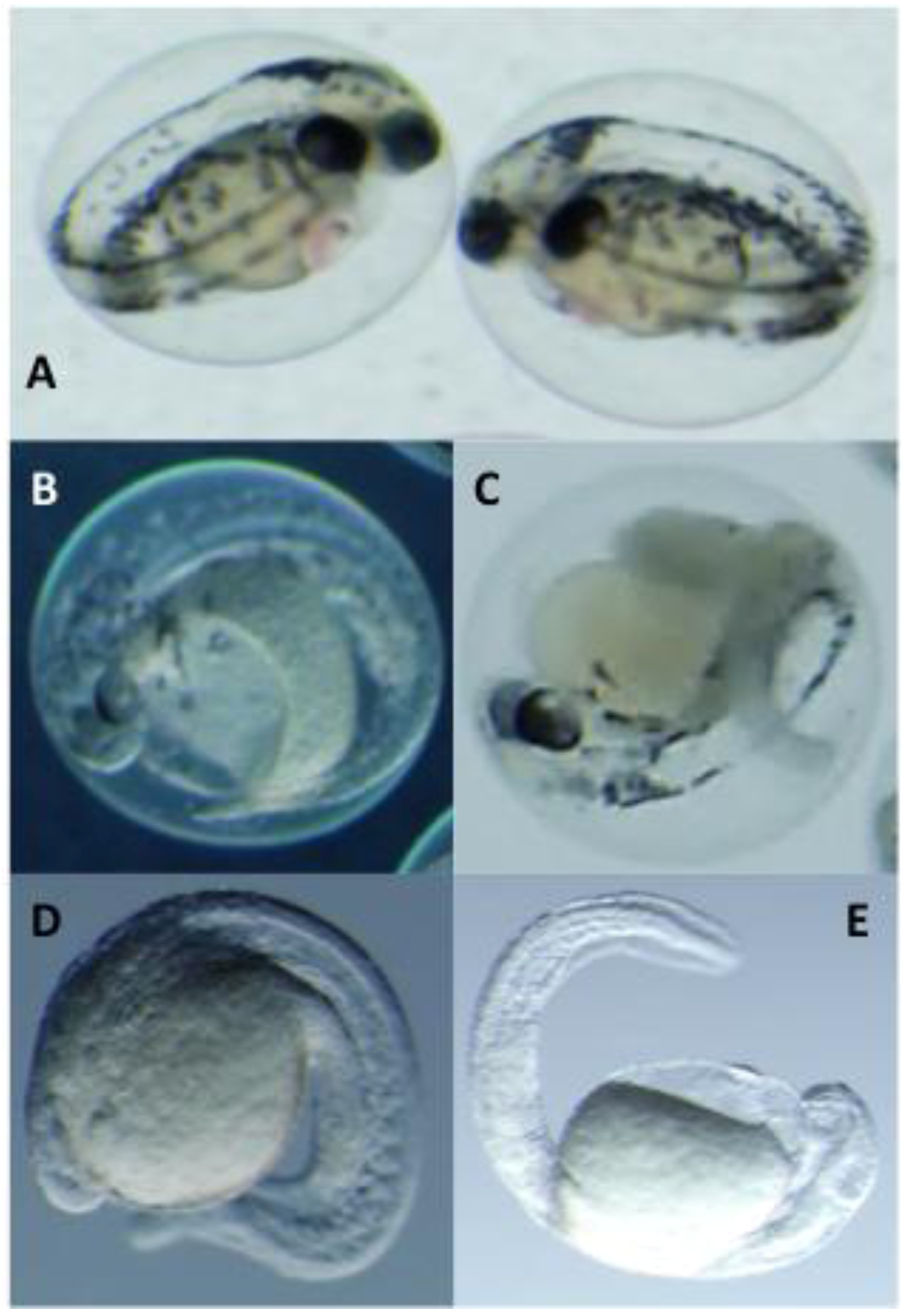
2.3. Toxicological Studies of PMAs
| % Mortality (5 dpf) b | Hatching Rate (4 dpf) b | |||||||
|---|---|---|---|---|---|---|---|---|
| PMA | ||||||||
| a Conc.: | 10 | 25 | 50 | 100 | 10 | 25 | 50 | 100 |
| 1 | 20% | 0% | 100% | 100% | 100% | 100% | 0% | 0% |
| 2 | 0% | 0% | 100% | 100% | 100% | 80% | 0% | 0% |
| 3 | 0% | 0% | 100% | 100% | 100% | 80% | 40% | 0% |
| 4 | 0% | 0% | 40% | 20% | 100% | 100% | 60% | 60% |
| 5 | 0% | 0% | 0% | 0% | 100% | 100% | 80% | 80% |
3. Experimental Section
3.1. Culturing of the Cyanobacteria
3.2. Extraction and Isolation of Polymethoxy-1-alkenes
3.3. Zebrafish Embryo Bioassay
3.4. Characterization of Polymethoxy-1-alkenes
4. Conclusions
Acknowledgements
References
- Valério, E.; Chaves, S.; Tenreiro, R. Diversity and impact of prokaryotic toxins on aquatic environments: A review. Toxins 2010, 2, 2359–2410. [Google Scholar] [CrossRef]
- Carmichael, W.W. A world overview-One-hundred-twenty-seven years of research on toxic cyanobacteria-Where do we go from here? Adv. Exp. Med. Biol. 2008, 619, 105–125. [Google Scholar] [CrossRef]
- Ferrão-Filho, A.; Kozlowsky-Suzuki, B. Cyanotoxins: Bioaccumulation and effects on aquatic animals. Mar. Drugs 2011, 9, 2729–2772. [Google Scholar] [CrossRef]
- Cox, P.A.; Banack, S.A.; Murch, S.J.; Rasmussen, U.; Tien, G.; Bidigare, R.R.; Metcalf, J.S.; Morrison, L.F.; Codd, G.A.; Bergman, B. Diverse taxa of cyanobacteria produce β-methylamino-l-alanine, a neurotoxic amino acid. Proc. Natl. Acad. Sci. USA 2005, 102, 5074–5078. [Google Scholar]
- Krüger, T.; Mönch, B.; Oppenhäuser, S.; Luckas, B. LC-MS/MS determination of the isomeric neurotoxins BMAA (β-N-methylamino-l-alanine) and DAB (2,4-diaminobutyric acid) in cyanobacteria and seeds of Cycas revolute and Lathyrus latifolius. Toxicon 2010, 55, 547–557. [Google Scholar] [CrossRef]
- Berry, J.P.; Gantar, M.; Gawley, R.E.; Wang, M.; Rein, K.S. Pharmacology and toxicology of pahayokolide A, a bioactive metabolite from a freshwater species of Lyngbya Isolated from the Florida everglades. Comp. Biochem. Physiol. C Pharmacol. Toxicol. 2004, 139, 231–238. [Google Scholar]
- Berry, J.; Gantar, M.; Gibbs, P.; Schmale, M. The zebrafish (Danio rerio) embryo as a model system for identification and characterization of developmental toxins from marine and freshwater microalgae. Comp. Biochem. Physiol. C Pharmacol. Toxicol. 2007, 145, 61–72. [Google Scholar] [CrossRef]
- Berry, J.; Gibbs, P.; Schmale, M.; Saker, M. Toxicity of cylindrospermopsin, and other apparent metabolites from Cylindrospermopsis raciborskii and Aphanizomenon ovalisporum, to the zebrafish (Danio rerio) embryo. Toxicon 2009, 53, 289–299. [Google Scholar] [CrossRef]
- Teraoka, H.; Dong, W.; Hiraga, T. Zebrafish as a novel experimental model for developmental toxicology. Congenit. Anom. 2003, 43, 123–132. [Google Scholar] [CrossRef]
- Hill, A.J.; Teraoka, H.; Heideman, W.; Peterson, R.E. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. 2005, 86, 6–19. [Google Scholar] [CrossRef]
- Wang, P.J.; Chien, M.S.; Wu, F.J.; Chou, H.N.; Lee, S.J. Inhibition of embryonic development by microcystin-LR in zebrafish, Danio rerio. Toxicon 2005, 45, 303–308. [Google Scholar] [CrossRef]
- Banker, R.; Carmeli, S.; Hadas, O.; Teltsch, B.; Porat, R.; Sukenik, A. Identification of cylindrospermopsin in Aphanizomenon ovalisporum (Cyanophceae) isolated from Lake Kinneret, Israel. J. Phycol. 1997, 33, 613–616. [Google Scholar]
- Banker, R.; Teltsch, B.; Sukenik, A.; Carmeli, S. 7-Epicylindrospermopsin, a toxic minor metabolite of the cyanobacterium Aphanizomenon ovalisporum from Lake Kinneret, Israel. J. Nat. Prod. 2000, 63, 387–389. [Google Scholar] [CrossRef]
- Mynderse, J.S.; Moore, R.E. Isotactic polymethoxy-1-Alkenes from the blue-green alga Tolypothrix conglutinata var. chlorata. Phytochemistry 1979, 18, 1181–1183. [Google Scholar] [CrossRef]
- Mori, Y.; Kohchi, Y.; Suzuki, M. Isotactic polymethoxy-1-alkenes from blue-green algae. Synthesis and absolute stereochemistry. J. Org. Chem. 1991, 56, 631–637. [Google Scholar] [CrossRef]
- Mori, Y.; Kohchi, Y.; Noguchi, H.; Suzuki, M.; Carmeli, S.; Moore, R.E.; Patterson, G.M.L. Isotactic polymethoxy-1-alkenes from the terrestrial blue-green alga, Scytonema ocellatum: Structure and synthesis. Tetrahedron 1991, 47, 4889–4904. [Google Scholar] [CrossRef]
- Staunton, J.; Weissman, K.J. Polyketide biosynthesis: A millenium review. Nat. Prod. Rep. 2001, 18, 380–416. [Google Scholar] [CrossRef]
- Rao, M.R.; Faulkner, D.J. Isotactic polymethoxydienes from the Philippines sponge Myriastra clavosa. J. Nat. Prod. 2002, 65, 1201–1203. [Google Scholar] [CrossRef]
- Leão, P.; Pereira, A.R.; Liu, W.T.; Ng, J.; Pevzner, P.A.; Dorrestein, P.C.; König, G.M.; Vasconcelos, V.M.; Gerwick, W.H. Synergistic allelochemicals from a freshwater cyanobacterium. Proc. Natl. Acad. Sci. USA 2010, 107, 11183–11188. [Google Scholar]
- MacMillan, J.B.; Ernst-Russell, M.A.; de Ropp, J.S.; Molinski, T.F. Lobocyclamides A-C, lipopeptides from a cryptic cyanobacterial mat containing Lyngbya confervoides. J. Org. Chem. 2002, 67, 8210–8215. [Google Scholar] [CrossRef]
- Gantar, M.; Berry, J.P.; Thomas, S.; Wang, M.; Perez, R.; Rein, K.S. Allelopathic activity among cyanobacteria and microalgae isolated from Florida freshwater habitats. FEMS Microbiol. Ecol. 2008, 64, 55–64. [Google Scholar] [CrossRef]
- Brand, M.; Granato, M.; Nüsslein-Volhard, C. Keeping and Raising Zebrafish. In Zebrafish; Nüsslein-Volhard, C., Dahm, R., Eds.; Oxford University Press: Oxford, UK, 2002; pp. 7–37. [Google Scholar]
- Samples Availability: Available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jaja-Chimedza, A.; Gantar, M.; Gibbs, P.D.L.; Schmale, M.C.; Berry, J.P. Polymethoxy-1-alkenes from Aphanizomenon ovalisporum Inhibit Vertebrate Development in the Zebrafish (Danio rerio) Embryo Model. Mar. Drugs 2012, 10, 2322-2336. https://doi.org/10.3390/md10102322
Jaja-Chimedza A, Gantar M, Gibbs PDL, Schmale MC, Berry JP. Polymethoxy-1-alkenes from Aphanizomenon ovalisporum Inhibit Vertebrate Development in the Zebrafish (Danio rerio) Embryo Model. Marine Drugs. 2012; 10(10):2322-2336. https://doi.org/10.3390/md10102322
Chicago/Turabian StyleJaja-Chimedza, Asha, Miroslav Gantar, Patrick D. L. Gibbs, Michael C. Schmale, and John P. Berry. 2012. "Polymethoxy-1-alkenes from Aphanizomenon ovalisporum Inhibit Vertebrate Development in the Zebrafish (Danio rerio) Embryo Model" Marine Drugs 10, no. 10: 2322-2336. https://doi.org/10.3390/md10102322
APA StyleJaja-Chimedza, A., Gantar, M., Gibbs, P. D. L., Schmale, M. C., & Berry, J. P. (2012). Polymethoxy-1-alkenes from Aphanizomenon ovalisporum Inhibit Vertebrate Development in the Zebrafish (Danio rerio) Embryo Model. Marine Drugs, 10(10), 2322-2336. https://doi.org/10.3390/md10102322